Abstract
Objective
We conducted a systematic review and meta-analysis to assess the diagnostic value of the electrocardiogram (ECG) method in detecting cardiac amyloidosis (CA) to indicate its clinical application.
Methods
We searched PubMed, Web of Science, OVID Medline, and Cochrane Library databases for clinical trials assessing the diagnostic performance of ECG in detecting CA. We employed the risk ratio and 95% confidence interval (CI) to explicit estimates. QUADAS-2 was applied to evaluate the bias risk and the clinical applicability of the included studies. Reviewer Manager (RevMan) 5.3 and Stata 16.0 were employed to complete all statistical analyses.
Results
This meta-analysis included ten studies (N = 6353 patients). Overall, the findings of the study exposed that, for CA patients in whom the ECG method was used, the sensitivity and specificity were 0.49 and 0.91, respectively. The positive likelihood ratio (LR) and negative LR were 5.17 and 0.57, respectively. The diagnostic odds ratio (DOR) and diagnostic score of the ECG in detecting CA were 9.11 and 2.21. The area under the curve (AUC) was 0.83(95% CI = 0.79–0.86). The hierarchical summary receiver operating characteristic (HSROC) curve further confirmed the diagnostic accuracy of the ECG, demonstrating a high prediction and confidence interval for the pooled estimate. No significant publication bias was detected, as confirmed by funnel plot analysis. Sensitivity analysis confirmed that the pooled estimates for ECG remained stable after the exclusion of individual studies, underscoring the robustness of the findings. The combined DOR and diagnostic score were 9.11 and 2.21, respectively.
Conclusions
ECM has low sensitivity and high specificity in the diagnosis of CA. AUC > 0.5, indicating that ECM has accuracy and diagnostic value in the diagnosis of CA to some extent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Cardiac amyloidosis (CA) is mainly caused by the misfolding and progressive deposition of fibrillar amyloid precursor protein in heart tissue [1]. Endomyocardial biopsy (EMB) is the gold standard for diagnosing CA. However, EMB is an invasive examination method with high technical requirements and cost [2]. Therefore, there is a critical need for non-invasive diagnostic methods.
Currently, the most prevalent methods for non-invasive diagnosis of CA include the detection of serological markers, electrocardiogram (ECG), echocardiogram (ECHO), cardiovascular magnetic resonance imaging (CMR), and nuclear imaging, among others [3, 4]. Among these, and radionuclide imaging, ECG stands out as the simplest and most frequently utilized auxiliary diagnostic tool. It offers the benefits of ease of use, short duration, and affordability [5]. The ECG manifestations in CA patients can be summarized as follows: presence of low voltage, absence of poor R wave progression in precordial leads, and occurrence of false infarction waves, [6]. Distinctive ECG findings can supply critical hints for the diagnosis of CA [7]. Rencuzogullari et al. [8] found that a prolonged R-S interval in patients with pulmonary embolism (PE) might indicate the condition. The study focused on high-risk patients and used digital ECG results, which are valuable for clinical diagnosis. Another study investigated the relationship between the fQRS-T angle and blood pressure (BP) patterns in newly diagnosed hypertensive individuals without left ventricular hypertrophy (LVH) [9]. Similarly, Karakayali et al. [10]. evaluated the efficacy of P-wave indices (PWIs) from ECG in predicting atrial high-rate episodes (AHRE) in cardiovascular implanted electronic devices (CIEDs) patients, noting that prolonged P wave frontal plane duration (PFWPD) could be linked to AHRE.
Currently, there is no relevant meta-analysis on the diagnostic value of ECG in detecting CA. Therefore, we searched for and analyzed relevant studies on the sensitivity and specificity of ECG in diagnosing CA, summarized these studies through meta-analysis, and validated the diagnostic value of ECG in CA.
Methods
Protocol
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
Eligibility criteria
Two reviewers independently assessed the titles and abstracts from the literature search to identify eligible articles. Articles were excluded if they were animal or cell experiments, case reports, reviews, lectures, or if they contained insufficient data. Studies were included if they met the following criteria: (1) The study was a diagnostic accuracy experiment. (2) Patients in the study were diagnosed with CA. Diagnostic criteria: Myocardial pathological specimens were obtained by endocardial biopsy, and the Congo red staining was positive. Alternatively, patients were diagnosed combined with ECG, ECHO, CMR, nuclear imaging, and other related examinations for clinical diagnosis of CA. (3) Studies that evaluated the accuracy of ECG. (4) The data for true positive, false positive, true negative, and false negative outcomes were available for extraction.
Search strategy and data extraction
We conducted an extensive search of the databases PubMed, Web of Science, OVID Medline, and Cochrane Library for articles published in English from January 1, 2010, to December 31, 2021. Key words or phrases were employed for the search: [(Cardiac amyloidosis) OR (Transthyretin Amyloidosis)]AND[(Electrocardiography) OR (Electrocardiogram) OR (ECG) OR (Electrocardiograph)]. Two independent reviewers carried out the original search, which was then reiterated and refined by a third researcher to validate the retrieved articles against the search strategy. The data extraction process included capturing information such as the author, publication year, country, design type, sample size of case and control group, age, time interval, blind method, diagnostic criteria, sensitivity, specificity, true positive (TP), false positive (FP), true negative (TN), false negative (FN), among others. The Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool was used for assessing the quality of the diagnostic accuracy studies.
Statistical analysis
Revman V.5.3 (The Cochrane Collaboration) was employed to assess the bias risk of the included articles. Stata 16.0 (StataCorp, USA) was used for meta-analysis. We employed the risk ratio and 95% confidence interval (CI) to explicit estimates. The consequences were exhibited as a forest plot, and heterogeneity among the articles was calculated by the I2 statistic. The publication bias was assessed by the funnel plot with Begg’s test. SROC curve was employed to evaluate and imagine the diagnostic performance of involved studies.
Results
Study selection
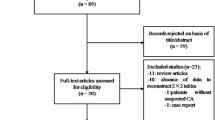
Figure 1 demonstrates the flowchart of the literature selection process. The initial search yielded 568 publications from electronic databases. Duplicates and inappropriate articles were excluded, generating 471 articles for title and abstract assessment. After eliminating 369 articles for irrelevant study aims, the remaining 102 were evaluated through the full-text review. Subsequently, 92 studies were excluded for the following reasons: unavailability of the full text, inappropriate study design, population consisting of individuals under the age of 18, and insufficient data. Finally, The full-text assessment ultimately yielded ten articles that met the selection criteria, and these ten studies were included in the meta-analysis.
Study characteristics and quality control
The characteristics and general information of the involved studies are demonstrated in Tables 1 and 2. Among these articles, two were conducted in Italy, 3 in China, 1 in France, and 4 in the United States.
Quality control
The quality of the involved articles was assessed using the QUADAS-2 scale. Two researchers conducted the quality evaluation, and the findings are exhibited in Fig. 2.
Diagnostic performance of ECG
The combined sensitivity reflects the ability of ECG in the detection of CA. As demonstrated in Figs. 3 and 4, the combined sensitivity was 0.49 (95%CI = 0.36–0.61, P = 0.00, I2 = 97.70%), and the combined specificity was 0.91 (95%CI = 0.85–0.94, P = 0.00, I2 = 96.33%). In addition, the positive LR was 5.17 (95%CI = 3.26–8.19, P = 0.00, I2 = 94.51%); and negative LR was 0.57 (95%CI = 0.45–80.71, P = 0.00, I2 = 98.43%).
Diagnostic odds ratio and diagnostic score
The forest map of the DOR of the ECG in detecting CA is demonstrated in Fig. 5 (DOR merge = 9.11) (95% CI: 5.08–16.34). The forest map of the diagnostic score of the ECG in detecting CA was 2.21 (95% CI: 1.63–2.79).
Summary receiver operative characteristic curve analysis
The area under the SROC curve reflects the accuracy of the diagnostic trial. The pooled diagnostic value of ECG in detecting CA was evaluated by SROC curve analysis; the area under the SROC was 0.83 (95% CI 0.79–0.86, Fig. 6).
Hierarchical summary receiver operating characteristic curve analysis
The pooled diagnostic value of ECG in detecting CA was evaluated by HSROC curve analysis; the 95% confidence region and 95% prediction region for each study estimate and merger estimate are shown in Fig. 7. The findings confirmed that ECG detection might exhibit good diagnostic accuracy for differentiating CA from healthy controls.
Publication bias
Deek’s funnel plot assessment for publication bias showed that Bias coefficient = 7.13 (95%CI= -4.15-18.30), P = 0.16, suggesting no publication bias in the meta-analysis (Fig. 8).
Sensitivity analysis
Sensitivity analysis was conducted to estimate the prospective influence of a single study on the overall estimate. After excluding each study one by one, the pooled consequences of ECG persisted robustly.
Discussion
The clinical manifestations of CA are diverse and lack specificity. Endocardial biopsy (EMB) is the gold standard for diagnosing CA [11]. However, EMB is an invasive examination. Therefore, non-invasive diagnostic methods play a crucial role in the diagnosis of CA. At present, non-invasive diagnostic approaches include ECG, cardiac Doppler ultrasound, cardiac MRI, and radionuclide imaging [12]. Among them, ECG is the most simple and commonly used auxiliary diagnostic tool, providing important clues for the preliminary diagnosis of CA [13]. With this simple and feasible non-invasive diagnosis method, early diagnosis and early treatment can be achieved by screening this rare disease.
Recently, the incidence of CA has been on the rise, and many studies are constantly exploring and improving the methods of diagnosing CA [14]. However, there is a need for detection methods that possess the same high specificity as myocardial biopsy. Therefore, patients with clinically unexplained heart failure or refractory heart failure should be monitored with heightened vigilance, and attention should be paid to the possibility of CA. Currently, it is necessary to comprehensively analyze and evaluate the relevant examination results comprehensively. Numerous studies have suggested that abnormalities in ECG are nearly universal in CA patients, but the specificity is low. An ECG with extensive low voltage and a poor R wave increases in precordial leads may also indicate CA [15]. Eicher et al. [16] performed a trial on 276 patients with CA; they found that the specificity of ECG in diagnosing CA was very high. Sharma et al. [17] have suggested that in the diagnosis of CA, the sensitivity of ECM is about 54%. For patients suspected of CA, the diagnostic significance of ECM cannot be overlooked. It can reduce the need for invasive detection methods such as EMB, thus significantly improving the diagnosis rate of CA. However, research into the accuracy of ECG in diagnosing CA needs to be more consistent.
We conducted this meta-analysis to search and analyze the literature about the application of ECG in diagnosing CA. This meta-analysis revealed that the sensitivity and specificity were 0.49 and 0.91, respectively. The positive LR and negative LR were 5.17 and 0.57, respectively. The area under the SROC curve was 0.83. The sensitivity of ECG reflects its diagnostic ability in detecting CA. Our study revealed that the sensitivity was low, indicating that the diagnostic ability for this disease could be improved. Lijima et al. [18] observed t a specificity of 43% for ECG in the diagnosis of CA, which was consistent with our findings. Furthermore, we observed that the combined DOR and diagnostic score of the ECG in detecting CA were 9.11 and 2.21, suggesting that the ECG has a particular value in diagnosing CA. PLR refers to the ratio of the probability of positive results between the CA group and the non-CA group. The combined PLR was 5.17 (95%CI = 3.26–8.19). A higher PLR indicates a stronger ability to diagnose diseases accurately. The PLR of this study was 5.17, suggesting that the diagnostic ability of ECG in diagnosing CA was moderate. In this study, the NLR was 0.57, suggesting that ECM has a solid ability to rule out the disease. AUC was 0.83(95% CI = 0.79–0.86), suggesting that ECG had accuracy in diagnosing CA. HSROC curve, reflecting the discriminative ability of the diagnostic test, had an estimated value of 1.89 (0.94–2.84). These findings indicated that ECM had low sensitivity and high specificity in the diagnosis of CA, with an AUC greater than 0.5, suggesting that it has accuracy and diagnostic value to some extent.
However, this study had some limitations. The source of heterogeneity was not explored in this study. Diagnostic cut-offs varied among the different studies, and some studies included did not demonstrate that small sample sizes were a source of heterogeneity. The majority of the included literature was based on single-center data analysis; thus, the representativeness could be improved.
Conclusions
In brief, this meta-analysis found that ECM has low sensitivity and high specificity in diagnosing CA. With an AUC greater than 0.5, this indicates that ECM has a certain level of accuracy and diagnostic value in the diagnosis of CA. The diagnosis by ECM, combined with other noninvasive diagnostic methods, can help achieve the goal of early detection, diagnosis, and treatment for CA. The high specificity of the ECG suggests that any abnormal findings should prompt further diagnostic testing, likely including echocardiography, MRI, or biopsy, to confirm CA. Treatment for CA depends on the subtype and severity, with various options including chemotherapy, immunotherapy, supportive care, or heart transplantation. We stress that while the ECG is a useful initial screen, a multimodal diagnostic strategy is essential to avoid delayed diagnosis and suboptimal treatment. The ECG’s diagnostic performance, particularly its low sensitivity, necessitates a multifaceted approach to patient management.
Data availability
The datasets used during the present study are available from the corresponding author upon reasonable request.
Abbreviations
- ECG:
-
Electrocardiogram
- CA:
-
Cardiac amyloidosis
- CI:
-
Confidence interval
- LR:
-
Likelihood ratio
- DOR:
-
Diagnostic odds ratio
- AUC:
-
Area under the curve
- SROC:
-
Summary receiver operating characteristic
- EMB:
-
Endomyocardial biopsy
- ECHO:
-
Echocardiogram
- CMR:
-
Cardiovascular magnetic resonance imaging
- TP:
-
True positive
- FP:
-
False positive
- TN:
-
True negative
- FN:
-
False negative
References
Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial diseases. Eur Heart J. 2021;42(16):1554–68.
Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, et al. Endomyocardial Biopsy characterization of heart failure with preserved ejection fraction and prevalence of Cardiac Amyloidosis. JACC Heart Fail. 2020;8(9):712–24.
Hotta VT, Giorgi MCP, Fernandes F, Abduch MCD, Falcão A, Mady C. Cardiac amyloidosis: non-invasive diagnosis. Rev Assoc Med Bras (1992) 2020, 66(3):345–352.
Garcia-Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, et al. Diagnosis and treatment of cardiac amyloidosis. A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial diseases. Eur J Heart Fail. 2021;23(4):512–26.
Ng PLF, Lim YC, Evangelista LKM, Wong RCC, Chai P, Sia CH, Loi HY, Yeo TC, Lin W. Utility and pitfalls of the electrocardiogram in the evaluation of cardiac amyloidosis. Ann Noninvasive Electrocardiol. 2022;27(4):e12967.
Povar-Echeverría M, Auquilla-Clavijo PE, Povar-Marco BJ, Moreno-Esteban EM, Figueras-Villalba MP. Cardiac amyloidosis: a review of a series of cases. Arch Cardiol Mex. 2020;90(3):259–65.
Mavrogeni SI, Vartela V, Ntalianis A, Vretou R, Ikonomidis I, Tselegkidou M, Paraskevaidis I, Markousis-Mavrogenis G, Noutsias M, Rigopoulos A, et al. Cardiac amyloidosis: in search of the ideal diagnostic tool. Herz. 2021;46(Suppl 1):9–14.
Rencuzogullari I, Çağdaş M, Karabağ Y, Karakoyun S, Çiftçi H, Gürsoy MO, Karayol S, Çinar T, Tanik O, Hamideyin Ş. A novel ECG parameter for diagnosis of acute pulmonary embolism: RS time: RS time in acute pulmonary embolism. Am J Emerg Med. 2019;37(7):1230–6.
Karakayali M, Artac I, Omar T, Rencuzogullari İ, Karabag Y, Cinar T, Altunova M, Hamideyin S. The association between frontal QRS-T angle and reverse dipper status in newly diagnosed hypertensive patients. Blood Press Monit. 2023;28(2):96–102.
Karakayali M, Artac I, Omar T, Rencuzogullari I, Karabag Y, Hamideyin S. Assessment of the efficacy of the electrocardiographic P-wave peak time in predicting atrial high rate episode in patients with cardiac implantable electronic devices. J Electrocardiol. 2023;80:40–4.
Sennott J, Ananthasubramaniam K. Multimodality imaging approach to cardiac amyloidosis: part 2. Heart Fail Rev. 2022;27(5):1515–30.
Rigopoulos AG, Ali M, Abate E, Torky AR, Matiakis M, Mammadov M, Melnyk H, Vogt A, de Vecchis R, Bigalke B, et al. Advances in the diagnosis and treatment of transthyretin amyloidosis with cardiac involvement. Heart Fail Rev. 2019;24(4):521–33.
Dominguez F. Predicting pacemaker implantation in cardiac amyloidosis: let’s start with an ECG. Eur J Heart Fail. 2022;24(7):1237–8.
Erbel-Khurtsidze S, Seibel R, Moka D, Brandt-Mainz K, Erbel R, Lichtenberg A. [Cardiac amyloidosis and aortic valve stenosis]. Herz. 2021;46(5):485–96.
Finocchiaro G, Sheikh N, Biagini E, Papadakis M, Maurizi N, Sinagra G, Pelliccia A, Rapezzi C, Sharma S, Olivotto I. The electrocardiogram in the diagnosis and management of patients with hypertrophic cardiomyopathy. Heart Rhythm. 2020;17(1):142–51.
Eicher JC, Audia S, Damy T. [Transthyretin cardiac amyloidosis]. Rev Med Interne. 2020;41(10):673–83.
Sharma S, Labib SB, Shah SP. Electrocardiogram Criteria to diagnose Cardiac Amyloidosis in Men with a Bundle Branch Block. Am J Cardiol. 2021;146:89–94.
Iijima T, Sawa N, Wake A, Kono K, Kinowaki K, Ubara Y, Ohashi K. Linear discriminant analysis on electrocardiogram achieved classification of cardiac involvement status in amyloid light-chain amyloidosis. J Cardiol. 2023;82(5):349–55.
Rahman JE, Helou EF, Gelzer-Bell R, Thompson RE, Kuo C, Rodriguez ER, Hare JM, Baughman KL, Kasper EK. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43(3):410–5.
Austin BA, Tang WH, Rodriguez ER, Tan C, Flamm SD, Taylor DO, Starling RC, Desai MY. Delayed hyper-enhancement magnetic resonance imaging provides incremental diagnostic and prognostic utility in suspected cardiac amyloidosis. JACC Cardiovasc Imaging. 2009;2(12):1369–77.
Cheng Z, Kang L, Tian Z, Chen W, Guo W, Xu J, Chen T, Fang L, Zeng Y, Cheng K, et al. Utility of combined indexes of electrocardiography and echocardiography in the diagnosis of biopsy proven primary cardiac amyloidosis. Ann Noninvasive Electrocardiol. 2011;16(1):25–9.
Cheng Z, Zhu K, Tian Z, Zhao D, Cui Q, Fang Q. The findings of electrocardiography in patients with cardiac amyloidosis. Ann Noninvasive Electrocardiol. 2013;18(2):157–62.
Mussinelli R, Salinaro F, Alogna A, Boldrini M, Raimondi A, Musca F, Palladini G, Merlini G, Perlini S. Diagnostic and prognostic value of low QRS voltages in cardiac AL amyloidosis. Ann Noninvasive Electrocardiol. 2013;18(3):271–80.
Di Bella G, Minutoli F, Piaggi P, Casale M, Mazzeo A, Zito C, Oreto G, Baldari S, Vita G, Pingitore A, et al. Usefulness of combining Electrocardiographic and echocardiographic findings and brain natriuretic peptide in early detection of Cardiac Amyloidosis in subjects with transthyretin gene mutation. Am J Cardiol. 2015;116(7):1122–7.
Huang J, Zhao S, Chen Z, Zhang S, Lu M. Contribution of Electrocardiogram in the differentiation of Cardiac Amyloidosis and Nonobstructive Hypertrophic Cardiomyopathy. Int Heart J. 2015;56(5):522–6.
Nicol M, Baudet M, Brun S, Harel S, Royer B, Vignon M, Lairez O, Lavergne D, Jaccard A, Attias D, et al. Diagnostic score of cardiac involvement in AL amyloidosis. Eur Heart J Cardiovasc Imaging. 2020;21(5):542–8.
Zhang KW, Zhang R, Deych E, Stockerl-Goldstein KE, Gorcsan J 3rd, Lenihan DJ. A multi-modal diagnostic model improves detection of cardiac amyloidosis among patients with diagnostic confirmation by cardiac biopsy. Am Heart J. 2021;232:137–45.
Grogan M, Lopez-Jimenez F, Cohen-Shelly M, Dispenzieri A, Attia ZI, Abou Ezzedine OF, Lin G, Kapa S, Borgeson DD, Friedman PA, et al. Artificial Intelligence-enhanced Electrocardiogram for the early detection of Cardiac Amyloidosis. Mayo Clin Proc. 2021;96(11):2768–78.
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
Haitao Sun, and Zheng Shi were responsible for research design, and conducting the experiments. Wei Liu was responsible for data acquisition, data analysis, and writing the manuscript. All the authors have contributed to the completion of this paper.
Corresponding author
Ethics declarations
Ethics approval
This meta-analysis was based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Conflict of interest
No conflicts of interest exit in the submission of this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sun, H., Shi, Z. & Liu, W. The value of the electrocardiogram in the recognition of cardiac amyloidosis: a systematic meta-analysis. BMC Cardiovasc Disord 24, 488 (2024). https://doi.org/10.1186/s12872-024-04143-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-024-04143-z