Abstract
Background
Direct-acting antivirals (DAAs) show high cure rates in treating chronic hepatitis C virus (HCV). However, the effect of DAAs on patients infected with genotype 2 (GT2) is difficult to determine despite the availability of several DAA regimens.
Methods
A systematic search of six databases (PubMed, Embase, Cochrane Library, Web of Science, CNKI, and Clinicaltrial.gov) was conducted through April 20, 2022. We considered the sustained virological response 12 weeks after treatment (SVR12) as the efficacy outcome, and adverse events (AEs) as the safety outcome. By calculating the mean SVR12 and the proportion of AEs among patients, we considered the intervention effect for each DAA regimen. The random effect model was then used in all meta-analyses. This systematic review and meta-analysis aimed to summarize the evidence on efficacy and safety of DAAs in patients infected with HCV GT2. The Bayesian Markov Chain Monte Carlo (MCMC) network metanalysis was used to indirectly compare regimen in GT2 patients.
Results
Among 31 articles included (2,968 participants), consisting of 1,387 treatment-naive patients and 354 patients with cirrhosis. The overall pooled SVR12 rate was 94.62% (95% CI: 92.43-96.52%) among the participants who received all doses of treatment. Meta-analysis results of AEs revealed that fatigue was the most common AE (14.0%, 95% CI: 6.4-21.6%), followed by headache (13.1%, 95% CI: 9.2-17.1%), whereas death and serious adverse events were uncommon.
Conclusions
We compared DAA-based treatments indirectly using meta-analysis and found the combination of Sofosbuvir plus Velpatasvir and Glecaprevir plus Pibrentasvir, each administered over a 12-week period, were identified as the most effective and relatively safe in managing chronic hepatitis C virus genotype 2 (HCV GT2) infection. Both treatments achieved a SVR12 of 100% (95% CI 99–100%).
Similar content being viewed by others
Background
Hepatitis C virus (HCV) is a blood-borne pathogen that could cause both chronic and acute hepatitis infection; around 50–80% of patients would develop a chronic inflammatory condition, which may lead to liver cirrhosis and hepatocellular carcinoma [1, 2]. Despite the implementation of universal precautions and blood safety measures, HCV infection continues to be a severe global public health burden [3]. The global estimated viraemic prevalence for hepatitis C virus infection was 0.7% (95% UI, 0.7–0.9), corresponding to 56.8 million (95% UI, 55.2–67.8) people infected with HCV in 2020 [4]. Besides, no prophylactic vaccine is available to prevent HCV infection, so the strategy to control HCV has to follow the treatment-as-prevention principle [1].
Since the emergence of well-tolerated oral direct-acting antivirals (DAAs) in early 2014, several major guidelines have recommended DAAs as the first-line treatment for patients infected with HCV instead of pegylated interferon (PEG-IFN) treatment, but therapy options vary according to factors such as HCV genotypes and patients’ status [5,6,7]. As a member of the Flaviviridae family, HCV is heterogeneous and can be classified into 7 genotypes and 67 subtypes, with genotype 1 being the most prevalent in the Americas, Europe, Australia, New Zealand, Central Asia and East Asia [8, 9]. Hepatitis C virus genotype 2 (HCV GT2) is the third predominant genotype in Asia, Africa and America, whereas the prevalence rate varies by geographical distribution, ranging from 62.9% in West Sub-Saharan Africa to 0.8% in North Africa and Middle East [10].
Nevertheless, compared with genotype 1 and 3, the evidence base for DAA therapies in GT2 patients is less extensive. Although there has been clinical evidence to support the novel DAAs entering the market, the majority of trials are open-label, single-arm studies that lack placebo comparators and primarily focus on individual DAA regimens. The comparative efficacy of individual combination therapies remains largely undetermined, primarily due to the scarcity of head-to-head trials Thus far, no studies have compared the efficacy and safety of all DAAs regimens for treating patients infected with HCV GT2.
To fill these gaps, this systematic review and meta-analysis focused on the studies about DAAs treatment for HCV GT2, to assess the comparative efficacy of DAAs regimens, and to identify the benefits and AEs associated with each DAAs intervention.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols guidelines (PRISMA-P) were adhered to this meta-analysis and systematic review [11]. This systematic review was registered on the PROSPERO database for systematic reviews (CRD: 42022344032).
Eligibility criteria
We predefined criteria for inclusion in accordance with PICOS principles [12]. Clinical studies which investigated DAAs regimens for treating patients infected with HCV GT2 were eligible for inclusion. The involved population must be adults with diagnosed HCV GT2 infection, who never had HIV/HCV co-infected or decompensated cirrhosis. The detailed eligibility criteria are listed in Table 1.
Search strategy
Six databases, including PubMed, Embase, Cochrane Library, Web of Science, CNKI, and Clinicaltrial.gov were searched for the relevant clinical trials. The last systematic search was performed on April 20, 2022. Search strategies were constructed on: (i) population: Chronic, Hepatitis C virus; (ii) Interventions: direct-acting antivirals, DAAs; (iii) study design: clinical trial, including single-arm studies (Supplementary Table S1).
Study selection
The process of selecting studies included two parts, through the literature software EndNote X9. Two reviewers independently screened articles by title and abstract based on eligibility criteria after removing duplicate records. Then, two researchers kept reviewing the remaining literature by full text. Any disputes between the two independent reviewers were resolved through discussion with the senior author.
Data extraction
Two researchers extracted information from the included studies independently. A third researcher resolved any further disagreements. The data extraction form comprises:
-
i.
study design: author, NCT number, published year and country.
-
ii.
study design: population and design.
-
iii.
characteristics of patients: intervention, duration, with/without cirrhosis, treatment-experienced, treatment-naïve.
-
iv.
outcome measures: SVR12, the number of AEs and SAEs.
-
v.
any information for the assessment of the risk of bias.
Quality assessment
The RoB 2 tool was used for the methodological quality evaluation of the randomized controlled trials (RCTs), which evaluated the following five domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result [13]. An outcome of low risk, high risk, or some concerns was reported for each domain. An overall assessment of the risk of bias was then determined for each study.
As for non-randomized studies, we used the risk of bias tool for nonrandomized studies (ROBINS-I) proposed by Cochrane Collaboration to assess the following five domains: (i) misclassification of interventions; (ii) deviations from intended intervention; (iii) missing data; (iv) measurement of outcomes; and (v) selection of the reported result [14]. An outcome of low, moderate, serious, critical, or no information for bias risk was reported for each domain. The combination of the five domains was then used to determine the overall risk of bias, whereas judgement for each domain was based on information extracted from each article.
Two researchers assessed the quality of all included studies for the quality assessment process independently. Any disagreements between the two independent reviewers were resolved by discussion and/or consultation with another researcher.
Statistical analyses
The SVR12 was regarded as the primary efficacy outcome, while the AEs was regarded as the secondary safety outcome. We extracted study-arm data from each study and summarised the intervention effect by calculating the proportion of patients reaching the SVR12 or with any AEs over the number of involved patients. Firstly, the overall pooled arm-specific proportion was conducted regardless of the cirrhosis status of patients, previous history of treatment, or treatment type. Then, subgroup analyses by all the above variables were conducted for specific efficacy outcomes. As for safety outcomes, any AEs reported were recorded and considered. Meta-analyses for SVR12 were conducted by using Freeman-Tukey double arcsine transformation, and a random effect model was performed for all meta-analyses because SVR12 rates were expected to follow the binomial distribution and approach the extreme boundaries [15]. The Bayesian Markov Chain Carlo (MCMC) was used to conduct the network meta-analysis. The MCMC approach was utilized three chains and was refined through 200,000 simulations, with every tenth simulation retained and the first 10,000 discarded as burn-in. We used software R v.4.1.2 and package ‘Meta’ v.5.2-0 & ‘gemtc’ to perform the meta-analysis, estimating and pooling the rate of SVR12 and AEs [16]. Unless otherwise stated, we set two-tailed statistical significance as P values < 0.05 for all analyses.
Assessment of heterogeneity
Heterogeneity of treatment effects was quantified by I2 statistics, with thresholds of 25%, 50% and 75%, where heterogeneity suggests low if (25%≤ I2 < 50%), moderate (50%≤ I2 < 75%), or high (I2 ≥ 75%), respectively [17].
Results
Characteristics of included studies
A total of 4,486 studies were yielded from database search, of which 703 duplicates were removed. After applying our inclusion criteria, we identified 31 articles for our meta-analysis, which included 35 clinical studies. All included articles were published within the past 10 years, with the majority being recent studies published within the past 5 years.
In accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, our search strategy and selection process are depicted in Fig. 1. Our search yielded a total of 31 articles, consisting of 9 randomized controlled trials (RCTs) and 26 non-randomized studies. Of the 35 clinical trials, 13 were conducted in phase 2, while 21 were conducted in phase 3; 1 clinical trial was conducted in phase 4. All 35 included studies were multicenter clinical trials.
2,968 participants with HCV GT2 from these 35 studies were eligible for the data synthesis, as presented in Table 2, consisting of 1,389 treatment-naive patients and 354 patients with cirrhosis. The sample size of the included clinical trials ranged from 18 to 458. All participants were diagnosed with HCV GT2 infection and received DAA therapy as their primary treatment.
A total of 23 combinations of DAAs were investigated, duration of therapy ranged from 6 to 16 weeks with or without the addition of ribavirin. We excluded the 6-week regimens from our meta-analysis due to a limitation on sample size (n = 6). Among the 35 clinical trials included in this study, DAAs were regarded as the first-line treatment. According to the mechanism of action, DAAs were divided into four types [18]:
-
i.
NS5A Inhibitor (Daclatasvir, Elbasvir, Ledipasvir, Ombitasvir, Ledipasvir, Pribrentasvir, Velpatasvir).
-
ii.
Protease NS3/4A Inhibitor (Glecaprevir, Paritaprevir, Simeprevir, Grazoprevir, Voxilaprevir).
-
iii.
Non-Nucleoside NS5B Polymerase Inhibitor (Dasabuvir, Deleobuvir).
-
iv.
NS5B Polymerase Inhibitor (Sofosbuvir).
Quality assessment
There are two domains were considered at low risk of bias in all studies, including classification of intervention and measurement of outcome (Supplementary Table S2 & Table S3). Of the 26 non-randomized trials included in our analysis, the quality was evaluated using the ROBINS-I tool, which assesses bias from five perspectives. Among these 26 studies, 12 were judged to be at moderate risk of bias due to deviations from the intended interventions. Bias due to missing data was considered moderate in 10 studies. Of the 9 randomized controlled trials assessed using the ROB 2 tool, 2 were judged to be at high risk of bias due to selection and performance. Overall, only 1 out of the 9 RCTs was considered to be at high risk of bias (Supplementary Figure S1).
Overall pooled SVR12 for DAA therapies
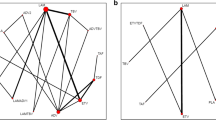
Since 2014, multiple regimens have been utilized for the treatment of hepatitis C virus genotype 2, with overall response rates showing considerable success. The characteristics of the 35 included studies (58 study-arms, n = 2,968) are reported (Supplementary Table S4). A total of 2,968 GT2 patients who received DAA therapy were included. As seen in Supplementary Figure S2, the pooled SVR12 was 94.62% (95% CI: 92.43-96.51%), but with substantial heterogeneity (I2 = 72.0%, P < 0.0001) caused by subgroups. Our network connected 10 mainstream treatment regimens for further indirectly comparison. Figure 2 illustrates the network of studies included in our network meta-analysis, comparing five different regimens. Notably, all regimens were compared directly to at least one other regimen, forming a well-connected network. The connection between SOF + RBV 12 and GLE + PIB 12 is depicted with a moderately thick line, indicating several studies have directly compared these two regimens. In contrast, the thinner line between SOF + VEL + VOX 8 and GLE + PIB 12 suggests fewer direct comparisons, relying more on indirect evidence. The assessment of inconsistency showed no significant differences between direct and indirect comparisons within this network. By comparing the main treatment regimens, we have confirmed that the 12-week SOF + VEL therapy and the 12-week GLE + PIB therapy are essentially equivalent in efficacy and are the treatment regimens with the highest response rates (Supplementary Figure S3).
We also did further analysis to indirectly compare the results between every two regimens (Table 3). Each cell in Table 3 shows the estimated difference in response rates between regimens with the corresponding 95% confidence intervals. For instance, the comparison between SOF + VEL-12 and GLE + PIB-12 shows a value of -1.05 (95% CI: -7.80, 5.58), suggesting that there is no significant difference in response rates between these regimens. We also highlighted interesting findings such as the comparison between SOF + VEL-12 and SOF + VEL + VOX-12, noting that adding voxilaprevir does not significantly change the response rate but may affect other clinical considerations like the duration of treatment or side-effect profile.
Forest plot of proportions of HCV GT2 patients reaching SVR12 weeks after the end of treatment with DAAs over patients receiving all doses of treatment, according to type of treatment. DAA regimen of SOF + VEL-12(a), SOF + RBV-12(b), SOF + RBV-16(c) LDV + SOF-12(d), GLE + PIB-8(e) and GLE + PIB-12(f)
SVR12 analysis by participants’ treatment history
Totally, 48 study-arms provided data on whether patients had been treated or not, including 1,387 treatment-naïve patients and 414 treatment-experienced patients. As shown in Fig. 3, the pooled SVR12 estimated were 93.18% (95% CI 89.88–95.95%) for the treatment-naïve group and 95.08% (95% CI 89.54–98.94%) for the treatment-experienced group. Both groups show substantial and moderate heterogeneity in I2 = 70% and I2 = 62%, respectively. Compared with treatment-naive patients, treatment-experienced patients had a narrowly 1.9% higher chance of achieving SVR12.
Networks of studies. Evidence network of DAA-based regimens studied in chronic hepatitis C genotype 2 patients (The nodes represent each regimen, with the thickness of the connecting lines represents the number of studies. The edges between nodes indicate direct comparisons, with thicker edges denoting multiple study comparisons)
SVR12 analysis by participants’ cirrhosis status
Due to the indistinctive difference in treatment history, we moved to focus on the subgroup analysis by cirrhosis status for 381 patients with cirrhosis and 1,709 patients without cirrhosis. In total, 55 study-arms provided data for 20 different regimens in the cirrhotic status subgroup analysis (Fig. 4). Out of expectations, we found that cirrhotic patients had a comparatively high SVR12 of 97.17% (95% CI 93.01%, 99.73%), while non-cirrhotic patients only had 92.77% (95% CI 89.48%, 95.57%).
Analysis of adverse events for DAA therapies
Meta-analysis of 1198 DAA-treated patients’ AEs showed that fatigue was the most common adverse event (14.0%, 95% CI: 6.4-21.6%) followed by headache (13.1%, 95% CI: 9.2-17.1%), while death and serious AEs were uncommon (Table 4). Most AEs related to DAA regimens were transient, and specific medical intervention was unnecessary.
Sofosbuvir plus Velpatasvir with a duration of 12 weeks
The highest SVR12 rate was estimated for SOF + VEL for 12 weeks, with SVR12 100% (95% CI 99–100%; Fig. 5a). Among the 311 HCV GT2 patients who treated with SOF + VEL included from 4 arms, any virologic failure was not observed, even in the population with cirrhosis and previous treatment failure [19,20,21,22]. Only one study-arm reported the safety profile of SOF + VEL with 9 common AEs being headache (17.9%), fatigue (14.9%), nasopharyngitis (6.0%), nausea (10.4%), pruritus (4.5%), insomnia (4.5%), irritability (3.0%), cough (3.0%), and dyspepsia (0.7%) [23]. The rate of AEs is 68.7%, which was slightly lower among patients treated with SOF + VEL than patients’ pooled level (Supplementary Table S5). However, it should be noted that although 2 out of 134 participants were reported to have died during the post-treatment follow-up, further investigation revealed that the cause of death was attributed to metastatic cancer and cardiac arrest rather than the SOF + VEL treatment itself [20].
Sofosbuvir plus Ribavirin with a duration of 12 and 16 weeks
As for the SOF + RBV regimen, the treatment of SOF + RBV for 12 weeks was used in 793 patients with HCV GT2 from 9 study-arms, which resulted in a pooled SVR12 of 94.6% (95% CI 92.8–96.4% I2 = 47%; Fig. 5b and c) [20, 24,25,26,27,28,29,30]. And the SVR12 of SOF + RBV for 16 weeks is 92.0% (95% CI 81.72–98.74%), which shows lower efficacy with longer treatment duration. Among the 425 patients who received SOF + RBV, 74 (17.4%) had anaemia, 69 (16.2%) had fatigue, 65 (15.3%) had headaches, and 26 types of AEs were reported in 5 studies totally. AEs were experienced by 63.8% of patients, and 13 SAEs occurred among 425 patients.
Ledipasvir plus Sofosbuvir with a duration of 8 and 12 weeks
The difference was observed when considering patients who received all doses of LDV + SOF for 8 or 12 weeks included from 4 study-arms [29, 31]. Treatment with LDV + SOF for 12 weeks resulted in an SVR12 (96.6%, 95% CI: 92.8-99.2%; Fig. 5d), compared with only 74.1% (95% CI: 54-89%) for a shorter duration of 8 weeks therapy. The most common AEs related to LDV + SOF are nasopharyngitis (14.6%) and headache (11.5%), while pruritus is not common. Specifically, some AEs like upper respiratory tract infection (URTI) (2.5%), gastroenteritis (2.1%), rash (1.9%), diarrhea (1.3%), hyperhidrosis (1.3%), pyrexia (1.3%) and back pain (0.6%) were only recorded in patients who received LDV + SOF.
Glecaprevir plus Pibrentasvir with a duration of 8 and 12 weeks
For the GLE + PIB regimen, the SVR12 rates were pooled from 4 studies with 693 patients infected with HCV GT2 [30, 32,33,34]. Comparing the pooled SVR12 with a random effect model between the two duration treatments shows no significant difference in SVR12 rates among the patients treated with 8 weeks (98.0%, 95% CI: 96.6-99.4%; Fig. 5e) and 12 weeks (100%, 95% CI: 99.0-100.0%; Fig. 5f). For safety, 10 kinds of AEs were observed in 455 patients treated with GLE + PIB for 8 or 12 weeks in total. Headache (11.9%), fatigue (8.8%) and nausea (7.9%) are the top three common AEs of patients after receiving all durations of GLE and PIB, while death cases were never observed. The rate of AEs in patients who received treatment with GLE + PIB (60.7%) is lower than the pooled rate (73.1%, 95% CI: 66.6-79.1%).
Discussion
In this up-to-date, evidence-based systematic review and meta-analysis, we combined SVR12 and AE data from 31 included studies wherever feasible through April 2022. Out of total 3,783 studies identified, we included 13 phase 2 studies and 22 phase 3 or 4 studies. Overall, our meta-analysis confirmed that DAAs therapy is highly effective and safe in the treatment of adult patients with HCV GT2.
The key finding of this study was that both SOF + VEL and GLE + PIB regimens, administered over 12 weeks, achieved the highest efficacy in treating HCV GT2. Additionally, the SOF + VEL regimen demonstrated a high safety profile for patients with GT2, evidenced by the lowest incidence of serious adverse events (1.5%) and comparatively milder side effects. Although both treatments exhibited a relatively high prevalence of adverse events (AEs), the GLE + PIB regimen reported a slightly lower overall AE rate (60.7%) compared to SOF + VEL (68.7%). Among the 58 study-arms, DAAs therapy allows around 80% of GT2 patients reach cure rates ≥ 90%, including those with cirrhosis and who had been treated before. Further analysis revealed an overall pooled SVR12 rate of 94.62% among 2,968 GT2 patients, which aligns with similar results reported for other genotypes [35, 36]. Besides, the therapy against HCV continues evolve. The DAAs regimen like GLE + PIB could shorten the duration into 8 weeks, while reaching 98% SVR12 rate among the GT2 patients. Compared to the 12-week duration of GLE + PIB, the rate of any adverse event in the 8-week group decreased from 65.0 to 56.6%. Shortening the DAA treatment duration for HCV patients can reduce the rate of side effects, there is also research demonstrating that shortened DAA treatment strategies are cost-effective for GT1 patients [37]. There was no observed difference in efficacy among subgroups based on previous treatment history or disease status. These findings align with results from a previous study on other genotypes, in which all patients with GT2 or GT3 were treated with sofosbuvir 400 mg and Velpatasvir 100 mg for 12 weeks and achieved SVR12 [38]. In clinical settings, the selection of a treatment regimen is influenced by various factors, including the accessibility and expense of DAAs, the patient’s tolerance, the potential for adverse reactions or drug-drug interactions, and the occurrence of resistance-associated substitutions (RAS).
However, achieving SVR12 is not the only goal of HCV treatment. While it is an important immediate goal, the ultimate goal is to reduce liver-related mortality and the incidence of hepatocellular carcinoma (HCC) resulting from chronic HCV infection [39]. Among the leading factors of HCC, chronic HCV infection is the primary cause of HCC in Australia [40]. More clinical benefits have been proven with the advent of DAA therapy, which could decrease the recurrence rate of HCC and improve survival [41]. HCV infection is not limited to liver-related symptoms and can also lead to extrahepatic manifestations (EHMs). These manifestations may include mixed cryoglobulinemia, non-Hodgkin lymphomas (NHL), cardiovascular disease, insulin resistance, type 2 diabetes, neurological and psychiatric diseases, and other rheumatic diseases [42]. Studies have shown that DAA treatment can improve or even resolve some EHMs associated with HCV infection [43,44,45,46].
In terms of safety, our analysis showed that fatigue (14.0%) was the most common side effect in GT2 patients, followed by headache (13.1%), and the pooled SAE rate was 1.5% (95% CI, 0.8-2.1%). These results confirm that DAAs are safe and well-tolerated. However, some SAEs reported in studies were considered irrelevant to the treatment itself, and therefore, they will not affect the safety evaluation of the DAAs regimen. The most frequent AEs, which included fatigue, headache, pruritus, and nasopharyngitis, were similar across regimens and occurred more frequently in regimens containing RBV. Only the sofosbuvir plus ledipasvir regimen had data for GT2 specifically, with nasopharyngitis (14.6%) as the most common adverse event. Even though the overall AE rates were higher than 50% and may reach 73.1%, the severity was considered mild to moderate, suggesting that treatment interruption or dose adjustment is not required. In assessing the safety profiles of SOF + VEL and GLE + PIB for treating Hepatitis C Virus genotype 2, SOF + VEL showed a 68.7% AE rate over 12 weeks with no treatment-related deaths, while GLE + PIB reported a slightly lower AE rate of 60.7% with no deaths across both 8 and 12-week durations. Despite similar efficacy, GLE + PIB’s marginally better safety profile, characterized by fewer AEs and no severe events, suggests it might be the preferable option, contingent on patient-specific considerations.
Our research has several strengths. It is the first systematic review and meta-analysis involving HCV GT2 patients treated with DAA, which included 35 studies. Moreover, it has a comprehensive subgroup analysis, including different regimens, treatment duration, the presence of cirrhosis, and treatment history. These data also seem to substantiate the suggestion that INF-free DAAs treatment is preferable for patients with any HCV genotypes [47].
There are also limitations to this study. Firstly, two-thirds of the included studies were not randomized controlled trials and were considered to have a low-moderate risk of bias. The single-arm study design has gradually replaced RCTs and becomes the mainstay of DAA therapy clinical trials, resulting in the infeasibility of directly comparing the major agents in DAA combinations. Secondly, the population of GT2 is relatively small, especially when compared to genotypes 1 and 3. Importantly, few studies have categorized patients based on specific genotypes or reported data according to patients’ characteristics, such as genotypes, treatment history, and cirrhosis status. Due to the absence of detailed data, we were unable to conduct further subgroup analyses to assess the efficacy and safety of DAA-based therapies across varying conditions (i.e., treatment history and cirrhosis status). Consequently, it was not feasible to aggregate the sustained virologic response at 12 weeks (SVR12) and the adverse events/serious adverse events (AE/SAE) data for all DAA regimen types. Moreover, only eight of the thirty-five eligible studies provided specific safety data for genotype 2, leading to potential incompleteness and bias in our findings.
Conclusions
Our systematic review and meta-analysis indicate that DAAs are effective in adults infected with chronic HCV GT2. The efficacy of the DAAs regimen in HCV GT2 patients is independent of patients’ previous treatment history and disease status. Among the various regimens evaluated, the combination of Sofosbuvir plus Velpatasvir for 12 weeks, as well as Glecaprevir plus Pibrentasvir for the same duration, were identified as the most effective and comparatively safe in managing chronic HCV GT2 infection. Both regimens achieved a sustained virological response 12 weeks post-treatment (SVR12) of 100% (95% CI 99–100%).
Data availability
The data presented in this study are available on request from the corresponding author.
References
Manns MP, Buti M, Gane E, Pawlotsky JM, Razavi H, Terrault N, Younossi Z. Hepatitis C virus infection. Nat Rev Dis Primers. 2017;3:17006.
World Health Organization, Hepatitis C. https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. 2016. https://www.who.int/publications/i/item/WHO-HIV-2016.06
Polaris Observatory HCVC. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7(5):396–415.
Asahina Y, Drafting Committee for Hepatitis Management Guidelines tJSoH. JSH guidelines for the management of Hepatitis C virus infection, 2019 update; protective effect of antiviral therapy against Hepatocarcinogenesis. Hepatol Res. 2020;50(7):775–90.
Chinese Society of Hepatology and Chinese Society of Infectious Diseases CMA. Guidelines for the prevention and treatment of hepatitis C (2019 version). J Clin Hepatol. 2019;35(12):2670–86.
Ghany MG, Morgan TR, Panel A-IHCG. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and treating Hepatitis C virus infection. Hepatology. 2020;71(2):686–721.
Polaris Observatory HCV Collaborators. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2(3):161–176.
Smith DB, Bukh J, Kuiken C, Muerhoff AS, Rice CM, Stapleton JT, Simmonds P. Expanded classification of hepatitis C virus into 7 genotypes and 67 subtypes: updated criteria and genotype assignment web resource. Hepatology. 2014;59(1):318–27.
Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: an up-date of the distribution and circulation of hepatitis C virus genotypes. World J Gastroenterol. 2016;22(34):7824–40.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2016; 354:i4086.
Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: a comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014;14:579.
Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919.
Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72(1):39.
Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–60.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60.
Asselah T, Marcellin P, Schinazi RF. Treatment of hepatitis C virus infection with direct-acting antiviral agents: 100% cure? Liver Int. 2018;38(Suppl 1Suppl 1):7–13.
Feld JJ, Jacobson IM, Hezode C, Asselah T, Ruane PJ, Gruener N, Abergel A, Mangia A, Lai CL, Chan HL, et al. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015;373(27):2599–607.
Foster GR, Afdhal N, Roberts SK, Bräu N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–17.
Jacobson IM, Lawitz E, Gane EJ, Willems BE, Ruane PJ, Nahass RG, Borgia SM, Shafran SD, Workowski KA, Pearlman B, et al. Efficacy of 8 weeks of Sofosbuvir, Velpatasvir, and Voxilaprevir in patients with chronic HCV infection: 2 phase 3 Randomized trials. Gastroenterology. 2017;153(1):113–22.
Asselah T, Shafran SD, Bourgeois S, Lai CL, Mathurin P, Willems B, Nguyen MH, Davis MN, Huang KC, Svarovskaia E, et al. Deferred treatment with a fixed-dose combination of sofosbuvir-velpatasvir for chronic hepatitis C virus genotype 1, 2, 4 and 6 infection. J Viral Hepat. 2019;26(10):1229–32.
Foster GR, Afdhal N, Roberts SK, Brau N, Gane EJ, Pianko S, Lawitz E, Thompson A, Shiffman ML, Cooper C, et al. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 infection. N Engl J Med. 2015;373(27):2608–17.
Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, Shiffman ML, Lawitz E, Everson G, Bennett M, et al. Sofosbuvir for Hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368(20):1867–77.
Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878–87.
Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al. Sofosbuvir plus Ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014;21(11):762–8.
Zeuzem S, Dusheiko GM, Salupere R, Mangia A, Flisiak R, Hyland RH, Illeperuma A, Svarovskaia E, Brainard DM, Symonds WT, et al. Sofosbuvir and Ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993–2001.
Ho SB, Monto A, Peyton A, Kaplan DE, Byrne S, Moon S, Copans A, Rossaro L, Roy A, Le H, et al. Efficacy of Sofosbuvir Plus Ribavirin in Veterans with Hepatitis C Virus Genotype 2 infection, compensated cirrhosis, and multiple comorbidities. Clin Gastroenterol Hepatol. 2017;15(2):282–8.
Asahina Y, Itoh Y, Ueno Y, Matsuzaki Y, Takikawa Y, Yatsuhashi H, Genda T, Ikeda F, Matsuda T, Dvory-Sobol H, et al. Ledipasvir-sofosbuvir for treating Japanese patients with chronic hepatitis C virus genotype 2 infection. Liver Int. 2018;38(9):1552–61.
Toyoda H, Chayama K, Suzuki F, Sato K, Atarashi T, Watanabe T, Atsukawa M, Naganuma A, Notsumata K, Osaki Y, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology. 2018;67(2):505–13.
Gane EJ, Hyland RH, Yang Y, Svarovskaia E, Stamm LM, Brainard DM, McHutchison JG, Stedman CAM. Efficacy of Ledipasvir Plus Sofosbuvir for 8 or 12 weeks in patients with Hepatitis C Virus genotype 2 infection. Gastroenterology. 2017;152(6):1366–71.
Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, Colombo M, Calinas F, Aguilar H, de Ledinghen V, et al. Efficacy of Glecaprevir/Pibrentasvir for 8 or 12 weeks in patients with Hepatitis C Virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018;16(3):417–26.
Brown RS Jr., Buti M, Rodrigues L, Chulanov V, Chuang WL, Aguilar H, Horvath G, Zuckerman E, Carrion BR, Rodriguez-Perez F, et al. Glecaprevir/pibrentasvir for 8weeks in treatment-naive patients with chronic HCV genotypes 1–6 and compensated cirrhosis: the EXPEDITION-8 trial. J Hepatol. 2020;72(3):441–9.
Wei L, Wang G, Alami NN, Xie W, Heo J, Xie Q, Zhang M, Kim YJ, Lim SG, Fredrick LM, et al. Glecaprevir–pibrentasvir to treat chronic hepatitis C virus infection in Asia: two multicentre, phase 3 studies—a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol Hepatol. 2020;5(9):839–49.
Berden FA, Aaldering BR, Groenewoud H, IntHout J, Kievit W, Drenth JP. Identification of the best direct-acting antiviral regimen for patients with Hepatitis C Virus genotype 3 infection: a systematic review and network Meta-analysis. Clin Gastroenterol Hepatol. 2017;15(3):349–59.
Due OT, Chaikledkaew U, Genuino AJM, Sobhonslidsuk A, Thakkinstian A. Systematic Review with Meta-Analysis: Efficacy and Safety of Direct-Acting Antivirals for Chronic Hepatitis C Genotypes 5 and 6. Biomed Res Int. 2019;2301291.
Fawsitt CG, Vickerman P, Cooke G, Welton NJ. A cost-effectiveness analysis of shortened direct-acting antiviral treatment in genotype 1 noncirrhotic treatment-naive patients with chronic Hepatitis C Virus. Value Health. 2019;22(6):693–703.
Pisaturo M, Russo A, Onorato L, Coppola N. Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis. Acta Biomed. 2019;90(2):187–96.
Asahina Y. JSH guidelines for the management of Hepatitis C virus infection, 2019 update; protective effect of antiviral therapy against Hepatocarcinogenesis. Hepatol Res. 2020;50(7):775–90.
Australian Institute of Health and Welfare. Cancer data in Australia. Canberra: AIHW (AIHW Cat. No. CAN 122). 2020. https://www.aihw.gov.au/reports/cancer/cancer-data-in-australia/contents/about
Liu H, Yang XL, Dong ZR, Chen ZQ, Hong JG, Wang DX, Li T. Clinical benefits of direct-acting antivirals therapy in hepatitis C virus patients with hepatocellular carcinoma: a systematic review and meta-analysis. J Gastroenterol Hepatol. 2022;37(9):1654–65.
Mazzaro C, Quartuccio L, Adinolfi LE, Roccatello D, Pozzato G, Nevola R, Tonizzo M, Gitto S, Andreone P, Gattei V. A review on extrahepatic manifestations of Chronic Hepatitis C virus infection and the impact of direct-acting antiviral therapy. Viruses. 2021;13(11).
Adinolfi LE, Petta S, Fracanzani AL, Coppola C, Narciso V, Nevola R, Rinaldi L, Calvaruso V, Staiano L, Di Marco V, et al. Impact of hepatitis C virus clearance by direct-acting antiviral treatment on the incidence of major cardiovascular events: a prospective multicentre study. Atherosclerosis. 2020;296:40–7.
Artemova M, Abdurakhmanov D, Ignatova T, Mukhin N. Persistent hepatitis C virus-associated cryoglobulinemic vasculitis following virus eradication after direct-acting antiviral therapy. Hepatology. 2017;65(5):1770–1.
Bohorquez H, Velez JCQ, Lusco M, Scheuermann J, Cohen AJ. Hepatitis C-associated focal proliferative glomerulonephritis in an aviremic recipient of a hepatitis C-positive antibody donor liver. Am J Transpl. 2021;21(8):2895–9.
Chen YC, Tseng CW, Tseng KC. Rapid platelet count improvement in chronic hepatitis C patients with thrombocytopenia receiving direct-acting antiviral agents. Med (Baltim). 2020;99(19):e20156.
Ding YJ, Lu CK, Chen WM, Tung SY, Wei KL, Shen CH, Hsieh YY, Yen CW, Chang KC, Chiu WN, et al. Pangenotypic direct-acting antiviral agents for mixed genotype hepatitis C infection: a real-world effectiveness analysis. J Gastroenterol Hepatol. 2021;36(10):2911–6.
Lawitz E, Sullivan G, Rodriguez-Torres M, Bennett M, Poordad F, Kapoor M, Badri P, Campbell A, Rodrigues L Jr., Hu Y, et al. Exploratory trial of ombitasvir and ABT-450/r with or without Ribavirin for HCV genotype 1, 2, and 3 infection. J Infect. 2015;70(2):197–205.
Foster GR, Chayama K, Chuang WL, Fainboim H, Farkkila M, Gadano A, Gaeta GB, Hezode C, Inada Y, Heo J, et al. A randomized, controlled study of peginterferon lambda-1a/ribavirin +/- daclatasvir for hepatitis C virus genotype 2 or 3. Springerplus. 2016;5(1):1365.
Brown A, Hezode C, Zuckerman E, Foster GR, Zekry A, Roberts SK, Lahser F, Durkan C, Badshah C, Zhang B, et al. Efficacy and safety of 12 weeks of elbasvir +/- grazoprevir +/- Ribavirin in participants with hepatitis C virus genotype 2, 4, 5 or 6 infection: the C-SCAPE study. J Viral Hepat. 2018;25(5):457–64.
Dore GJ, Lawitz E, Hezode C, Shafran SD, Ramji A, Tatum HA, Taliani G, Tran A, Brunetto MR, Zaltron S, et al. Daclatasvir plus Peginterferon and Ribavirin is noninferior to Peginterferon and Ribavirin alone, and reduces the duration of treatment for HCV genotype 2 or 3 infection. Gastroenterology. 2015;148(2):355–66. e351.
Everson GT, Towner WJ, Davis MN, Wyles DL, Nahass RG, Thuluvath PJ, Etzkorn K, Hinestrosa F, Tong M, Rabinovitz M, et al. Sofosbuvir with Velpatasvir in treatment-naive noncirrhotic patients with genotype 1 to 6 Hepatitis C virus infection: a Randomized Trial. Ann Intern Med. 2015;163(11):818–26.
Foster GR, Pianko S, Brown A, Forton D, Nahass RG, George J, Barnes E, Brainard DM, Massetto B, Lin M, et al. Efficacy of Sofosbuvir plus Ribavirin with or without peginterferon-alfa in patients with hepatitis C virus genotype 3 infection and treatment-experienced patients with cirrhosis and hepatitis C virus genotype 2 infection. Gastroenterology. 2015;149(6):1462–70.
Gane EJ, Kowdley KV, Pound D, Stedman CA, Davis M, Etzkorn K, Gordon SC, Bernstein D, Everson G, Rodriguez-Torres M, et al. Efficacy of Sofosbuvir, Velpatasvir, and GS-9857 in patients with Hepatitis C Virus genotype 2, 3, 4, or 6 infections in an Open-Label, phase 2 trial. Gastroenterology. 2016;151(5):902–9.
Lawitz E, Buti M, Vierling JM, Almasio PL, Bruno S, Ruane PJ, Hassanein TI, Muellhaupt B, Pearlman B, Jancoriene L, et al. Safety and efficacy of a fixed-dose combination regimen of grazoprevir, ruzasvir, and uprifosbuvir with or without Ribavirin in participants with and without cirrhosis with chronic hepatitis C virus genotype 1, 2, or 3 infection (C-CREST-1 and C-CREST-2, part B): two randomised, phase 2, open-label trials. Lancet Gastroenterol Hepatol. 2017;2(11):814–23.
Lawitz E, Gane E, Feld JJ, Buti M, Foster GR, Rabinovitz M, Burnevich E, Katchman H, Tomasiewicz K, Lahser F, et al. Efficacy and safety of a two-drug direct-acting antiviral agent regimen ruzasvir 180 mg and uprifosbuvir 450 mg for 12 weeks in adults with chronic hepatitis C virus genotype 1, 2, 3, 4, 5 or 6. J Viral Hepat. 2019;26(9):1127–38.
Lawitz E, Poordad F, Anderson LJ, Vesay M, Kelly MM, Liu H, Gao W, Fernsler D, Asante-Appiah E, Robertson MN, et al. Efficacy and safety of ruzasvir 60 mg and uprifosbuvir 450 mg for 12 weeks in adults with chronic hepatitis C virus genotype 1, 2, 3, 4 or 6 infection. J Viral Hepat. 2019;26(6):675–84.
Wei L, Lim SG, Xie Q, Văn KN, Piratvisuth T, Huang Y, Wu S, Xu M, Tang H, Cheng J, et al. Sofosbuvir–velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol Hepatol. 2019;4(2):127–34.
Izumi N, Takehara T, Chayama K, Yatsuhashi H, Takaguchi K, Ide T, Kurosaki M, Ueno Y, Toyoda H, Kakizaki S, et al. Sofosbuvir-Velpatasvir plus Ribavirin in Japanese patients with genotype 1 or 2 hepatitis C who failed direct-acting antivirals. Hepatol Int. 2018;12(4):356–67.
Rao H, Song G, Li G, Yang Y, Wu X, Guan Y, Mao Q, Jiang X, Wang C, Zhang Y, et al. Safety and efficacy of coblopasvir and sofosbuvir in patients with genotypes 1, 2, 3 and 6 HCV infections without or with compensated cirrhosis. J Viral Hepat. 2020;27(1):45–51.
Shafran SD, Shaw D, Charafeddine M, Agarwal K, Foster GR, Abunimeh M, Pilot-Matias T, Pothacamury RK, Fu B, Cohen E, et al. Efficacy and safety results of patients with HCV genotype 2 or 3 infection treated with ombitasvir/paritaprevir/ritonavir and sofosbuvir with or without Ribavirin (QUARTZ II-III). J Viral Hepat. 2018;25(2):118–25.
Gao Y, Kong F, Li G, Li C, Zheng S, Lin J, Wen X, Hu J, Wang X, Wu X, et al. Coblopasvir and sofosbuvir for treatment of chronic hepatitis C virus infection in China: a single-arm, open-label, phase 3 trial. Liver Int. 2020;40(11):2685–93.
Hua R, Kong F, Wen X, Xiong Q, Chen J, Meng C, Ma H, Tan Y, Huang Y, Jiang Y, et al. Efficacy and safety of alfosbuvir plus daclatasvir in Chinese patients with hepatitis C virus genotypes 1, 2, 3, and 6 infection: an open-label, phase 2 study. J Viral Hepat. 2022;29(6):455–64.
Acknowledgements
We would like to thank the advice from our colleagues at the University of Macau.
Funding
This research is supported by the funding of the University of Macau (MYRG2022-00113-ICMS; MYRG-GRG2023-00059-ICMS).
Author information
Authors and Affiliations
Contributions
PKL, COLU, and HH conceived and designed the study. PKL and ZL performed the systematic search and contributed to eligible articles selection, data, quality assessment, and drafted the manuscript. COLU and HH supervised the work and re-vised the manuscripts. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
As a systematic review and meta-analysis, our study did not require referral to our institutional clinical ethics committee, nor did it necessitate clinical trial registration.
Clinical trial number
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lei, P.K., Liu, Z., Ung, C.O. et al. Efficacy and safety of direct-acting antiviral regimen for patients with hepatitis C virus genotype 2: a systematic review and meta-analysis. BMC Gastroenterol 24, 331 (2024). https://doi.org/10.1186/s12876-024-03414-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-024-03414-5