Abstract
Background
The number of people receiving second-line antiretroviral therapy (ART) has increased as global access to ART has expanded. Data on the burden and factors associated with second-line ART virologic failure (VF) from India remain limited.
Methods
We conducted cross-sectional viral load (VL) testing among adults (≥ 18 years) who were registered at a publicly funded ART center in western India between 2014 and 2015 and had received second-line ART for at least 6 months. Sociodemographic and clinical characteristics were abstracted from routinely collected programmatic data. Logistic regression evaluated factors associated with VF (defined as VL > 1000 copies/mL).
Results
Among 400 participants, median age was 40 years (IQR 34–44), 71% (285/400) were male, and 15% (59/400) had VF. Relative to participants without VF, those with VF had lower median CD4 counts (230 vs 406 cells/mm3, p < 0.0001), lower weight at first-line failure (49 vs 52 kg, p = 0.003), were more likely to have an opportunistic infection (17% vs 3%, p < 0.0001) and less likely to have optimal ART adherence (71% vs 87%, p = 0.005). In multivariable analysis, VF was associated with opportunistic infection (aOR, 4.84; 95% CI, 1.77–13.24), lower CD4 count (aOR 4.15; 95% CI, 1.98–8.71) and lower weight at first-line failure (aOR, 2.67; 95% CI, 1.33–5.34).
Conclusions
We found second-line VF in about a sixth of participants in our setting, which was associated with nearly fivefold increased odds in the context of opportunistic infection. Weight could be a useful clinical indicator for second-line VF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Globally, the prevalence of failure of first-line antiretroviral therapy (ART) regimens for adult HIV infection ranges between 20% and 82% [1]. This has concurrently increased the need for countries to expand the rollout of second-line ART regimens. In India, approximately 50,000 people living with HIV (PLHIV) are on second-line ART [2]. However, country-specific estimates of second-line ART failure and associated factors have not been well described.
The National AIDS Control Organization (NACO), India’s premier HIV program has provided publicly funded second-line ART since 2008 [3]. Second-line ART regimens in India have evolved with recommendations of the World Health Organization’s (WHO’s) guidelines. Prior to 2019, Protease Inhibitor (PI)-based regimens were the mainstay of second-line ART, comprising a ritonavir (r)-boosted PI atazanavir (ATV/r) or lopinavir (LPV/r); with ATV/r as the PI of choice, and two nucleoside reverse transcriptase inhibitors (NRTI) [Tenofovir disoproxil fumarate (TDF)/abacavir [ABC] + Lamivudine (3TC)] [4]. Since 2019, regimens comprising the integrase strand transfer inhibitor (INSTI) dolutegravir (DTG) have been introduced (together with two NRTI: zidovudine (AZT)/TDF + 3TC) [5].
Prior to 2018, viral load (VL) monitoring in India was targeted only at PLHIV that were suspected to have immunological failure on first-line ART [4]. Since 2018, NACO has designated VL as the preferred approach to monitor treatment response to second-line ART, with testing recommended at 6 months, 12 months, and yearly thereafter, following second-line ART initiation [6, 7]. The prevalence of virological failure (VF, i.e., high VL) on second-line ART reported across 19 studies from low-and-middle income countries (LMICs) included in a meta-analysis, was between 6% and 38%. This meta-analysis of 2035 PLHIV found poor adherence to second-line ART to be the primary factor associated with VF [8]. Older age, lower CD4 counts, and socioeconomic disadvantage are other factors reported to be associated with second-line VF [9,10,11]. A limitation of these studies is that none of them included data from India, making the generalizability of their findings to the country questionable.
Third-line ART regimens (following second-line failure) constitute the last line of treatment for HIV. To identify geographically relevant strategies to reduce the number of people that will require third-line ART, it is important to generate data on second-line ART failure. Here, we evaluate the prevalence of, and factors associated with VF among PLHIV receiving second-line ART under programmatic conditions in western India.
Methods
Study design, study site and study population
This was a convenience sample of PLHIV aged ≥ 18 years registered for HIV care at the ART center affiliated with Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals (BJGMC-SGH).
The BJGMC-SGH ART center was established in 2004 in Pune, Maharashtra (a state in western India). Maharashtra has one of the highest HIV burdens in India, with approximately 330,000 PLHIV, and the adult HIV prevalence is higher than the national average (0.36% vs 0.22%) [12]. The ART center currently has over 5000 PLHIV in active care, including approximately 1800 receiving second-line ART.
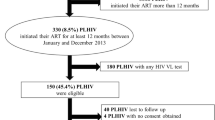
Eligible PLHIV were those that had received second-line ART for ≥ 6 months between August 2014 and September 2015 due to first-line ART failure [4]. PLHIV receiving second-line regimens as an alternate regimen due to toxicity of the first-line regime were excluded. We conducted cross-sectional VL testing among all consenting eligible PLHIV, henceforth called study participants. Since study accrual occurred prior to 2019, all study participants in this analysis exclusively received PI-based (ATV/r or LPV/r) second line ART.
Data variables and definition
Details of all PLHIV registered for care under India’s national HIV care and treatment program are routinely collected using a standard paper form and subsequently entered into an electronic database. VL tests conducted through the study were linked to this database.
Outcome
Consistent with published literature, we defined VF as VL > 1000 copies/mL at a single time point [13, 14].
Predictors
These were variables hypothesized to be associated with the outcome, and available to be abstracted from the electronic database. Predictors included (a) sociodemographic characteristics: age, sex, income, education, occupation, marital status, history of smoking and alcohol consumption and (b) clinical characteristics: time-updated CD4 count, years on first and second-line ART, past and present opportunistic infections (OIs), time-updated WHO HIV stage, weight at first-line failure, ART regimen and adherence.
Median weight was used to dichotomize weight at first-line failure into two categories (< 51 kg, ≥ 51 kg). Past OI were those occurring before second-line ART initiation, and present OI were those occurring after second-line ART initiation. Treatment adherence was calculated as percentage of pills consumed to those prescribed. Adherence was categorized as suboptimal (< 95%) or optimal (> 95%), consistent with NACO and WHO guidelines [7, 15].
Statistical analysis
We used Wilcoxon rank sum test and Fisher’s exact test to compare continuous and categorical variables, respectively. Statistical significance was set to a two-sided p-value of 0.05. Associations between VF and the predictors were assessed using univariate and multivariable logistic regression. Multivariable models were adjusted for age, sex and variables found to be significant in univariate analysis.
All statistical analyses were performed with STATA® version 14.2 (STATA Corp, College Station, TX, USA).
Ethics approval and consent to participate
The Institutional Review Board of Johns Hopkins University and the Ethics Committee of BJGMC-SGH approved the study (Approval number IRB00026444). Written informed consent for VL testing was obtained from all participants.
Results
Study population and characteristics
Among 400 PLHIV enrolled, median age was 40 years [interquartile range (IQR) 34–44], 71% (285/400) were male, median time-updated CD4 count was 385 cells/mm3 (IQR 228–547) and median VL was 42.5 copies/mL (IQR 40–299). Second-line ART was initiated at a median 4 years (IQR 3–6) after first-line ART initiation. A majority (96%, 385 of 400) were on ATV/r-based regimen. Past OI was reported by 57% (224/400) and 5% (20/400) reported a present OI. Tuberculosis (TB) was the most reported past (135/224) and present (8/20) OI (Additional file 1: Table S1). Suboptimal adherence was observed in 15% (62/400) of study participants (Table 1).
Prevalence of virological failure
VF was observed in 15% (59/400) of study participants. Compared to participants without VF, those with VF had a lower median CD4 count [230 cells/mm3 (IQR 114–354) vs 406 cells/mm3 (IQR 251–562), p < 0.0001], lower median weight at first-line failure [49 kg (IQR 42–54) vs 52 kg (IQR 45–60), p = 0.003], were more likely to have present OI (17% vs 3%, p < 0.0001) and suboptimal adherence (29% vs 13%, p = 0.005) (Table 1). Half of the participants with VF and present OI (n = 05/10) had suboptimal adherence.
Associations of virological failure with predictors
In univariate analysis VF was associated with: (a) time-updated CD4 count level ≤ 350 cells/mm3 [Odds ratio [OR] for 200–350 cells/mm3: 3.67; 95% confidence interval (CI) 1.82–7.41 and OR for < 200 cells/mm3: 4.74; 95% CI, 2.34–9.60]; (b) present OI (OR 6.73; 95% CI 2.66–17); (c) weight < 51 kg at first-line failure (OR 2.36; 95% CI 1.32–4.22); and (d) suboptimal adherence (OR 2.66; 95% CI 1.39–5.07) (Table 2).
In multivariable analysis adjusted for age, sex, time-updated CD4 count, present OI, weight at first-line failure and adherence, (a) CD4 count level ≤ 350 cells/mm3 [adjusted OR (aOR), for 200–350 cells/mm3: 3.39; 95% CI, 1.63–7.05), and aOR for CD4 count < 200 cells/mm3: 4.15; 95% CI, 1.98–8.71], (b) present OI (aOR 4.84; 95% CI 1.77–13.24) and (c) weight < 51 kg at first-line failure (aOR 2.67; 95% CI 1.33–5.34) remained significantly associated with VF (Table 2).
Discussion
Our cross-sectional study found that around one-sixth of participants met criteria for VF on second-line ART at a large tertiary ART center in western India. In addition, our analysis identified significant associations between VF and lower time-updated CD4 count (≤ 350 cells/mm3), present OI and low weight (< 51 kg). Overall, this study adds to the limited evidence on second-line VF and associated factors in India.
The prevalence of VF in our study population is consistent with that reported in the only other study from north India on second-line ART associated VF [3]. However, our estimate was obtained using a larger sample size from a different geographical region in India with higher HIV prevalence. Additionally, compared to the study from north India that included data only till 2012, the data we use is updated to 2015. Our prevalence estimate is also consistent with reports of VF from other LMICs, although varying definitions of VF across studies, makes it challenging to directly compare the burden of second-line associated VF across countries [9].
Similar to previous studies, our analysis indicates that low CD4 count at the time of VL testing is associated with VF [9, 10]. The cross-sectional nature of our data precludes us from commenting on the directionality of this association. The WHO has recommended that in situations where VL measurement is routinely available and PLHIV are clinically stable on ART, VL be exclusively used to monitor second-line ART treatment outcomes [16]. However, in many LMICs, the regularity of VL testing remains suboptimal. A recent study from Asia of 31,346 PLHIV (with > 60% of the data originating from India) showed that VL testing was performed less than annually in > 50% of PLHIV [17]. At present, NACO recommends CD4 monitoring among adult PLHIV on second-line ART with CD4 count < 350 cells/mm3 in addition to 6-monthly VL testing [6, 7]. Given the implementational challenges of regular VL testing in India, CD4 count measurement will continue to remain an important aspect of monitoring PLHIV on second-line ART and may be useful to identify PLHIV at potential risk of VF.
The presence of an OI at the time of diagnosis of VF was associated with second-line failure in our cohort. However, as this was a cross-sectional study, it was not possible to assess whether OIs were the cause or effect of VF. As in previous studies from India, TB was the most common OI [18, 19]. As per NACO guidelines, PLHIV diagnosed with TB are treated with rifabutin-based antituberculosis therapy, and the additional pill burden could affect adherence in this subset of PLHIV [20]. Our analysis indicates suboptimal adherence to ART among half of PLHIV with second-line VF and an OI. These findings suggest that patients with active TB on second-line ART may require additional adherence support.
We found that lower weight at first-line failure was associated with second-line VF. A study from Ethiopia reported that weight loss after starting second-line ART was associated with treatment failure [21]. The measurement of weight is easy and inexpensive to perform. Therefore, in LMICs, where the burden of HIV is high and resources are limited, it may be of value for future studies to better understand the role of weight as a predictor of treatment response to second-line ART.
There are several limitations to our study findings. A major limitation is that we used a data set that does not include DTG-based second-line therapy. Currently 80% of PLHIV at BJGMC-SGH ART center receiving second-line ART are on PI-based regimens, making these findings relevant to a majority. Moreover, as recently as September 2022, there were DTG stockouts across India increasing the reliance on PI-based second-line regimens [22]. As already mentioned, to our knowledge, this remains the most updated dataset from India to describe VF among those receiving second-line ART. Future analyses should work on updating our findings. We used a single VL measurement to define VF. WHO defines second-line treatment failure as plasma VL above 1000 copies/mL on two consecutive measurements within 3 months along with adherence support [23]. However, our study definition was based on published literature, allowing us to compare our findings to similar previous studies. We found no association with adherence; a primary factor for VF in previous studies [8]. We were able to characterize adherence only at cut-off levels of < 95% and ≥ 95%, as recommended by NACO. Therefore, it is possible that very few participants had truly low adherence (i.e., adherence levels < 80%) which resulted in the non-significant adjusted association with VF. Additionally, pill counting may not be the optimal method to measure adherence to second-line ART. The use of two non-invasive methods or plasma therapeutic drug concentration levels may be better adherence assessment measures [24]. We did not perform HIV genotyping to determine resistance to second-line drugs, on those with VF. However, in a study of 47 PLHIV from Mumbai (150 km from Pune), relative to drug resistance, decreased adherence was observed to be a more crucial factor for second-line associated VF [25]. This further highlights the need for better and more granular adherence measures among PLHIV receiving second-line ART. Our study is cross-sectional, limiting the exploration of causal relationships. Future longitudinal analyses are required to address this limitation. Lastly, our findings may be generalizable only to western India and more specifically Pune. We advocate for studies from different regions of India to corroborate our findings.
Conclusions
We found second-line ART failure in 15% of our study population. In India, a combination of immunological surveillance and virological monitoring is crucial to identify patients at potential risk of VF on second-line ART. Improvements in adherence measurements and management of OIs for those on second-line ART are important to avoid VF and switch to third-line ART. Weight can be a useful clinical indicator to predict VF, but additional studies are needed to better understand the association between weight and second-line ART treatment response.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ART:
-
Antiretroviral therapy
- PLHIV:
-
People living with HIV
- NACO:
-
National AIDS Control Organization
- WHO:
-
World Health Organization
- PI:
-
Protease inhibitor
- r:
-
Ritonavir
- ATV:
-
Atazanavir
- LPV:
-
Lopinavir
- NRTI:
-
Nucleoside reverse transcriptase inhibitors
- TDF:
-
Tenofovir disoproxil fumarate
- ABC:
-
Abacavir
- 3TC:
-
Lamivudine
- INSTI:
-
Integrase strand transfer inhibitor
- DTG:
-
Dolutegravir
- AZT:
-
Zidovudine
- VL:
-
Viral load
- VF:
-
Virologic failure
- LMICs:
-
Low-and-middle income countries
- BJGMC-SGH:
-
Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals
- OI:
-
Opportunistic infection
- IQR:
-
Interquartile range
- TB:
-
Tuberculosis
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- aOR:
-
Adjusted OR
References
Gregson J, Tang M, Ndembi N, et al. Global epidemiology of drug resistance after failure of WHO recommended first-line regimens for adult HIV-1 infection: a multicentre retrospective cohort study. Lancet Infect Dis. 2016;16(5):565–75.
Ministry of Health & Family Welfare, Government of India. Annual Report 2018–2019. 2019. https://main.mohfw.gov.in/sites/default/files/24%20Chapter%20496AN2018-19.pdf. Accessed 12 Apr 2021.
Chakravarty J, Sundar S, Chourasia A, et al. Outcome of patients on second line antiretroviral therapy under programmatic condition in India. BMC Infect Dis. 2015;14(15):517.
National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. Antiretroviral Therapy Guidelines for HIV-infected Adults and Adolescents. May 2013. 2013. http://naco.gov.in/sites/default/files/Antiretroviral%20Therapy%20Guidelines%20for%20HIV-Infected%20Adults%20and%20Adolescents%20May%202013%281%29_0.pdf. Accessed 1 Jun 2022.
National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. Sankalak Status of National AIDS Response (Second edition). 2020. http://naco.gov.in/sites/default/files/Sankalak Status of National AIDS Response, Second Edition (2020).pdf. Accessed 17 Apr 2021.
National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. National Guidelines for HIV care and Treatment. 2021. http://naco.gov.in/sites/default/files/National_Guidelines_for_HIV_Care_and_Treatment_2021.pdf. Accessed 01 Jun 2022.
National AIDS Control Organisation. National Operational Guidelines For Viral Load Testing. 2018. http://www.naco.gov.in/sites/default/files/National Operational Guidelines for Viral Load Testing Mar%2718.pdf. Accessed 17 April 2021.
Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. AIDS. 2012;26(8):929–38.
Pujades-Rodríguez M, Balkan S, Arnould L, Brinkhof MA, Calmy A. Treatment failure and mortality factors in patients receiving second-line HIV therapy in resource-limited countries. JAMA. 2010;304(3):303–12.
Thao VP, Quang VM, Wolbers M, et al. Second-line HIV therapy outcomes and determinants of mortality at the largest HIV referral center in Southern Vietnam. Medicine (Baltimore). 2015;94(43): e1715.
Burch LS, Smith CJ, Phillips AN, Johnson MA, Lampe FC. Socioeconomic status and response to antiretroviral therapy in high-income countries: a literature review. AIDS (London, England). 2016;30(8):1147–62.
National AIDS Control Organisation, Ministry of Health and Family Welfare, Government of India. India HIV Estimates 2019 Report. 2019. http://naco.gov.in/sites/default/files/Estimation Report 2019.pdf. Accessed 12 Apr 2021.
Collier D, Iwuji C, Derache A, et al. Virological outcomes of second-line protease inhibitor-based treatment for human immunodeficiency virus type 1 in a high-prevalence rural south African setting: a competing-risks prospective cohort analysis. Clin Infect Dis. 2017;64(8):1006–16.
Leierer G, Grabmeier-Pfistershammer K, Steuer A, et al. A single quantifiable viral load is predictive of virological failure in human immunodeficiency virus (HIV)-infected patients on combination antiretroviral therapy: the Austrian HIV Cohort Study. Open Forum Infect Dis. 2016;3(2):ofw089.
World Health Organization. HIV testing, treatment and prevention generic tools for operational research. World Health Organization, Geneva, 2009. https://www.who.int/hiv/pub/operational/or_generic_tools.pdf?ua=1. Accessed 12 Apr 2021.
World Health Organization. What’s new in treatment monitoring: viral load and CD4 testing. World Health Organization, Geneva, 2017. https://www.who.int/publications/i/item/WHO-HIV-2017.22. Accessed 21 Oct 2022.
Bijker R, Kumarasamy N, Kiertiburanakul S, et al. An expanded HIV care cascade: ART uptake, viral load suppression and comorbidity monitoring among adults living with HIV in Asia. Antivir Ther. 2020;25(5):275–85. https://doi.org/10.3851/IMP3379.
Chavan VR, Chaudhary V, Ahir P, et al. Current scenario of opportunistic and co-infections in HIV-infected individuals at a tertiary care hospital in Mumbai, India. Indian J Med Microbiol. 2015;33(1):78–83.
Ghate M, Deshpande S, Tripathy S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis. 2009;13(1):e1-8.
National AIDS Control Organization, Ministry of Health and Family Welfare, Government of India. National Guidelines on Second-line and Alternative First-line ART For Adults and Adolescents. 2013. http://naco.gov.in/sites/default/files/NationalGuidelinesonSecond-lineandAlternativeFirst-lineARTForAdultsandAdolescentsMay2013_0.pdf. Accessed 12 Apr 2021.
Tsegaye AT, Wubshet M, Awoke T, Addis AK. Predictors of treatment failure on second-line antiretroviral therapy among adults in northwest Ethiopia: a multicentre retrospective follow-up study. BMJ Open. 2016;6(12):e012537.
Recent ARV Stockouts in India Created a Health Crisis for People Living with HIV [Internet]. amfAR, The Foundation for AIDS Research. Available from: https://www.amfar.org/news/recent-arv-stockouts-in-india-created-a-health-crisis-for-people-living-with-hiv/. Accessed 26 Oct 2022.
World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection; recommendations for a public health approach. World Health Organization, Geneva, 2016. http://apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1. Accessed 12 Apr 2021.
Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–22. https://doi.org/10.15386/mpr-1201.
Khan S, Das M, Andries A, et al. Second-line failure and first experience with third-line antiretroviral therapy in Mumbai. India Glob Health Action. 2014;7:24861.
Acknowledgements
The authors would like to thank NACO, the staff at the BJGMC-SGH ART center who were involved in data collection, the counsellors who were involved in the informed consent process and all study participants. We would also like to acknowledge Katherine McIntyre for reviewing and editing the manuscript.
Funding
This work was supported by amfAR, The Foundation for AIDS Research, with support from the U.S National Institutes of Health’s National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute of Mental Health, the National Institute on Drug Abuse, the National Heart, Lung, and Blood Institute, the National Institute on Alcohol Abuse and Alcoholism, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Fogarty International Center as part of the International Epidemiology Databases to Evaluate AIDS [IeDEA; U01AI069907]. The content and views expressed are those of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Author information
Authors and Affiliations
Contributions
VM, SS1, SS2, DK, IM, and NG conceived the study. SS1, NN, SP and SN contributed to data collection. AC, NG, and IM analyzed the data. SS1, PR, IM, and VM wrote the manuscript. All authors critically reviewed the manuscript for intellectual content and approved the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board of Johns Hopkins University and the Ethics Committee of Byramjee Jeejeebhoy Government Medical College and Sassoon General Hospitals approved the study (Approval number IRB00026444). Written informed consent for VL testing was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors have no competing interests to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Past and present opportunistic infections among adult PLHIV on second-line ART at BJGMC-SGH ART center, August 2014–September 2015.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Salvi, S., Raichur, P., Kadam, D. et al. Virological failure among people living with HIV receiving second-line antiretroviral therapy in Pune, India. BMC Infect Dis 22, 951 (2022). https://doi.org/10.1186/s12879-022-07894-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-022-07894-2