Abstract
Peritoneal dialysis-associated peritonitis is a serious complication of peritoneal dialysis, and the prevention and treatment of this condition are important for improving the long-term survival and quality of life of patients. However, peritoneal dialysis-associated peritonitis due to Mycobacterium tuberculosis infection is relatively rare and not easily diagnosed. Here, we present a case of peritoneal dialysis-associated peritonitis caused by Mycobacterium tuberculosis identified by pathogenic microbial DNA high-throughput genetic sequencing. This case demonstrates that pathogenic microbial DNA high-throughput genetic sequencing could be used to improve the detection rate of pathogenic microorganisms in patients with complex conditions, thereby allowing for earlier initiation of treatment.
Similar content being viewed by others
Introduction
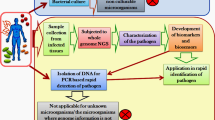
Peritoneal dialysis-associated peritonitis (PDAD) is a common complication of peritoneal dialysis (PD) and is a major cause of treatment failure in PD patients. Pathogens can be isolated from dialysis effluent in more than 90% of patients with signs and symptoms of peritonitis and increased dialysate neutrophils, with gram-positive bacteria (40–50%) being the most common, fungal peritonitis (2–4%) being uncommon, and Mycobacterium tuberculosis (MTB) and other branching mycobacteria being extremely rare [1]. Because of limitations in traditional pathogen detection and clinical aspects, PDAD patients often have negative results in dialysis effluent cultures, making the identification of the causative pathogen challenging. We report a case of refractory PDAD that was diagnosed as MTB infection via pathogenic microorganism DNA high-throughput genetic sequencing (PMseq-DNA). This detection method can extract all nucleic acids from a variety of infectious samples and perform rapid sequencing on a sequencing platform. A comparison of the sequencing results with a database enables the accurate identification of pathogenic microorganisms within 24 h. This diagnostic method is effective and rapidly produces results.
Case introduction
Chief complaint and medical history
A 52-year-old woman with end-stage kidney disease (ESKD) secondary to essential hypertension started PD on September 10, 2021. On June 15, 2022, the patient was admitted to the hospital due to abdominal pain. The color of the dialysis effluent was light yellow and slightly turbid, with a cell count of 1051/mm3. The patient, who was diagnosed with PDAD, was treated with Etimicin in the dialysis solution once a day via peritoneal perfusion for 12 days and then discharged. Antifungals were not administered during this period for treatment or prevention. On July 20, 2022, her abdominal pain worsened, with flocculent material observed in the dialysis effluent. She was admitted to the emergency department. Her body temperature was 36.7 °C, with tenderness upon abdominal palpation, and rebound tenderness was provoked. The patient had no known past history of tuberculosis or tuberculosis exposure.
Laboratory and ancillary tests
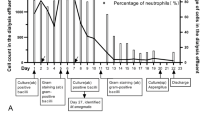
Upon hospital admission, the patient’s initial dialysis effluent cell count was recorded at 555/mm³, with polymorphonuclear cells accounting for 77.1% and mononuclear cells accounting for 22.9%. A routine blood examination revealed a white blood cell count of 6.98 × 109/L. Comprehensive details of the remaining laboratory test results are provided in Table 1. The dynamic changes in the cell count in the dialysis effluent and C-reactive protein (CRP) concentration during the second treatment period are depicted in Fig. 1. Dialysis effluent culture revealed no pathogenic bacteria on the 13th day. On the 14th day, the result of the fungal 1,3-β-D-glucan test (G test) was 56.1 pg/mL (normal value < 100.5), and the Aspergillus galactomannan antigen test (GM test) result was 0.35 S/CO (normal value 0–0.5). On the same day, PMseq-DNA was conducted on the patient’s dialysis effluent. One day later, 3052 sequences were detected for the Mycobacterium tuberculosis complex, whereas 3 sequences were detected for MTB. Because the extraction of MTB DNA is difficult and the detection rate of MTB in mNGS is low, specific sequences of the MTB complex are usually detected to increase the detection rate. The MTB complex refers to a group of mycobacteria with highly homologous genomes that can cause tuberculosis, including Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium africanum, and Mycobacterium microtus. If the specific DNA sequences of the MTB complex are detected, the possibility of pathogenic Mycobacterium infection is very high. If some specific sequences of MTB are detected, the possibility of MTB infection is very high, even if only one specific sequence is detected.
Main treatment
There were multiple changes in antimicrobials used in the management of this patient’s care (Fig. 1). Initial dialysis effluent testing confirmed PDAD, and intraperitoneal Teicoplanin and Etimicin were used. Constipation was successfully treated with Mosapride and a glycerin enema. Intraperitoneal antibiotics were continued for a total of 7 days, and improvements in abdominal pain and dialysis effluent cell counts were noted. On the 8th day, the patient’s abdominal pain was significantly relieved, and the dialysis effluent was slightly turbid. Antibiotics were reinitiated with intraperitoneal Teicoplanin and intravenous Etimicin. On the 9th day after admission, the patient developed a fever with a body temperature of 38.5 °C, which was subsequently reduced following the administration of physical cooling measures. On the 12th day, as the patient’s abdominal pain intensified, the anti-infection treatment regimen was modified to include intraperitoneal Teicoplanin and Etimicin, along with intravenous Piperacillin. However, the patient’s abdominal pain worsened on Day 14 with an increasing dialysis effluent cell count. Intraperitoneal Vancomycin, oral Moxifloxacin, and intravenous Fluconazole were added. On the 15th day, PMseq-DNA revealed that the number of MTB sequences was high, indicating tuberculous peritonitis. Considering the patient’s symptoms of nausea and loss of appetite as well as the significant gastrointestinal side effects that Pyrazinamide may cause, the antituberculosis treatment plan was changed to oral Isoniazid, Rifampicin, and Ethambutol, supplemented with intravenous Levofloxacin. To reduce the potential liver damage caused by antituberculosis drugs, we routinely take preventive measures by administering Bicyclol to patients. After the 20th day, the patient’s abdominal pain was significantly relieved, the cell count of the dialysis effluent decreased significantly, and systematic standardized antituberculosis treatment was continued. After the 26th day, the patient’s symptoms improved significantly, and the patient only sometimes experienced nausea, which was considered related to oral antituberculosis drugs. Vitamin B6 was administered to ameliorate the side effects of the antituberculosis drugs. The patient underwent three months of antituberculous treatment after being discharged because of severe gastrointestinal reactions.
Discussion
Continuous improvements in peritoneal dialysis equipment and technology have increased the survival rate of PD patients. However, the occurrence of complications during treatment cannot be ignored, the most common of which is PDAD. This condition is the direct or main cause of death in more than 15% of PD patients [2]. A diagnosis of tuberculous peritonitis is extremely rare among PD patients and is characterized by a low incidence and nonspecific symptoms. A systematic review of 216 cases of tuberculous peritonitis in PD patients revealed that the predominant clinical manifestations are abdominal pain (81.8%), fever (67.2%), and cloudy dialysis effluent (38%). Although distinct from the usual bacterial peritonitis presentation, specific symptoms include hypercalcemia (3.6%), weight loss (27.7%), extraperitoneal tuberculosis (30.1%), and a lymphocyte predominant peritoneal dialysate (31.6%) [3]. In this case, the patient did not present with distinctive symptoms of tuberculous peritonitis that differ from those of typical bacterial infections.
Tuberculous peritonitis is often associated with a significant time delay in diagnosis from the onset of symptoms to confirmation, with an average duration of 6.1 weeks [3]. Tuberculous peritonitis is often diagnosed in patients undergoing PD with a culture and smear of the dialysis effluent. Other rapid diagnostic techniques, such as dialysis effluent polymerase chain reaction (PCR) and peritoneal biopsy, are less commonly used, which may be related to their cost-effectiveness in clinical practice. The advantages and disadvantages of various diagnostic methods are shown in Table 2. Dialysis effluent culture plays a crucial role in diagnosing peritonitis because of its high specificity and ability to conduct antibiotic sensitivity testing. However, a major limitation of this method is the relatively long time required for culture, with MTB culture potentially needing up to 40 days of culture. Although dialysis effluent smears are less expensive and provide preliminary results more quickly, they have limitations in terms of detection sensitivity and specificity. The results of the smear examination are influenced by the volume of the sample: at least 5000 bacteria/mL are needed to detect mycobacteria. In contrast, culture positivity has a lower threshold for the number of bacteria, with a diagnosis possible with as few as 10 bacteria [4]. In addition, adenosine deaminase (ADA) and neutrophil-to-lymphocyte ratio (NLR) can also assist in the diagnosis of tuberculous peritonitis. Liu et al. evaluated 191 patients with suspected tuberculous peritonitis and reported that ADA in peritoneal effusion, with a cutoff value of 31.5 U/l, exhibited a sensitivity of 89.6% and specificity of 92.1%. Additionally, the ADA concentration in peritoneal effusion samples from tuberculous peritonitis patients was significantly greater than that in those from nontuberculous peritonitis patients and was positively correlated with the mycobacterial load [5]. Winston et al. demonstrated that an NLR < 15 in the dialysis effluent was the optimal cutoff value for differentiating tuberculous/nontuberculous peritonitis from bacterial peritonitis, with a sensitivity of 81% and a specificity of 70% [6]. Dialysis effluent PCR requires the design of specific short DNA segments (primers), which are tailored according to the unique gene sequences of the target microorganisms. Therefore, its limitation lies in the need to assume the type of pathogenic microorganism in advance. PCR can be used to detect low-concentration microorganisms, and this process is typically completed within a few hours. Xpert MTB/RIF, which uses heminested real-time PCR, enables the rapid detection of MTB and resistance to Rifampin. Vishal et al. conducted a systematic review of the diagnostic accuracy of Xpert MTB/RIF in abdominal tuberculosis and reported that Xpert MTB/RIF has moderate sensitivity and good specificity for diagnosing peritoneal tuberculosis [7]. When ascitic fluid culture is used as a test indicator, the pooled sensitivity and specificity of Xpert MTB/RIF are 64% and 97%, respectively. Although laparoscopy and tissue biopsy are invasive methods for diagnosing tuberculous peritonitis, they have the advantage of a high diagnostic accuracy. Division et al. conducted a systematic review of tuberculous peritonitis and reported that when the macroscopic appearance of laparoscopy is combined with histological results, the sensitivity and specificity are 93% and 98%, respectively [4].
PMseq-DNA is a method based on metagenomic next-generation sequencing (mNGS) technology. mNGS is a powerful tool for analyzing the entire genomes of both human and microbial organisms in clinical samples. It allows the unbiased detection of various pathogenic microorganisms, including viruses, bacteria, fungi, and parasites [8]. PMseq-DNA offers a broad detection range, including 6350 bacterial species (including 133 Mycobacterium species and 122 Mycoplasma/Chlamydia/Rickettsia species), 1798 DNA viruses, 1064 fungi, and 234 parasites. PMseq-DNA testing, costing approximately 463 USD, enabled a diagnosis confirmation within just one day from the time the sample was submitted. It can be applied to various samples, such as cerebrospinal fluid, bronchoalveolar lavage fluid, bone marrow, and blood. The short turnaround time and wide detection range of PMseq-DNA make clinical diagnosis more convenient and reliable, especially for patients with complex conditions. Since its emergence in 2004, the cost of high-throughput or next-generation sequencing has decreased by several orders of magnitude [9]. It has become a key technological platform for detecting microorganisms in clinical samples from patients. For some difficult to treat and critically ill patients, the use of high-throughput genomic sequencing of pathogenic microorganisms is advisable. In a comparative study of the diagnostic performance between mNGS and traditional culture methods, mNGS demonstrated greater sensitivity (50.7% vs. 35.2%), whereas the difference in specificity between the two methods was not significant (85.7% vs. 89.1%). Additionally, mNGS has a clear advantage in detecting MTB, viruses, anaerobic bacteria, and fungi and maintains a relatively high positive rate in samples exposed to antibiotics (52.7% vs. 34.4%), which further demonstrates the potential and value of mNGS in clinical diagnostics [10]. In light of the excellent performance of mNGS in detecting MTB, we hope that some foundations can support this testing to reduce its cost and make it a routine detection method in high-MTB burden countries.
PMseq-DNA has been applied in various clinical fields, including central nervous system infections, bloodstream infections, respiratory infections and gastrointestinal infections, demonstrating significant advantages over traditional detection methods. PMseq-DNA can be used to analyze a variety of sample types and is not affected by the patient’s prior antibiotic use. Its extensive detection range eliminates the need to presume the type of pathogenic microorganism, and it can even detect new types of pathogens. The time from sample submission to result generation is only 24 h. Although the cost of detection is high at approximately 460 dollars, with the advancement of technology, mNGS is expected to become a routine detection method in the future.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- PDAD:
-
Peritoneal dialysis-associated peritonitis
- PD:
-
Peritoneal dialysis
- MTB:
-
Mycobacterium tuberculosis
- PMseq-DNA:
-
Pathogenic microorganism DNA high-throughput genetic sequencing
- ESKD:
-
End-stage kidney disease
- CRP:
-
C-reactive protein
- G test:
-
1, 3-β-D-glucan test
- GM test:
-
Galactomannan antigen test
- mNGS:
-
Metagenomic next-generation sequencing
- PCR:
-
polymerase chain reaction
References
Changlin Mei XG. Chaoyang Ye: practical Dialysis Handbook. Beijing: People’s Medical Publishing House; 2017.
Szeto C-C, Li PK-T. Peritoneal Dialysis–Associated Peritonitis. Clin J Am Soc Nephrol. 2019;14(7):1100–5.
Thomson BKA, Vaughan S, Momciu B. Mycobacterium tuberculosis peritonitis in peritoneal dialysis patients: a scoping review. Nephrology. 2021;27(2):133–44.
Sanai FM, Bzeizi KI. Systematic review: tuberculous peritonitis – presenting features, diagnostic strategies and treatment. Aliment Pharmacol Ther. 2005;22(8):685–700.
Liu R, Li J, Tan Y, Shang Y, Li Y, Su B, Shu W, Pang Y, Gao M, Ma L. Multicenter evaluation of the acid-fast bacillus smear, mycobacterial culture, Xpert MTB/RIF assay, and adenosine deaminase for the diagnosis of tuberculous peritonitis in China. Int J Infect Dis. 2020;90:119–24.
Fung WW-S, Chow K–M, Ng JK-C, Chan GC-K, Li PK-T, Szeto C-C. The Clinical Utility of the Neutrophil-to-Lymphocyte Ratio as a Discriminatory Test among Bacterial, Mycobacterium Tuberculosis, and Nontuberculous Mycobacterium Peritoneal Dialysis–Related Peritonitis. Kidney360 2022, 3(6):1031–1038.
Sharma V, Soni H, Kumar-M P, Dawra S, Mishra S, Mandavdhare HS, Singh H, Dutta U. Diagnostic accuracy of the Xpert MTB/RIF assay for abdominal tuberculosis: a systematic review and meta-analysis. Expert Rev Anti-infective Therapy. 2020;19(2):253–65.
Li S. Metagenomic next generation sequencing used in immunocompromised patients with kidney disease. Chin J Nephrol Dialysis Transplantation. 2022;31:151–2.
Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14(1):319–38.
Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, Yao Y, Su Y, Huang Y, Wang M, et al. Microbiological Diagnostic performance of Metagenomic Next-generation sequencing when Applied to Clinical Practice. Clin Infect Dis. 2018;67(suppl2):S231–40.
Acknowledgements
The authors thank the patient for allowing us to publish this case report. We would like to thank American Journal Experts for providing linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the General Project of Inner Mongolia Natural Science Foundation (No. 2022MS08063), the Inner Mongolia Health Science and Technology Project in 2022 (No. 202201293), the Key Research and Development and Achievement Transformation Project in the Social Welfare Field of the 14th Five-Year Plan in the Inner Mongolia Autonomous Region (No. 2022YFSH0087), the Inner Mongolia “Grassland Talents” Program Young Innovative Talent Project (No. Q2022082), the National Natural Science Foundation of China (No. 81960143), the “ Going Far” Talent Program of Inner Mongolia Medical University (No. ZY0130015), and the Trinity College Students Innovation and Entrepreneurship Cultivation Project of Inner Mongolia Medical University (No. SWYT2020008).
Author information
Authors and Affiliations
Contributions
R.X conducted a literature search on the topic and drafted and revised the paper. W.G, T.H and X.W conducted a literature search on the topic and revised the paper. J.Z gives guidance and revises the paper. Y.M supervised the work and revised the paper.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent was obtained from the patient in both written and verbal formats to publish this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, Rf., Gao, Wn., Hu, Tl. et al. Pathogenic microorganism DNA high-throughput genetic sequencing to diagnose peritoneal dialysis-associated peritonitis due to Mycobacterium tuberculosis infection. BMC Nephrol 25, 290 (2024). https://doi.org/10.1186/s12882-024-03727-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03727-3