Abstract
Background
Study objectives included the development of a practical nomogram for predicting live birth following frozen-thawed embryo transfers in ovulatory women.
Methods
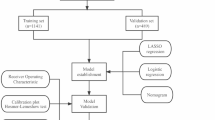
Totally, 2884 patients with regular menstrual cycles in our center were retrospectively enrolled. In an 8:2 ratio, we randomly assigned patients to training and validation cohorts. Then we identified risk factors by multivariate logistic regression and constructed nomogram. Finally, receiver operating characteristic curve analysis, calibration curve and decision curve analysis were performed to assess the calibration and discriminative ability of the nomogram.
Results
We identified five variables which were related to live birth, including age, anti-Müllerian hormone (AMH), protocol of frozen-thawed embryo transfer (FET), stage of embryos and amount of high-quality embryos. We then constructed nomograms that predict the probabilities of live birth by using those five parameters. Receiver operating characteristic curve analysis (ROC) showed that the area under the curve (AUC) for live birth was 0.666 (95% CI: 0.644–0.688) in the training cohort. The AUC in the subsequent validation cohorts was 0.669 (95% CI, 0.625–0.713). The clinical practicability of this nomogram was demonstrated through calibration curve analysis and decision curve analysis.
Conclusions
Our nomogram provides a visual and simple tool in predicting live birth in ovulatory women who received FET. It could also provide advice and guidance for physicians and patients on decision-making during the FET procedure.
Similar content being viewed by others
Background
Infertility is a serious human reproductive health issue, which affects about fifteen percent of married couples [1]. Nowadays, In vitro fertilization and embryo transfer (IVF-ET) is applied more and more widely worldwide. In some circumstances, when the patients have a tendency to suffer ovarian hyperstimulation syndrome or their endometrium is too thin in fresh IVF cycle, or they have a plan to conduct preimplantation genetic testing, frozen-thawed embryo transfer (FET) become an effective alternative. The proportion of FET is increasing gradually and now accounts for about 30–40% of all the transfer cycles. Therefore, more and more patients are receiving FET treatment.
Although the live birth rate (LBR) of FET improved recently in the world, the average success rate remains low. It is hard to predict clinical pregnancy rate and live birth rate objectively because of the heterogeneity in etiology and treatment protocols et cl. How to identify the risk factors and predict live birth after embryo transfer has received widespread attention both from the infertility population and clinical researchers.
As we all knows, pathophysiology and pregnancy outcomes between ovulatory infertility and anovulatory infertility are different to some extent. For example, the live-birth rate of FET is higher than that of fresh-embryo transfer in anovulatory patients who suffered polycystic ovary syndrome (PCOS) [2]. Frozen single blastocyst transfer resulted in a higher singleton livebirth rate than did fresh single blastocyst transfer in ovulatory women [3]. However, there is no difference in ovulatory women between FET and fresh-embryo transfer of cleavage-stage embryos [4]. Recently, there are studies trying to figure out risk factors and construct predictive models in specific infertility population [5]. For example, obesity and metabolism factors were used to develop model to predict the pregnancy outcomes in PCOS patients who received IVF/ICSI or FET [6, 7]. In ovulatory women, there are some studies trying to figure out the best FET protocols [8, 9]. However, until now, studies trying to predict clinical pregnancy outcome in ovulatory women remain limited.
Many mathematical models such as the nomogram have been used in the field of assisted reproduction technology [10,11,12]. Nomogram converts complex regression analyses into visual figures, making predictive models more readable and convenient. The purpose of this study was to identify factors that affect ovulatory women's live birth rate. By logistic regression and nomogram, we tried to create a convenient tool for predicting the live birth in ovulatory women undergoing FET.
Methods
Study design and population
We enrolled patients younger than 42 years old with regular length of menstrual cycles between 24 and 38 days. They received their first or second FET cycles from June 2015 to December 2021 in the reproductive center of Qilu hospital, Shandong University. Exclusion criteria include recurrent spontaneous abortion (defined as three or more spontaneous abortions prior to 20–28 weeks gestation), abnormal anatomy of uterine, intrauterine adhesions, endometriosis, polycystic ovary syndrome, endocrine disorders and chromosomal diseases (exclude chromosome polymorphism). A total of 2884 patients were enrolled and assigned randomly into training (n = 2307) and validation cohort (n = 577). The former was used for the construction of the model and the latter was used for internal validation. Indicators for IVF/ICSI treatments were tubal factor (64.9%), male factor (12.7%), unexplained (4.5%), mixed factor (13.6%) and diminished ovarian reserve [13] (4.3%).
Ethics Committee approval for this study has been granted by Qilu Hospital, Shandong University (registration number: KYLL-202306–019). As a retrospective study, we were exempt from informed consent. Our protocol of research was performed according to the Declarations of Helsinki.
Data collection
We collected clinical data including male and female age, duration and type of infertile history, gravidity and labor history, body mass index (BMI), reproductive endocrine hormones and anti-Müllerian hormone (AMH). The protocols of FET, detailed information of embryos transferred and endometrial thickness on transplant day were recorded. The outcome was live birth. We defined live birth as live newborn ≥ 28th week of pregnancy.
Embryo score
Morphological score of embryos was assessed before embryo transfer. The qualities of the Cleavage-stage embryo were evaluated into four grades according to their speed of development, degree of fragmentation and evenness of cleavage sphere. We scored blastocysts according to Gardner morphological criteria [14].
Protocol of FET
Natural cycle (NC) or artificial cycle (AC) were selected based upon patients’ individual prefer and the experience of physicians in charge. For NC, clinicians examined the follicle size and endometrial thickness in order to get the ovulation day by transvaginal ultrasound examination. HCG or GnRH-a was administrated for ovulation induction in some patients. FET was scheduled based on ovulation day. For AC, estrogens are administered for approximately 14 days from cycle day 3. Endometrial thickness and the serum estrogen level are monitored from cycle day 13. Patients were given progesterone when their endometrial thickness were at least 7 mm and the serum estrogen level of over 100 pg/ml. Thawing and transferring of embryo was scheduled based on the supplementation of progesterone. On the day of ovulation, intramuscular injection of progesterone 40–80 mg/d or oral dydrogesterone 20-40 mg/d was administered for luteal support of NC. Intramuscular injection of progesterone 40–100 mg/d, or progesterone vaginal sustained-release gel 90 mg/d or micronized progesterone 300–800 mg/d was administered for luteal support of AC. The luteal support was maintained until intrauterine pregnancy is seen on ultrasound, and then the dose was reduced until 8 ~ 10 weeks.
Data analysis
We performed statistical analyses with statistical packages R and SPSS 26.0. For continuous variables, mean ± SD (for normally distributed variables) or median and IQR (for skewed distributed variables) was used and normality test was detected by the Kolmogorov–Smirnov test. Student t test or Mann–Whitney U tests were applied to compare continuous variables. Frequencies (percentages) were presented and Chi-square test was applied to compare categorical variables. An analysis of the relationship between variables and live birth was conducted by using logistic regression. We first selected variables (P < 0.1) in univariate analyses to avoid missing important risk factors [15, 16]. Then we screened out final variables by multivariable logistic regression analysis (MLR) (p < 0.05) and enrolled them into the nomogram model. Variance inflation factor was used to detect the multicollinearity with the criterion of less than 10.
Nomogram was constructed by the data in training cohort to predict the probability of live birth in ovulatory women receiving FET. The variables enrolled in nomogram are female age, AMH, number of high-quality embryos, FET protocol and stage of embryos. Each variable was mapped on its scale to get a score, then the total score of all the covariates was calculated and the probability of live birth was finally obtained. The area under the receiver operating characteristic (ROC) curve (AUC) was used to detect the discriminatory ability. Calibration curve was conducted with a bootstrapping technique (1,000 repetitions) to detect the calibration. Decision curve analyses (DCA) was applied to calculate the net benefit of making a decision [17].
Results
Baseline characteristics
Patient characteristics and parameters related to FET cycle were shown in Table 1. Clinical pregnancy rate was 55.61% and 57.71%, separately. Live birth rate was 45.60% and 48.01%, separately. The characteristics observed between the two cohorts were not significant.
As shown in Table 2, the characteristics of women with and without live birth in the training cohort were displayed. Women with live birth had younger age, lower BMI and baseline FSH, higher AMH, thicker endometrium on the day of ET. They transferred more high-quality embryos and higher percentage of blastocyst. The FET protocol is also different. The percentage of patients with live birth and their physician who chose natural cycle is 72.24%, while the percentage of patients without live birth and their physician who chose natural cycle is 68.13%.
ROC analyses were conducted to analyze the proper cutoff values of variables. The cutoff point of the female age related to live birth was 32.5 and the optimal cutoff point of AMH was 2.265 (Supplementary Table 1). For the cutoff point of the female age, the sensitivity and specificity for live birth is 0.525 and 0.641. For the cutoff point of AMH, the sensitivity and specificity for live birth is 0.717 and 0.440.
Logistic analysis of the risk factors of live birth
The univariable and multivariable logistic analyses in the training cohort revealed that female age, AMH, protocol of FET, stage of embryos and number of high-quality embryos were significantly correlated with live birth. The MLR showed that the live birth was associated with age > 32 (OR: 0.629; 95% CI:0.511–0.773, P = 0.000), AMH > 2.26 (OR: 1.406; 95% CI: 1.143–1.730, P = 0.001), number of high-quality embryos (OR:1.896; 95% CI: 1.602–2.243, P = 0.000), artificial FET cycle (OR: 0.734; 95% CI:0.600–0.897, P = 0.003) and blastocyst embryos transferred (OR, 3.829; 95% CI, 2.850–5.145, P = 0.000) (Table 3).
Construction of nomogram to predict live birth
Based on the logistic regression analyses in the training cohort, five factors including age, AMH, cleavage embryo/blastocyst, number of high-quality embryos and protocol of FET were selected. we incorporated these factors into nomogram which could predict live birth (Fig. 1A).
Nomogram to predict the probability of live birth for ovulatory women undergoing FET. A To calculate a patient’s live birth rate, first label her value on each variable axis and get the corresponding points through the top Points scale. Then, add the points of each variable value together and label it on the Total Points scale. Finally, obtain the personalized probability of live birth by the bottom axis. B The ROC curves of the nomogram for probability of live birth in the training cohort. C The ROC curves of the nomogram for probability of live birth in the validation cohort. D Calibration curve of the nomogram in the training cohort. E Calibration curve of the nomogram in the validation cohort. After 1000 repetitions of bootstrap, the calibration curves showed a good correlation between the predicted probability and actual probability. ROC: receiver operating characteristics curves
Validation of the nomogram
ROC curves were shown. In the training dataset, AUC for live birth (Fig. 1B) was 0.666. In the validation dataset, the AUC was 0.669 (Fig. 1C). The sensitivity of the training cohort was 73.5% and specificity was 50.1%. The sensitivity of the validation cohort was 56.3% and specificity was 68.3%.The calibration curves for live birth were shown in Fig. 1D and E. Decision curve analyses were used to calculate the net benefit which were shown in Fig. 2. The black line means the net benefit of no patients got live birth. The gray one means the net benefit of all patients got live birth. The closer the curve to these two reference lines, the less valuable the model is. Our curve is above the reference line in a relative wide threshold interval.
The decision curve analysis of the nomogram for probability of live birth in the training cohort and validation cohort. A The decision curve analysis of the nomogram for probability of live birth in the training cohort. B The decision curve analysis of the nomogram for probability of live birth in the validation cohort
Discussion
Live birth is the most important issue concerned by patients who received FET and their physician. Based on 2884 patients with regular menstrual cycles, we constructed an easy-used nomogram to predict the live birth probability in this study. The indicators enrolled in the nomogram include female age, AMH, embryo transfer protocols, cleavage embryos/blastocysts and number of high-quality embryos. Moreover, we evaluated our nomogram by calibration curve, discrimination curve and decision curve analysis. Our model showed moderate discriminatory ability and well-calibrated ability overall. Decision curve analyses showed that our model could get net benefit in a relative wide threshold interval.
In recent years, there are many predictive models to predict the outcome of infertility population or those who received assisted reproductive technology. For example, Scientists constructed different predictive models to calculate cumulative live birth rate in patients who accepted fresh IVF treatment [18, 19]. However, only a few predictive models were constructed to predict pregnancy outcomes in patients who received FET treatment. Yu et al. tried to establish a predictive nomogram for early clinical pregnancy but not live birth in FET cycles [20]. In another study, a data-driven predictive model using machine learning was constructed to calculate LBR in FET, with a satisfactory performance [21]. However, the infertile population is highly heterogeneous. The pathophysiology, treatment protocol and clinical outcome of ovulatory people and anovulatory people is different. It is necessary to construct predictive model for ovulatory people who received FET. Our model aims to do this and it helps those patients to have a reasonable expectation of their LBR. It also helps clinicians to choose proper transfer protocol and embryos.
As we all know, female fecundity declines with age [22]. Advanced maternal age reduces ovarian reserve, decreases oocyte/embryo competence and negatively associates with pregnancy outcomes [23]. No remedies could effectively antagonize the aging-related fertility decline [24]. It was reported that age is the only variable predicting live birth in patients undergoing IVF treatment [25]. Our result was in accordance with those previously published researches. In this study, we calculated the ideal cutoff values of age for live birth and found that the cut-off value was 32.5 years old.
AMH is a proper metric for ovarian reserve function or ovarian response. Research suggests that AMH could predict live birth independently of age in ART cycles, although the predictive ability is poor [26]. AMH was also proven to be related to implantation and clinical pregnancy [27]. We incorporated AMH in our model according to results of MLR. In our study, the OR for AMH as an indicator of live birth was 1.406 and the optimal cutoff value for AMH was 2.26 ng/ml. This shows a high degree of consistency with previous study. In another study predicting early pregnancy rate in FET, the cutoff value of AMH was 2.23 ng/ml [20].
The studies about the relationship between basal FSH and pregnancy outcome was inconsistent in previous studies. It was reported that basal FSH was independent predictive factor of live birth in poor ovarian response patients [28] and women elder than forty years [29]. However, in donor/recipient pairs undergoing oocyte donation IVF, donor basal FSH was not associated with pregnancy outcomes [30]. In our study, after adjusting the confounding factors, multivariable logistic analysis revealed that basal FSH level was not related with live birth. This difference may be due to the different populations enrolled in different studies.
Artificial cycle (AC) and natural cycle (NC) are the main endometrial preparation regimen in FET. However, no consensus was reached as which protocol is optimal. Although previous researches were controversial, it seemed that NC was better in ovulatory women. For example, a study found that modified NC was linked to a higher LBR in patients with regular menstruation than AC [31]. A similar study found that AC was linked to higher ectopic pregnancy rate and lower LBR in ovulatory women [32]. A recent study found that AC was related with higher risk of large for gestational age, hypertensive disorders and higher birthweight in 3,639 patients who got live-born singletons after FET [33]. In our study, we found NC regimen had a higher probability of live birth in ovulatory women.
In our nomogram, cleavage embryo/blastocysts and number of high-quality embryos are two dominant factors. The OR of “stage of embryo” and “number of high-quality embryos” is 3.829 and 1.896, separately. These two indicators were also the common indicators in other models. For example, Zhang. et al. incorporated the “stage and number of transferred embryos” into the nomogram predicting the early pregnancy in women who received FET [20]. Zhou et al. found that “number of high-quality embryos” are related to live birth in artificial FET [34].
As we all know, the factors influencing live birth are complex. The discriminatory ability and calibration ability of the models predicting live birth in patients receiving IVF treatment are usually not so high. For example, Dhillon et al. constructed a predictive model of live birth probabilities with AUC of 0.62 in the validation cohort [35]. Qui et al. constructed a personalized prediction of live birth prior to the first IVF cycle with AUC of 0.73 by using complex machine learning method [36]. In our study, we analyzed risk factors of live birth in ovulatory women with regular menstruation in our center. To our knowledge, it is the first predictive model of live birth for ovulatory women receiving FET. Secondly, our nomogram is a visual and simple model. The indicators we incorporated in our model are all easily got clinical measurements, which made our model be more applicable than other complicated models.
However, there are also some disadvantages in our study. Firstly, our study did not analyze the female age at oocyte retrieval, which may neglect the potential relationship between female age at oocyte retrieval and live birth. Secondly, it is single-center research, and we still need outer validation from other center’s datasets. Thirdly, it is retrospective research, in which bias is unavoidable to some extent. Prospective study is needed to validate the sensitivity and specificity of our nomogram. Finally, our predictive model has a limited accuracy partly because the indicators we enrolled in the nomogram are all the simple and easy-got clinical data. The heterogeneity of the infertile population also makes it difficult to obtain an accurate predictive model. Further studies focusing on those limitations need to be conducted.
Conclusions
To sum up, we constructed a practical predictive nomogram for live birth in ovulatory women undergoing FET. We used five variables including age, AMH, protocol of FET, types of embryos and number of high-quality embryos, which were screened out by logistic regression analyses. Our model provided a reference for patients with regular ovulation and help them establish a reasonable expectation of their live birth. It aims to help the couples minimize their anxiety and confusion, which will further enhance their confidence in the long course of ART treatment. However, our data were from a single reproductive center and the accuracy of our predictive model is limited, outer validation and further exploration to improve the performance of our model are needed in the future.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AC:
-
Artificial cycle
- AMH:
-
Anti-Müllerian hormone
- AUC:
-
The area under the curve
- BMI:
-
Body mass index
- DCA:
-
Decision curve analyses
- FET:
-
Frozen-thawed embryo transfer
- IVF-ET:
-
In vitro fertilization and embryo transfer
- LBR:
-
The live birth rate
- MLR:
-
Multivariable logistic regression analysis
- NC:
-
Natural cycle
- PCOS:
-
Polycystic ovary syndrome
- ROC:
-
Receiver operating characteristic curve analysis
References
Sang Q, Ray PF, Wang L. Understanding the genetics of human infertility. Science. 2023;380(6641):158–63.
Chen ZJ, Shi Y, Sun Y, Zhang B, Liang X, Cao Y, et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2016;375(6):523–33.
Wei D, Liu JY, Sun Y, Shi Y, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310–8.
Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of Fresh versus Frozen Embryos in Ovulatory Women. N Engl J Med. 2018;378(2):126–36.
Weghofer A, Barad DH, Darmon SK, Kushnir VA, Albertini DF, Gleicher N. The ovarian sensitivity index is predictive of live birth chances after IVF in infertile patients. Hum Reprod Open. 2020;2020(4):hoaa049.
Gao L, Li M, Wang Y, Zeng Z, Xie Y, Liu G, et al. Overweight and high serum total cholesterol were risk factors for the outcome of IVF/ICSI cycles in PCOS patients and a PCOS-specific predictive model of live birth rate was established. J Endocrinol Invest. 2020;43(9):1221–8.
Jiang X, Liu R, Liao T, He Y, Li C, Guo P, et al. A Predictive Model of Live Birth Based on Obesity and Metabolic Parameters in Patients With PCOS Undergoing Frozen-Thawed Embryo Transfer. Front Endocrinol (Lausanne). 2021;12: 799871.
Li D, Khor S, Huang J, Chen Q, Lyu Q, Cai R, et al. Frozen Embryo Transfer in Mildly Stimulated Cycle With Letrozole Compared to Natural Cycle in Ovulatory Women: A Large Retrospective Study. Front Endocrinol (Lausanne). 2021;12: 677689.
Liu X, Wen W, Wang T, Sun T, Wang T, Zhang N, et al. Comparison of endometrial preparation protocols (natural cycle versus hormone replacement cycle) for frozen embryo transfer (COMPETE): a study protocol for a randomised controlled trial. BMJ Open. 2022;12(10): e063981.
Ballester M, Oppenheimer A, d’Argent EM, Touboul C, Antoine JM, Coutant C, et al. Nomogram to predict pregnancy rate after ICSI-IVF cycle in patients with endometriosis. Hum Reprod. 2012;27(2):451–6.
Liao Z, Cai L, Liu C, Li J, Hu X, Lai Y, et al. Nomogram for predicting the risk of preterm delivery after IVF/ICSI treatment: an analysis of 11513 singleton births. Front Endocrinol (Lausanne). 2023;14:1065291.
Qu P, Chen L, Zhao D, Shi W, Shi J. Nomogram for the cumulative live birth in women undergoing the first IVF cycle: Base on 26, 689 patients in China. Front Endocrinol (Lausanne). 2022;13: 900829.
Cohen J, Chabbert-Buffet N, Darai E. Diminished ovarian reserve, premature ovarian failure, poor ovarian responder–a plea for universal definitions. J Assist Reprod Genet. 2015;32(12):1709–12.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Dai M, Jahanzaib R, Liao Y, Yao F, Li J, Teng X, et al. Prognostic value of KRAS subtype in patients with PDAC undergoing radical resection. Front Oncol. 2022;12:1074538.
Zhou C, Yao Y, Zheng Z, Gong J, Wang W, Hu S, et al. Stenting technique, gender, and age are associated with cardioprotection by ischaemic postconditioning in primary coronary intervention: a systematic review of 10 randomized trials. Eur Heart J. 2012;33(24):3070–7.
Fitzgerald M, Saville BR, Lewis RJ. Decision curve analysis. JAMA. 2015;313(4):409–10.
McLernon DJ, Raja EA, Toner JP, Baker VL, Doody KJ, Seifer DB, et al. Predicting personalized cumulative live birth following in vitro fertilization. Fertil Steril. 2022;117(2):326–38.
Ratna MB, Bhattacharya S, van Geloven N, McLernon DJ. Predicting cumulative live birth for couples beginning their second complete cycle of in vitro fertilization treatment. Hum Reprod. 2022;37(9):2075–86.
Zhang Q, Wang X, Zhang Y, Lu H, Yu Y. Nomogram prediction for the prediction of clinical pregnancy in Freeze-thawed Embryo Transfer. BMC Pregnancy Childbirth. 2022;22(1):629.
Liang R, An J, Zheng Y, Li J, Wang Y, Jia Y, et al. predicting and improving the probability of live birth for women undergoing frozen-thawed embryo transfer: a data-driven estimation and simulation model. Comput Methods Programs Biomed. 2021;198: 105780.
Carson SA, Kallen AN. Diagnosis and Management of Infertility: A Review. JAMA. 2021;326(1):65–76.
Cimadomo D, Fabozzi G, Vaiarelli A, Ubaldi N, Ubaldi FM, Rienzi L. Impact of Maternal Age on Oocyte and Embryo Competence. Front Endocrinol (Lausanne). 2018;9:327.
Ubaldi FM, Cimadomo D, Vaiarelli A, Fabozzi G, Venturella R, Maggiulli R, et al. Advanced Maternal Age in IVF: Still a Challenge? The Present and the Future of Its Treatment. Front Endocrinol (Lausanne). 2019;10:94.
Mutlu MF, Erdem M, Erdem A, Yildiz S, Mutlu I, Arisoy O, et al. Antral follicle count determines poor ovarian response better than anti-Müllerian hormone but age is the only predictor for live birth in in vitro fertilization cycles. J Assist Reprod Genet. 2013;30(5):657–65.
Iliodromiti S, Kelsey TW, Wu O, Anderson RA, Nelson SM. The predictive accuracy of anti-Müllerian hormone for live birth after assisted conception: a systematic review and meta-analysis of the literature. Hum Reprod Update. 2014;20(4):560–70.
Tal R, Tal O, Seifer BJ, Seifer DB. Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: a systematic review and meta-analysis. Fertil Steril. 2015;103(1):119-30.e3.
Gong X, Zhang Y, Zhu Y, Wang P, Wang Z, Liu C, et al. Development and validation of a live birth prediction model for expected poor ovarian response patients during IVF/ICSI. Front Endocrinol (Lausanne). 2023;14:1027805.
Kim HO, Sung N, Song IO. Predictors of live birth and pregnancy success after in vitro fertilization in infertile women aged 40 and over. Clin Exp Reprod Med. 2017;44(2):111–7.
Barton SE, Missmer SA, Ashby RK, Ginsburg ES. Multivariate analysis of the association between oocyte donor characteristics, including basal follicle stimulating hormone (FSH) and age, and IVF cycle outcomes. Fertil Steril. 2010;94(4):1292–5.
Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, et al. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med. 2016;62(5):335–42.
Jing S, Li XF, Zhang S, Gong F, Lu G, Lin G. Increased pregnancy complications following frozen-thawed embryo transfer during an artificial cycle. J Assist Reprod Genet. 2019;36(5):925–33.
Zhou R, Zhang X, Huang L, Wang S, Li L, Dong M, et al. The impact of different cycle regimens on birthweight of singletons in frozen-thawed embryo transfer cycles of ovulatory women. Fertil Steril. 2022;117(3):573–82.
Zhou R, Zhang X, Dong M, Huang L, Zhu X, Wang S, et al. Association between endogenous LH level prior to progesterone administration and live birth rate in artificial frozen-thawed blastocyst transfer cycles of ovulatory women. Hum Reprod. 2021;36(10):2687–96.
Dhillon RK, McLernon DJ, Smith PP, Fishel S, Dowell K, Deeks JJ, et al. Predicting the chance of live birth for women undergoing IVF: a novel pretreatment counselling tool. Hum Reprod. 2016;31(1):84–92.
Qiu J, Li P, Dong M, Xin X, Tan J. Personalized prediction of live birth prior to the first in vitro fertilization treatment: a machine learning method. J Transl Med. 2019;17(1):317.
Acknowledgements
We appreciate Dr. Mao for his help in data analysis.
Funding
This study has received assistance from National Natural Science Foundation of China (No. 82001638, No. 82371695, No. 82071620).
Author information
Authors and Affiliations
Contributions
Lan Chao conceived and designed the experiments. Hengfei Li, Shan Dong and Ying Wang collected and analyzed the data. Ying Wang and Shan Dong drafted the manuscript. Yang Yang and Anliang Guo provided critical revision. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Qilu Hospital of Shandong University. Exemption from informed consent was approved by the Ethics Committee (registration number: KYLL-202306–019) as this study was a retrospective study of routinely collected clinical data. All experimental protocols were approved by the medical ethics committee of Qilu Hospital of Shandong University and all methods were carried out in accordance with the Declarations of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, Y., Dong, S., Li, H. et al. Nomogram for predicting live birth in ovulatory women undergoing frozen-thawed embryo transfer. BMC Pregnancy Childbirth 24, 559 (2024). https://doi.org/10.1186/s12884-024-06759-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06759-7