Abstract
Background
Group B Streptococcus (GBS) infection remains a leading cause of newborn morbidity and mortality. The study aimed to determine the adherence rate to the universal screening policy a decade after its introduction. Secondly, whether the timing of antibiotics given in GBS carriers reduces the incidence of neonatal sepsis.
Methods
Delivery records at Hong Kong Baptist Hospital in 2022 were examined to retrieve antenatal and intrapartum details regarding maternal GBS carrier status, previous maternal GBS carrier status, antibiotic treatment, timing of treatment, neonatal condition at birth and whether the neonate had sepsis. Univariate statistics was used to assess the relationship between maternal GBS carrier and neonatal sepsis overall. Incidence of neonatal sepsis was stratified according to mode of delivery and timing of antibiotic.
Results
The adherence rate to the universal GBS screening policy was 97%. The risk of neonatal sepsis was 5.45 (95% CI 3.05 to 9.75) times higher in women who were GBS screened positive when compared to non-GBS carriers (p < 0.001). Amongst term neonates from GBS carriers delivered by Caesarean section, the risk of neonatal sepsis significantly decreased by 70% after antenatal antibiotic treatment (p = 0.041) whereas in term neonates delivered vaginally, the risk of neonatal sepsis decreased by 71% (p = 0.022) if intrapartum antibiotic prophylaxis was given 4 or more hours.
Conclusion
Giving antenatal antibiotic treatment before Caesarean section or intrapartum antibiotic prophylaxis for 4 or more hours before vaginal delivery may decrease the risk of neonatal sepsis in term neonates delivered from GBS carriers.
Similar content being viewed by others
Background
Group B streptococcus (GBS) is a leading cause of newborn morbidity and mortality [1]. In order to reduce the risk from GBS infection, the Centers for Disease Control and Prevention (CDC) recommended universal screening of pregnant women between 35 and 37 weeks of gestation in 2002 [2]. The CDC recommendation to screen pregnant women for GBS was to allow those with GBS colonisation to receive intra-partum antibiotics in order to mitigate the risks for developing neonatal sepsis [2]. Although the Royal College of Obstetricians and Gynaecologists (RCOG) also recommended risk based screening programme [3], the universal screening program of GBS for all pregnant seeking antenatal care has been implemented in Hong Kong since 2012 [4]. Universal prenatal screening for GBS could assist health care providers to identify those pregnant women with GBS colonisation so as to provide intra-partum antibiotics before delivery [5].
Several studies have now shown that a universal GBS policy is effective in decreasing newborn GBS [5, 6]. Recent publications showed that the adherence rate to the universal GBS screening ranges from as low as 65% in Greece [7] to 68.3% in Germany [8] to 89% in USA [9]. A previous study has reported that 81% of patients in Hong Kong would accept screening for GBS with 62% willing to pay [10]. However, the study was conducted prior to the initiation of the Hong Kong-wide universal GBS carrier screening policy in 2012. There has been no information reported to indicate the adherence rate to this universal screening policy.
The aims of the current study were to assess: firstly, the adherence rate to the universal GBS screening after implementation of the policy; secondly, whether the rate of neonatal sepsis increased in those with GBS carrier and lastly, whether the timing of antibiotic treatment affected the rate of neonatal sepsis.
Methods
Participants
This is a real-world study of all the maternities who delivered at Department of Obstetrics and Gynaecology of the Hong Kong Baptist Hospital between 1st January to 31st December 2022. The study was approved by the clinical and Research Ethics Committee of Hong Kong Baptist Hospital (CREC- 2024-01).
On the delivery admission episode, the attending obstetrician or midwife completed a standardised medical history form to document maternal socio-demographics, past and current obstetric risk history, past and current medical history, whether GBS screening was performed and if so, the date of the screening, the screening result and any antenatal antibiotic treatment. After delivery, the nurses recorded whether intrapartum antibiotics were prescribed, the interval between prescribing antibiotics and delivery, mode of delivery, neonatal condition at birth, whether any neonatal investigations were done including C-reactive protein (CRP) blood tests, blood cultures and chest X-rays (CXR).
Neonates of mothers confirmed to be GBS carriers were managed according to departmental standard management protocol which is based on the American Academy of Pediatrics [5]. Under this protocol, neonates are assessed for signs and symptoms of GBS infection and/or neonatal sepsis. Neonates were diagnosed as having neonatal sepsis based on clinical evidence including apnea, respiratory distress, increased CRP, CXR and whether intravenous antibiotics were administered.
Statistical analysis
The statistical analysis was performed using the Statistical Packages of Social Sciences for Windows version 29.0 (SPSS, Illinois, USA). Continuous variables are presented as medians and Interquartile range (IQR) whilst qualitative data is presented as absolute frequency and percentage.
Gestational age was defined according to the last menstrual period unless it was not consistent with that of the ultrasound estimation. In that event, the ultrasound date was taken as the actual gestational age. Relative timing of administration of intrapartum antibiotics to that of the time of birth was partitioned as being either < 4 h or ≥ 4 h.
Contingency tables and chi-squared or Fishers Exact test were used to assess the association between the absence/presence of neonatal sepsis at birth and maternal GBS carrier status, timing of intrapartum antibiotic before delivery, mode of delivery and maternal antenatal treatment for GBS. All statistical tests were two sided with p values < 0.05 considered statistically significant.
Results
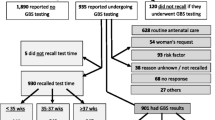
There were 1553 maternities and 1565 births (including 12 twin pregnancies) during the calendar year of 2022. 1472 (94.72%) had antenatal screening for GBS with 368 (23.70%) being screened positive. Amongst the 81 (5.21%) women who did not have GBS carrier screening, 29/1553 (1.87%) had a past history of GBS infection and were therefore managed and treated as carriers in their current pregnancy; 9/1553 (0.58%) were delivered preterm before carrier screening could be performed and 43/1553 (2.70%) did not have screening. Overall, the adherence rate to the GBS universal screening policy was 97.30% (1511/1553). Among the 1472 cases who had GBS screening, 86 cases had GBS detected before 35 weeks of gestation and 1 case had preterm delivery before 35 weeks of gestation. In the remaining 1385 cases, there were 1191 patients (85.99%) who had GBS screening performed between 35 and 37 weeks of gestation. The number of women who delivered within 5 weeks of the GBS screening was 1336. Therefore, only 49 (3.53%) of the 1385 women had a screening to delivery interval of greater than 5 weeks.
Clinical neonatal sepsis was diagnosed in 57 (3.64%) of the 1565 neonates. All the cases of neonatal sepsis were diagnosed as early onset disease (EOD) within 3 days of birth, with 55 (96.49%) cases being diagnosed within 48 h of birth. There were no confirmed cases of neonatal GBS infection from blood culture method. Table 1 summarizes maternal socio-demographics, obstetrics complications, group B streptococcus status, obstetric history, birth and neonatal condition at birth according to whether the neonate was diagnosed with neonatal sepsis or not.
Univariate analysis indicated that the risk of neonatal sepsis was 5.45 (95% CI 3.05 to 9.75) times higher in women who were GBS screened positive when compared to non-GBS carriers (p < 0.001). The risk is also greater in preterm delivery (p = 0.011). In further analysis, 9 cases of neonate from GBS carriers with preterm delivery were excluded.
Among 359 women (including 2 twin pregnancies) who were screened positive as GBS carriers and had term delivery, 122 had vaginal delivery and 237 had Caesarean section(CS). Table 2 showed the risk of neonatal sepsis according to the timing of intra-partum antibiotics given to GBS carriers during vaginal delivery at term. Intra-partum antibiotics were given in all 122 vaginal deliveries (all singleton pregnancies) and antibiotics were given ≥ 4 h before delivery in 88 women. The neonatal sepsis rate significantly decreased by 71% in this group of patients with a sepsis rate of 6/88 (6.81%) compared to those having antibiotics for less than 4 h before delivery 8/34 (23.52%) (p = 0.022). However, antenatal antibiotic treatment before vaginal delivery did not decrease the neonatal sepsis risk, as 8 out of 60 patients who had antenatal treatment had sepsis, compared to 6 out of 62 without antenatal treatment. (p = 0.580)
In the remaining 237 women (239 neonates) who had term CS, antenatal treatment with antibiotics was given to 99 (41.77%) women. Table 3 showed the risk of neonatal sepsis after CS of GBS carriers at term. Three out of 101 newborns (2.97%) were diagnosed with neonatal sepsis compared to 14/138 (10.14%) when their mother did not receive antenatal antibiotics treatment. There was a 70% decrease in neonatal sepsis and the difference was statistically significant (2.97% vs. 10.14%; p = 0.041).
Discussion
This study showed that the adherence rate of universal GBS screening was high (≈ 97%) in Hong Kong. Our data indicates that GBS screening acceptance amongst Hong Kong women has increased from the 81% reported in Chow et al. in 2013 [10]. A recent study performed in Greece showed the adherence rate was only 67% and they suggested that one of the possible explanations was the high CS rate [7]. However, in our study with a CS rate of 69%, the adherence rate of universal GBS screening was high. It is important as the American College of Obstetricians and Gynecologists (ACOG) has recommended that GBS screening should still be performed even in women with a planned CS, as a rupture of membranes could take place before planned birth [5].
The ACOG recommendation that universal GBS screening should be performed between 35 and 366/7 weeks of gestation [2] was revised to 36 to 376/7wks in 2020 in order to allow a five-week window for valid culture results to be reported and available, assuming that delivery would occur before 42 weeks of gestation [11]. However, In Hong Kong, there was no change in the guidelines and hence recommendations as to when GBS screening should be performed. In a real-world scenario, our data showed that under the present Hong Kong GBS screening policy, 3.55% of women did not have a culture result within 5 weeks of the GBS screening at time of delivery. Further study would be necessary to see whether changes to the timing of GBS screening in Hong Kong would improve it.
Our data and analysis indicated that the risk of neonatal sepsis was 3.6%. This is comparable to other published papers, which reported the risk of clinical neonatal sepsis ranging from 4.4 to 4.69% [12, 13]. 96.49% of the neonatal sepsis were diagnosed within 48 h. Our rate of neonatal sepsis within 48 h is consistent with 94.7% reported by Nanduri and colleagues in their multistate study [14]. There were no cases of late onset neonatal sepsis in our series. Late onset sepsis is known to be associated with the postnatal nosocomial or community environment, with the peak incidence reported to be between the 10th and 22nd day of life [15]. Within our local context, that would be after the baby had been discharged and under the care of their own paediatricians at other medical institution(s).
The risk for clinical neonatal sepsis was almost 6 times higher in those women who were screened positive compared to non-GBS carriers. Although no positive group B streptococcus or bacterial culture was identified, all these neonates were found to have clinical features of neonatal sepsis and required antibiotic treatment. Therefore, GBS carrier screening did identity a high-risk group for neonatal sepsis. It is important to increase the compliance of GBS screening.
At the same time, we found that there was a variation in practice that existed with respect to whether antenatal antibiotic treatment is prescribed or not. It is also interesting to note that antenatal treatment before caesarean section is associated with a lower risk of neonatal sepsis. Our study highlighted that despite current ACOG [5] and RCOG [3] guidelines not recommending antenatal antibiotic to eradicate bacteria, more than 40% of GBS carriers were still treated antenatally contrary to the current guidelines. The rationale for not giving antenatal antibiotic to GBS carriers in the current guidelines is that antenatal treatment does not reduce the likelihood of GBS colonization at the time of delivery. They usually quoted the couple study conducted by Gardner SE and colleagues [16]. This study was performed over 40 years ago in only 40 patients, which reported that 67% of women remained colonised with GBS despite receiving penicillin antenatally. A later study by Bland et al. [17] showed that the recolonised rate was only 25% with no case of ‘heavy’ growth amongst antenatal treated women who were GBS carriers. Recent studies have shown that the risk of neonatal sepsis was dose dependent instead of a dichotomous relationship [18]. A randomized control trial published in 2008 showed that antenatal amoxycillin reduced colonisation by 57% [19]. Even though all these studies showed that antenatal antibiotic treatment would decrease GBS colonisation rate, we believe one potential reason guidelines did not recommend the use of antenatal antibiotics was the worry of antibiotic resistance. Furthermore, all these studies claimed that the risk of neonatal sepsis was not affected by the antenatal treatment. However, with all these three studies combined, there were fewer than 150 participants and only 82 treated with antenatal antibiotic. Therefore, studies with a larger sample size are necessary to determine the effect of antenatal treatment on the rate of neonatal sepsis.
Our analysis, however, would suggest that antenatal treatment is useful in decreasing the neonatal sepsis risk in CS, but not in the vaginal delivery group where the timing of intrapartum antibiotic is more important. Amongst GBS carriers who delivered by CS at term, the rate of neonatal sepsis was decreased by 70% in those receiving antenatal treatment, suggesting a potential benefit. Our findings are especially important in countries that have very high neonatal sepsis rates, up to 16% [20].
The next question would be why antenatal antibiotics treatment can decrease the risk of neonatal sepsis. Epidemiologic data have indicated the potential of pre-labour invasion of the uterus by group B Streptococcus, and metagenomic analysis revealed the presence of group B Streptococcus in the placenta in approximately 5% of pregnant women at term before onset of labor and membrane rupture. However, the determinants and consequences of pre-labour invasion of the uterus by group B Streptococcus are yet to be established [21]. The question of whether any other bacteria were involved and whether antenatal antibiotics treatment may help in this way need further studies to be determined. Further and bigger studies are also needed to clarify whether antenatal antibiotic should be given or not and if so, which ones and for how long.
Intrapartum antibiotics have been shown to be useful in reducing neonatal GBS infection in both local and international studies. The introduction of universal GBS screening and intrapartum antibiotic treatment of GBS positive women in Hong Kong significantly reduced the incidence of early-onset neonatal sepsis (based on positive blood or cerebrospinal fluid) from 3.25 to 2.26 per 1000 livebirths [6]. Our analysis confirms and supports the earlier study by Turrentine and colleagues that intrapartum antibiotics should be administered for ≥ 4 h. Turrentine and colleagues [22] reported that women who received intrapartum antibiotics for ≥ 4 h had a 65% lower rate of neonatal sepsis which is consistent with the 58% we observed in our present study. We therefore recommend that patients who are GBS carriers go to the hospital earlier if there is any sign of labour, so antibiotics can be given as soon as possible.
Strengths and limitations
The strength of our study was that it showed the compliance rate of GBS screening in the setting where patients need to pay for the investigation. This study also included a larger number of GBS carriers receiving antenatal antibiotic treatment than the previous studies [16, 17, 19]. Also, the colonisation rate of GBS in our study was 23.7% which was compatible with both the 20% incidence reported in Hong Kong as well as internationally [6, 23]. Limitations of our study firstly included the retrospective nature of our study, which relied on the administrative database and electronic records to identify women and neonates who had neonatal sepsis. Whilst we documented whether antibiotics were given or not, we were unable to ascertain which one was given, the gestation at which it started, the duration for which it was given and whether the course was completed before delivery. Secondly, we determined the presence or absence of neonatal sepsis based on clinical findings alone, which could have to led to overdiagnosis. The alternative to clinical diagnosis of neonatal sepsis would be the isolation of pathogens from neonatal blood or cerebrospinal fluid which is considered as a gold standard for the diagnosis of neonatal sepsis. However, culture is also unreliable due to low sample volume, low bacterial density, culture contamination, or suppression of bacterial growth after antibiotic administration [24]. The diagnosis of neonatal sepsis was based on clinical signs but symptoms can be vague, nonspecific and subjected to individual paediatrician interpretation. Thirdly, the number of maternities assessed in this study was relatively small, so additonal larger studies would need to be conducted to confirm our real-world observation. Lastly, ≈ 70% of women were delivered by CS, the vast majority of which were elective CS performed at the request of the mother. Hong Kong mothers seeking delivery by CS are more likely to be delivered in a private as opposed to a public medical institution and therefore have greater ability to pay which may potentially limit the generalisability of our findings.
Conclusion
In conclusion, our study demonstrated that neonates delivered from women who screened positive for GBS are at high risk of neonatal sepsis. Giving antenatal antibiotic treatment before Caesarean section or intrapartum antibiotic prophylaxis for 4 or more hours before vaginal delivery may decrease the risk of neonatal sepsis in term neonates delivered from GBS carriers.
Data availability
The data is available from the corresponding author upon reasonable request.
References
Lawn JE, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, et al. Group B streptococcal disease worldwide for pregnant women, stillbirths, and children: why, what, and how to undertake estimates? Clin Infect Dis. 2017;65(suppl 2):S89–99.
Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1–22.
Hughes RG, Brocklehurst P, Steer PJ, Heath P, Stenson BM. On behalf of the Royal College of Obstetricians and gynaecologists. Prevention of early-onset neonatal group B streptococcal disease. Green-top Guideline 36 BJOG. 2017;124:e280–305.
Department of Health and the Hospital Authority. Prevention of Neonatal Group B Streptococcus Infection. https://www.fhs.gov.hk/english/health_info/woman/478.pdf; Accessed on 8th July 2024.
Prevention of Group B Streptococcal Early-Onset Disease in Newborns: ACOG Committee Opinion, Number 797 Obstet Gynecol. 2020;135(2):e51-e72.
Chan YTV, Lau SYF, Hui SYA, Ma T, Kong CW, Kwong LT, et al. Incidence of neonatal sepsis after universal antenatal culture-based screening of group B streptococcus and intrapartum antibiotics: a multicentre retrospective cohort study. BJOG. 2023;130(1):24–31.
Berikopoulou MM, Pana A, Liakopoulou-Tsitsipi T, Vlahos NF, Papaevangelou V, Soldatou A. Poor adherence to the screening-based strategy of group B streptococcus despite colonization of pregnant women in Greece. Pathogens. 2021;10(4):418.
Kunze M, Zumstein K, Markfeld-Erol F, Elling R, Lander F, Prömpeler H, et al. Comparison of pre- and intrapartum screening of group B Streptococci and adherence to screening guidelines: a cohort study. Eur J Pediatr. 2015;174:827–35.
Santillan DA, Hubb AJ, Nishimura TE, Rosenfeld-O’Tool SR, Schroeder KJ, Conklin JM, et al. Group B Streptococcus screening and treatment adherence in pregnancy: a Retrospective Cohort Study and opportunities for Improvement. AJPM Focus. 2022;28(2):100028.
Chow TY, To WKW, Chan LWD. Knowledge and attitudes of Hong Kong pregnant women on Group B Streptococcus Screening. HKJGOM. 2013;13:45–51.
Committee on Obstetric Practice. Prevention of early-onset group B streptococcal disease in newborns. Obstet Gynecol. 2019;134:e19–40.
Ma MKT, Leung KY. Timing of elective caesarean section at term on neonatal morbidities. Hong Kong J Gynaecol Obstet Midwifery. 2023;23(2):101–5.
Ortiz de Zárate M, Sáenz C, Cimbaro Canella R, Díaz M, Mucci J, Dinerstein A, et al. Prevalence of microbiologically confirmed neonatal sepsis at a maternity center in the City of Buenos Aires. Arch Argent Pediatr. 2023;121(3):e202202779.
Nanduri SA, Petit S, Smelser C, Apostol M, Alden NB, Harrison LH, et al. Epidemiology of Invasive Early-Onset and late-Onset Group B Streptococcal Disease in the United States, 2006 to 2015: Multistate Laboratory and Population-based surveillance. JAMA Pediatr. 2019;1(3):224–33.
Dong Y, Speer CP. Late-onset neonatal sepsis: recent developments. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F257–63.
Gardner SE, Yow MD, Leeds LJ, Thompson PK, Mason EO Jr, Clark DJ. Failure of penicillin to eradicate group B streptococcal colonization in the pregnant woman. A couple study. Am J ObstetGynecol. 1979;135:1062–5.
Bland ML, Vermillion ST, Soper DE. Late third-trimester treatment of rectovaginal group B Streptococci with benzathine penicillin G. Am J Obstet Gynecol. 2000;183(2):372–6.
Ann L, Jefferies. Management of term infants at increased risk for early onset bacterial sepsis. Paediatr Child Health. 2017, 223–8.
Baecher L, Grobman W. Prenatal antibiotic treatment does not decrease group B streptococcus colonization at delivery. Int J Gynaecol Obstet. 2008;101:125–8.
Milton R, Gillespie D, Dyer C, Taiyari K, Carvalho MJ, Thomson K, et al. Neonatal sepsis and mortality in low-income and middle-income countries from a facility-based birth cohort: an international multisite prospective observational study. Lancet Glob Health. 2022;10:e661–72.
Katie S, Charnock-Jones DS, Gordon CS. Smith. Group B Streptococcus and the risk of perinatal morbidity and mortality following term labor. Am J Obstet Gynecol. 2023;228(5):S1305–12.
Turrentine MA, Greisinger AJ, Brown KS, Wehmanen OA, Mouzoon ME. Duration of intrapartum antibiotics for group B streptococcus on the diagnosis of clinical neonatal sepsis. Infect Dis Obstet Gynecol. 2013; 525878.
Gopal Rao G, Hiles S, Bassett P, Lamagni T. Differential rates of group B streptococcus (GBS) colonisation in pregnant women in a racially diverse area of London, UK: a cross-sectional study. BJOG. 2019;126(11):1347–53.
Chiesa C, Panero A, Osborn JF, Simonetti AF, Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin Chem. 2004;50:279–87.
Acknowledgements
Thanks to Mei Yuk TO, Cherry Sze Wan TANG, Veii Mei Chun LEUNG and the midwives of Hong Kong Baptist Hospital for data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Author contributionsConcept or design: TY FungAcquisition of data: TY Fung.Analysis or interpretation of data: TY Fung, DS Sahota, Drafting of the manuscript: TY Fung and DS SahotaCritical revision of the manuscript for important intellectual content: TY Fung, DS SahotaAll authors had full access to the data, contributed to the study, approved the final version for publication, and take responsibility for its accuracy and integrity.
Corresponding author
Ethics declarations
Conflict of interest
All authors have disclosed no conflicts of interests.
Ethics approval and consent to participate
The study was approved by the Clinical and Research Ethics Committee of Hong Kong Baptist Hospital (CREC- 2024-01). Since it was a retrospective review of the records in the hospital, the Clinical and Research Ethics Committee of Hong Kong Baptist Hospital waived the need for consent to participate. The records and information of all patients in the study were anonymised and de-identified prior to analysis.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fung, T.Y., Sahota, D.S. How can we reduce neonatal sepsis after universal group B streptococcus screening?. BMC Pregnancy Childbirth 24, 586 (2024). https://doi.org/10.1186/s12884-024-06791-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-024-06791-7