Abstract
Background
Visceral adiposity index (VAI) and a body shape index (ABSI) were newly developed indices for visceral fat mass. Whether they are superior to conventional obesity indices in predicting colorectal cancer (CRC) remains unclear. We examined the associations of VAI and ABSI with CRC risk, and investigated their performance in discriminating CRC risk compared with conventional obesity indices in the Guangzhou Biobank Cohort Study.
Methods
A total of 28,359 participants aged 50 + years without cancer history at baseline (2003-8) were included. CRC were identified from the Guangzhou Cancer Registry. Cox proportional hazards regression was used to assess the association of obesity indices with the CRC risk. Discriminative abilities of obesity indices were assessed using Harrell’s C-statistic.
Results
During an average follow-up of 13.9 (standard deviation = 3.6) years, 630 incident CRC cases were recorded. After adjusting for potential confounders, the hazard ratio (95% confidence interval) of incident CRC for per standard deviation increment in VAI, ABSI, body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) was 1.04 (0.96, 1.12), 1.13 (1.04, 1.22), 1.08 (1.00, 1.17), 1.15 (1.06, 1.24), 1.16 (1.08, 1.25)and 1.13 (1.04, 1.22), respectively. Similar results for colon cancer were found. However, the associations of obesity indices with risk of rectal cancer were non-significant. All obesity indices showed similar discriminative abilities (C-statistics from 0.640 to 0.645), with WHR showing the highest whilst VAI and BMI the lowest.
Conclusions
ABSI, but not VAI, was positively associated with a higher risk of CRC. However, ABSI was not superior to the conventional abdominal obesity indices in predicting CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common cancer and the second leading cause of cancer death worldwide, with an estimated 1.9 million new cases and 935,000 death in 2020[1]. The incidence of CRC has been increasing over the last three decades, especially in low-and middle-income [2], which can be partly attributable to changes in lifestyles and some modifiable risk factors, further highlighting the importance of primary prevention.
Obesity is an established risk factor of CRC [3, 4]. Body mass index (BMI) and waist circumference (WC) are two most widely used conventional measures for obesity. However, BMI cannot indicate fat [5] while WC cannot distinguish between subcutaneous and visceral fat [6]. As it is suggested that visceral adipose tissue was more strongly associated with colorectal adenomas than subcutaneous adipose tissue [7], increasing research efforts have been made on identifying alternative obesity indices that have stronger predictive value for visceral fat mass.
Some alternative indices of visceral obesity have been introduced [8,9,10,11,12,13,14]. Of them, visceral adiposity index (VAI) [8] and a body shape index (ABSI) [9] were most commonly used in epidemiologic studies to examine the associations with all-cause mortality, cardiovascular disease or diabetes [15,16,17]. As both VAI and ABSI account for WC, height and weight simultaneously, they were suggested to have better discrimination of visceral adipose mass and distribution [8, 9]. Moreover, studies based on magnetic resonance imaging indicated that VAI and ABSI had significant correlation with visceral adipose [8, 18]. A recent study in Japan showed a positive association between VAI and the risk of CRC [19]. Furthermore, higher ABSI was also associated with a higher risk of CRC [20,21,22]. High ABSI corresponds to a greater level of visceral adipose tissue at a given height and weight, which may secrete more proinflammatory adipokines and is more heavily infiltrated with immune cells [23, 24], contributing to colon cancer development [25]. Meanwhile, the immune cells activated by adipose tissue can also promote insulin resistance and subsequent hyperinsulinemia [26], which may increase CRC risk by inducing tumor cell proliferation and decreasing apoptosis [27, 28]. However, no consensus was made in the predicting ability of VAI or ABSI with comparison to conventional obesity indices. A pooled collaborative analysis of 11 Australian cohorts reported similar predictive ability of CRC between ABSI and other obesity indices in both men and women [29] whereas another recent study from Sweden found that although ABSI had a weaker association with colon cancer than WC in both sexes, ABSI but not WC predicted the risk of rectal cancer significantly in men [30]. The discrepancies between the studies highlight the need for further research to better understand the discriminatory ability for each index, specifically among older people with a higher risk of CRC.
Therefore, we examined the associations of VAI and ABSI with CRC risk in middle-aged to older Chinese using data from the Guangzhou Biobank Cohort Study, and compared their performance in predicting CRC risk with conventional obesity indices, including body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR).
Methods
Study participants
Guangzhou Biobank Cohort Study (GBCS) is a 3-way collaboration among Guangzhou Twelfth People’s Hospital and the Universities of Hong Kong and Birmingham. Details of GBCS have been reported elsewhere [31]. In brief, all participants were recruited during 2003 to 2008 from the Guangzhou Health and Happiness Association for the Respectable Elders (GHHARE), which was a community social and welfare organization unofficially aligned with the municipal government. GHHARE included about 7% of Guangzhou residents in this age group, with branches in all districts of Guangzhou. Membership was open to Guangzhou residents aged 50 years or above for a minimal nominal fee of ¥4 (about 50 US cents) per month. The baseline examination included a face-to-face computer-assisted interview by trained nurses on demographic characteristics, lifestyle factors, family and personal medical history and assessment of anthropometric parameters, blood pressure, fasting plasma glucose and lipids. Reliability and validity of the questionnaire were tested 6 months into recruitment by recalling 200 randomly selected participants for re-interview [31]. Ethics approval was granted by the Guangzhou Medical Ethics Committee of the Chinese Medical Association and all participants provided written, informed consent before participation.
Obesity indices
Anthropometric measurements were performed by trained nurses in the morning before breakfast following standard protocols, including weight, standing height, WC and hip circumference (HC) with light indoor clothing and without shoes. BMI was calculated as weight in kilogram divided by height in meter squared (kg/m2). WC was measured horizontally around the smallest circumference between the ribs and iliac crest, or at the navel, if no natural waistline was present. HC was measured around the maximal girth of the hips. WHR was calculated by dividing WC (cm) by HC (cm), and WHtR was calculated by dividing WC (cm) by height (cm). ABSI was derived using the formula (a), with WC and height expressed in meter [9], as follows:
ABSI = WC/(BMI2/3 × height1/2) (a).
VAI in men and women was calculated using the following formula (c) and (d), respectively, where WC were expressed in cm, and triglycerides (TG) and high-density lipoprotein cholesterol (HDL-c) in mmol/L [8]:
VAI = (WC/(39.68 + 1.88 × BMI)) × (TG/1.03) × (1.31/HDL-c) (c).
VAI = (WC/(36.58 + 1.89 × BMI)) × (TG/0.81) × (1.52/HDL-c) (d).
The Chinese-specific cut-offs for general obesity, as recommended by the World Health Organization (WHO), were used, with underweight defined as BMI lower than 18.5 kg/m2, normal weight 18.5–24.9 kg/m2, overweight 25.0–27.4 kg/m2 and general obesity ≥ 27.5 kg/m2 [32]. Higher WC was defined according to Asian-specific cut-offs suggested by WHO with ≥ 90 cm for men and ≥ 80 cm for women [33]. Higher WHR and WHtR were defined by established cut-offs, i.e., men ≥ 0.9 and women ≥ 0.8 for WHR [34] and ≥ 0.5 for WHtR [35]. As no established cut-offs for VAI and ABSI, they were categorized into tertiles.
Outcomes
The study outcome was incident CRC (Tenth Revision of the International Classification of Diseases (ICD-10) C18-C20). Information on first diagnosis of CRC up to April 2021 was obtained from the Guangzhou Cancer Registry (GCR) of the Guangzhou Center for Disease Control and Prevention (GZCDC).
Potential confounders
As age [36], sex [37], lifestyle factors (physical activity [38], smoking [39, 40], alcohol drinking [41, 42], consumption of vegetable, fruits and red meat [43]) were associated with both obesity and CRC, these factors were considered as potential confounders and adjusted in the Cox regression models. Furthermore, education and household annual income were also included to partly account for confounding due to socioeconomic position [44]. Physical activity was categorized into inactive, moderate and active based on the short version of the International Physical Activity Questionnaire (IPAQ), which has been validated previously [45]. Consumption of vegetable, fruits and red meat intake were assessed using a validated food frequency questionnaire (FFQ), which covered the average frequency over the last seven days and amount of consumption of each item [46]. Vegetable, fruits and red meat intake was calculated by multiplying the frequency by the amount of intake each time.
Potential mediators
As chronic inflammation may involve in the pathway between obesity and development of CRC [47], we also assessed the potential mediating effects due to white blood cell count (WBC), a reliable biomarker for inflammation [48, 49]. WBC were assayed in an automated hematology analyzer (KX-21, SYSMEX, Japan) [31].
Statistical analysis
Differences of distribution in demographic and other characteristics by incident CRC were examined using t-test or Wilcoxon test for continuous variables and chi-square test for categorical variables. The correlations among ABSI, VAI and conventional obesity indices were evaluated using Pearson correlation coefficients (Pearson’s r) by sex. To enable comparisons between different obesity indices with different units, all indices were standardized using z-scores, calculated by the following equation (e). Sex-specific z-scores was used for WC and WHR.
z-score = (actual value-mean)/standard deviation (SD) (e).
Cox proportional hazard regression was used to estimate crude and adjusted hazard ratio (HR) and 95% confidence intervals (CI) for CRC risk from different obesity indices. The proportional hazard assumption was tested by the Schoenfeld residual method. No evidence for the violation of the assumption was found. Furthermore, the potential nonlinear relationships between obesity indices and risk of CRC were estimated by using restricted cubic splines with three knots (10th, 50th, 90th percentiles) based on Cox regression. Potential interactions between obesity indices and sex or selected lifestyle factors including smoking, alcohol drinking and physical activity were tested by comparing model fit with and without the interaction terms using likelihood ratio test. To investigate the potential mediation of inflammation in the association between obesity and CRC, WBC were further adjusted in Cox regression model. The discriminative abilities of the obesity indices for CRC were assessed using Harrell’s C-statistic. Sensitivity analyses were conducted by excluding participants with less than 5 years of follow-up and restricting analyses to never-smokers to limit possible reverse causality and residual confounding. All analyses were performed using R software (version 4.1.3) and all tests were two-sided with a significant level of P < 0.05.
Results
At baseline, a total of 30,430 participants were recruited. After excluding those with self-reported cancer at baseline (n = 585), missing information on anthropometric measures (n = 301) or other covariates (n = 733) and loss to follow-up for vital status (n = 452), 28,359 (72.3% women) were included in the current analyses. The mean age was 62.0 (SD = 7.1) years. During an average follow-up of 13.9 (SD = 3.6) years, 630 (61.3% women) incident CRC were recorded.
Table 1 shows that participants diagnosed with CRC were older, had higher proportion of men and had higher levels of WC, WHR, WHtR and ABSI (all P < 0.05). No significant association was found between other characteristics (education, household annual income, alcohol drinking, smoking, physical activity, vegetable intake, fruit intake, red meat intake, WBC, TG, HDL-c, BMI and VAI) and incident CRC (P > 0.05). ABSI was weakly correlated with WHtR and VAI (Pearson’s r from 0.06 to 0.16), but moderately correlated with BMI, WHR and WC (Pearson’s r from 0.54 to 0.71) (Supplementary Table 1). VAI had weak correlations with conventional obesity indices (Pearson’s r from 0.17 to 0.27) (Supplementary Table 1). Table 2 shows that, after adjustment for age, sex, smoking, alcohol drinking, household annual income, education, physical activity, intake of vegetable, fruits and red meat, per SD increment in BMI, WC, WHR, WHtR and ABSI was significantly associated with higher CRC risk, with HR (95% CI) being 1.08 (1.00, 1.17), 1.15 (1.06, 1.24), 1.16 (1.08, 1.25), 1.13 (1.04, 1.22) and 1.13 (1.04, 1.22), respectively. No significant association was found between per SD increment in VAI and CRC risk. The results were similar for colon cancer, with HR (95% CI) for per SD increment in BMI, WC, WHR, WHtR, VAI and ABSI being 1.11 (1.01, 1.21), 1.18 (1.07, 1.29), 1.21 (1.10, 1.33), 1.16 (1.06, 1.28), 1.06 (0.97, 1.15) and 1.15 (1.04, 1.26), respectively (Table 3). However, non-significant associations of obesity indices with the development of rectal cancer were found, with adjusted HR for per SD increment in BMI, WC, WHR, WHtR, VAI and ABSI being 1.04 (0.90, 1.19), 1.09 (0.95, 1.25), 1.07 (0.93, 1.23), 1.06 (0.92, 1.23), 0.98 (0.85, 1.15) and 1.08 (0.94, 1.25), respectively (Table 3). Similar results were found for risk of CRC and colon cancer when obesity indices were analyzed as categorical variables (Tables 2 and 3). Sensitivity analyses by excluding participants with smoking history (i.e., former and current smokers) and less than 5 years of follow-up yielded similar results (Supplementary Tables 2 to 5).
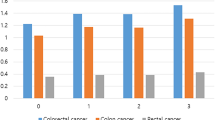
Table 4 shows the Harrell’s C-statistic of predicting CRC for all obesity indices ranging from 0.640 to 0.645. No significant difference in the C-statistic for CRC, colon and rectal cancers, respectively, across different obesity indices was found (P > 0.05). Results from the restricted cubic spline regression did not support the non-linearity assumptions between obesity indices and risk of CRC (all P for non-linearity > 0.05, Fig. 1). No significant interaction between obesity and potential moderators (sex, smoking, alcohol drinking and physical activity) was found (P for interaction from 0.15 to 0.89). The combination of general obesity (BMI) and each of the other obesity indices did not improve the predictive performance compared with each of indices alone (Supplementary Tables 6 to 8). The associations between obesity indices and CRC attenuated slightly after adjusting for WBC in the Cox regression models (Supplementary Table 9).
Association of baseline adiposity indices (2003-8) with risk of colorectal cancer on 28,359 participants
Note: Potential nonlinear relationships were examined using restricted cubic splines (three knots on 10th, 50th and 90th ), with hazard ratio (HRs) from Cox proportional hazard models. The HRs was adjusted for age, sex, smoking, alcohol drinking, household annual income, education, physical activity, intake of vegetable, fruits and red meat
Discussion
In this large population-based prospective cohort study, we found that ABSI, but not VAI were associated with increased risk of total CRC and colon cancer. The predictive ability of VAI or ABSI was not superior to the conventional visceral adiposity indices (i.e., WC, WHR and WHtR). To our knowledge, our study is the first to systematically compare the ability of VAI/ABSI and conventional obesity indices in predicting CRC risk. As ABSI and the other indices related to central obesity appeared to provide similar prediction for CRC risk, simple measures such as WC or WHR are recommended.
Obesity is one of the important modifiable risk factors for CRC [50]. The gold standard measurement of abdominal obesity was by computerized tomography (CT) or magnetic resonance imaging (MRI). However, they were costly, time consuming and not practical for large epidemiologic studies [51]. Therefore, some simple clinical anthropometric indices were proposed as alternative ways for measuring visceral fat mass, such as VAI and ABSI. We found three studies reported significant positive associations of VAI and ABSI with CRC risk in Japan [19] and United Kingdom [20, 21], respectively. The association of VAI with CRC risk in our study appeared to be lower to that from the Japanese cohort (NAGALA study), i.e., participants with the highest tertile of VAI scores had 25% higher risk of CRC in our study versus 78% in the NAGALA study [19]. The discrepancy could be due to different reference groups used and various follow-up duration. Notably, as the NAGALA study included 27,921 middle-aged adults (mean age = 45.7 years) with relatively short follow-up duration (4.4 years), the number of incident CRC events was relatively small [19]. Our study found that per SD increment in ABSI was associated with 13% greater risk of CRC, which was generally consistent with previous studies. Two studies using data from the UK Biobank with followed-up of 7 years reported that per SD higher ABSI was associated with a higher risk of CRC by 16% in men, 7% in women [20] and 9% in total [21].
Several possible mechanisms have been suggested for the adverse effects of adiposity on CRC. First, adiposity tissue, especially visceral adipose can increase concentrations of serum insulin and insulin-like growth factor- I (IGF-I), and lead to leptin and adiponectin secretion, which play important roles in the pathogenesis of cancer [52]. In addition, inflammatory cytokine released by adiposity tissue and oxidative stress triggered by excess fat might involve in CRC tumorigenesis [47, 53]. However, the adjustment for inflammatory marker (i.e., WBC) in our study did not attenuate the association between obesity and incident CRC, suggesting that other pathogenic pathways, apart from WBC, might play a role. Future studies are warranted to confirm these results and elucidate the underlying mechanisms.
Previous studies comparing the predictive ability between BMI and abdominal obesity indices including WC and WHR in terms of their prediction for CRC risk showed that WC and WHR were more strongly associated with risk of CRC [54, 55]. However, there was no consensus on whether VAI/ABSI had better discrimination for predicting risk of CRC than conventional obesity indices. Our results showed that VAI was not superior to WC, WHR or WHtR in predicting CRC risk. A study pooling 11 Australian cohorts showed that ABSI had moderate discriminative ability for CRC with C-statistic values between 0.60 and 0.71 in both men and women after adjusting for age, education and smoking [29]. In a Swedish cohort study, ABSI showed a weaker association with colon cancer than WC in men and women [30]. However, ABSI but not WC predicted the risk of rectal cancer significantly in men [30]. None of the selected obesity indices was associated with CRC risk in women in this Swedish study [30]. Another study of postmenopausal women showed that the HR of CRC for ABSI appeared to be lower than those of WC, WHR and WHtR [22], although direct comparison of the HRs might be problematic because of different unites of the indices used for analysis. Results from previous studies as well as ours consistently showed that ABSI was not superior to other obesity indices in predicting CRC. Moreover, although a previous study showed that cancer risk prediction was improved by using both ABSI and BMI simultaneously [21], our results did not support the combination of ABSI and BMI in improving CRC prediction. As most of the new obesity indices such as VAI and ABSI were generated based on populations with different ethical background (i.e., VAI was derived from Caucasian [8] and ABSI from the US populations [9]), direct application of these indices to other populations might be limited. Note that different ethnic populations have different patterns of fat distribution, e.g., Asians were at greater risk of abdominal obesity and had low muscle mass than Caucasians [56, 57]. Whether the diverse fat distribution has differential effects on CRC risk remains to be explored in large scale prospective cohort studies with diverse ethnic populations.
The strengths of this study included a large sample size with long-term follow-up, the employment of standardized anthropometric measurements by well-trained nurses, which minimized the potential bias from self-reported value, and the comprehensive adjustment for a wide range of confounding factors. There were also some limitations that should be mentioned. First, as adiposity indices were assessed at a one-time point, they might have changed during follow-up. Further studies using repeated measurements of obesity indices might allow for more in-depth analysis. Second, we did not have the information of family CRC history [58], although we found no evidence that family CRC history plays a role in adiposity or fat distribution. Finally, our study was observational and could not confirm causal relation between obesity indices and CRC risk. However, interventional studies examining the effect of weight reduction on CRC risk require large sample size and long-term follow-up, and are unlikely to have good compliance, which limit the feasibility.
In conclusion, higher levels of BMI, WC, WHR, WHtR, and ABSI were associated with higher risk of CRC, and the associations were stronger for WC, WHR, WHtR and ABSI. Although clinical perspective focusing on abdominal obesity is appealing, VAI nor ABSI was unlikely an appropriate substitute for conventional adiposity indices in predicting CRC.
Data Availability
Due to ethical restrictions protecting patient privacy, exposure data available on request from the Guangzhou Biobank Cohort Study Data Access Committee. Please contact us at gbcsdata@hku.hk for fielding data accession requests.
References
Sung H, Ferlay J, Siegel RL. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. 2021. 71(3): p. 209–249.
Collaborators GCC. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019.Lancet Gastroenterol Hepatol, 2022.
Ma Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE. 2013;8(1):e53916.
Dong Y et al. Abdominal obesity and colorectal cancer risk: systematic review and meta-analysis of prospective studies.Biosci Rep, 2017. 37(6).
Kruschitz R, et al. Detecting body fat-A weighty problem BMI versus subcutaneous fat patterns in athletes and non-athletes. PLoS ONE. 2013;8(8):e72002.
Shuster A, et al. The clinical importance of visceral adiposity: a critical review of methods for visceral adipose tissue analysis. Br J Radiol. 2012;85(1009):1–10.
Keum N, et al. Visceral adiposity and colorectal adenomas: dose-response meta-analysis of observational studies. Ann Oncol. 2015;26(6):1101–9.
Amato MC, et al. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–2.
Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS ONE. 2012;7(7):e39504.
Xia MF, et al. A indicator of visceral adipose dysfunction to evaluate metabolic health in adult chinese. Sci Rep. 2016;6:38214.
Thomas DM, et al. Relationships between body roundness with body fat and visceral adipose tissue emerging from a new geometrical model. Obes (Silver Spring). 2013;21(11):2264–71.
Consalvo V, et al. ABSI (a body shape index) and ARI (Anthropometric Risk Indicator) in bariatric surgery. First application on a bariatric cohort and possible clinical use. Obes Surg. 2018;28(7):1966–73.
Guerrero-Romero F, Rodríguez-Morán M. Abdominal volume index. An anthropometry-based index for estimation of obesity is strongly related to impaired glucose tolerance and type 2 diabetes mellitus. Arch Med Res. 2003;34(5):428–32.
Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44(9):955–6.
Ji M, Zhang S, An R. Effectiveness of a body shape index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19(5):737–59.
Leite NN, et al. Visceral adiposity index is positively associated with blood pressure: a systematic review. Obes Res Clin Pract. 2021;15(6):546–56.
Wang D, et al. Association between visceral adiposity index and risk of prediabetes: a meta-analysis of observational studies. J Diabetes Investig. 2022;13(3):543–51.
Anoop S et al. A Body shape index significantly predicts MRI-defined abdominal adipose tissue depots in non-obese Asian Indians with type 2 diabetes mellitus. 2020. 8(1).
Okamura T, Hashimoto Y, Hamaguchi M. Visceral Adiposity Index is a predictor of incident colorectal cancer: a population-based longitudinal study. 2020. 7(1).
Christakoudi S, Tsilidis KK. A Body Shape Index (ABSI), hip index, and risk of cancer in the UK Biobank cohort. 2021. 10(16): p.5614–5628.
Parra-Soto S, Malcomson FC. Associations of A Body Shape Index (ABSI) with Cancer Incidence, All-Cause, and at 23 Sites-Findings from the UK Biobank Prospective Cohort Study. 2022. 31(2): p.315–324.
Kabat GC, et al. Risk of breast, endometrial, colorectal, and renal cancers in postmenopausal women in association with a body shape index and other anthropometric measures. Cancer Causes Control. 2015;26(2):219–29.
Samaras K, et al. Subcutaneous and visceral adipose tissue gene expression of serum adipokines that predict type 2 diabetes. Obes (Silver Spring). 2010;18(5):884–9.
Bruun JM, et al. Monocyte chemoattractant protein-1 release is higher in visceral than subcutaneous human adipose tissue (AT): implication of macrophages resident in the AT. J Clin Endocrinol Metab. 2005;90(4):2282–9.
Meira LB, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice. J Clin Invest. 2008;118(7):2516–25.
Wensveen FM et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. 2015. 16(4): p.376–85.
Tran TT, et al. Hyperinsulinemia, but not other factors associated with insulin resistance, acutely enhances colorectal epithelial proliferation in vivo. Endocrinology. 2006;147(4):1830–7.
Wu X, et al. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95(4):1897–905.
Harding JL, et al. Comparison of anthropometric measures as predictors of cancer incidence: a pooled collaborative analysis of 11 Australian cohorts. Int J Cancer. 2015;137(7):1699–708.
Andreasson A et al. The prediction of colorectal cancer using anthropometric measures: A Swedish population-based cohort study with 22 years of follow-up. 2019. 7(9): p.1250–1260.
Jiang C, et al. Cohort profile: the Guangzhou Biobank Cohort Study, a Guangzhou-Hong Kong-Birmingham collaboration. Int J Epidemiol. 2006;35(4):844–52.
Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63.
Alberti, K.G., et al., Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation, 2009. 120(16):1640-5.
Huxley R, et al. Ethnic comparisons of the cross-sectional relationships between measures of body size with diabetes and hypertension. Obes Rev. 2008;9(Suppl 1):53–61.
Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0·5 could be a suitable global boundary value. Nutr Res Rev. 2010;23(2):247–69.
Sanford NN, et al. Obesity and younger versus older onset colorectal cancer in the United States, 1998–2017. J Gastrointest Oncol. 2020;11(1):121–6.
Kim H, Giovannucci EL. Sex differences in the association of obesity and colorectal cancer risk. Cancer Causes Control. 2017;28(1):1–4.
Morris JS, et al. Physical activity, sedentary behaviour and colorectal cancer risk in the UK Biobank. Br J Cancer. 2018;118(6):920–9.
Kim JH, et al. Cigarette smoking increases abdominal and visceral obesity but not overall fatness: an observational study. PLoS ONE. 2012;7(9):e45815.
Botteri E, et al. Smoking and colorectal Cancer risk, overall and by Molecular Subtypes: a Meta-analysis. Am J Gastroenterol. 2020;115(12):1940–9.
Sayon-Orea C, Martinez-Gonzalez MA, Bes-Rastrollo M. Alcohol consumption and body weight: a systematic review. Nutr Rev. 2011;69(8):419–31.
McNabb S et al. Meta-analysis of 16 studies of the association of alcohol with colorectal cancer. 2020. 146(3): p.861–873.
Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60e16.
Manser CN, Bauerfeind P. Impact of socioeconomic status on incidence, mortality, and survival of colorectal cancer patients: a systematic review. Gastrointest Endosc. 2014;80(1):42–60e9.
Deng HB, et al. Reliability and validity of the IPAQ-Chinese: the Guangzhou Biobank Cohort study. Med Sci Sports Exerc. 2008;40(2):303–7.
J., et al., A food frequency questionnaire for use in the Chinese population in Hong Kong: Description and examination of validity.Nutrition Research, 1997. 17(11–12): p.1633–1641.
Ye P et al. Linking Obesity with Colorectal Cancer: Epidemiology and Mechanistic Insights.Cancers (Basel), 2020. 12(6).
Lee YJ, et al. White blood cell count and the risk of colon cancer. Yonsei Med J. 2006;47(5):646–56.
Virdee PS et al. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal.Cancers (Basel), 2020. 12(9).
Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62(6):933–47.
Kuriyan R. Body composition techniques. Indian J Med Res. 2018;148(5):648–58.
Donohoe CL, Doyle SL, Reynolds JV. Visceral adiposity, insulin resistance and cancer risk. Diabetol Metab Syndr. 2011;3:12.
Martinez-Useros J, Garcia-Foncillas J. Obesity and colorectal cancer: molecular features of adipose tissue. J Transl Med. 2016;14:21.
Pischon T, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98(13):920–31.
Freisling H, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116(11):1486–97.
Lear SA, et al. The use of BMI and waist circumference as surrogates of body fat differs by ethnicity. Obes (Silver Spring). 2007;15(11):2817–24.
Lim U, et al. Asian women have greater abdominal and visceral adiposity than Caucasian women with similar body mass index. Nutr Diabetes. 2011;1(5):e6.
Schoen RE, et al. Incidence and mortality of colorectal cancer in individuals with a family history of colorectal cancer. Gastroenterology. 2015;149(6):1438–1445e1.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of China (81941019). The Guangzhou Biobank Cohort Study was funded by The University of Hong Kong Foundation for Educational Development and Research (SN/1f/HKUF-DC; C20400.28505200), the Health Medical Research Fund (Grant number: HMRF/13143241) in Hong Kong; Guangzhou Public Health Bureau (201102A211004011) Natural Science Foundation of Guangdong (2018A030313140), and the University of Birmingham, UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
The study was approved by the Guangzhou Medical Ethics Committee of the Chinese Medical Association. All participants provided written informed consent before participation. All methods in this study were performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Contributors
SYW, LX, CQJ, WSZ, TZ, YLJ, and ZF have substantial contributions to conception and design, acquisition of funding, data and interpretation of data; SYW analyzed the data, SYW, LX and WSZ drafted the article, LX and WSZ revised it critically for important intellectual content, and all authors contributed to final approval of the paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, S.Y., Zhang, W.S., Jiang, C.Q. et al. Association of novel and conventional obesity indices with colorectal cancer risk in older Chinese: a 14-year follow-up of the Guangzhou Biobank Cohort Study. BMC Cancer 23, 286 (2023). https://doi.org/10.1186/s12885-023-10762-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-023-10762-0