Abstract
Background
Renal cell carcinoma (RCC), one of the most fatal urologic tumors, accounts for approximately 3% of all adult cancers and exhibits a high metastatic index at diagnosis and a high rate of relapse. Radical or partial nephrectomy is a curative option for nonmetastatic RCCs. Targeted therapy has been shown to improve the survival of patients with metastatic RCCs. However, the underlying cellular and molecular events associated with RCC pathogenesis are not well known.
Methods
To investigate the clinical role of the transcription factor activator protein (AP)-2α in RCC, methylated CpG island recovery assays and microarray analysis were employed. COBRA and RT‒qPCR assays were performed to assess AP-2α expression in RCC.
Results
A negative correlation was noted between AP-2α mRNA expression levels and methylation status. Multivariate analyses showed that AP-2α mRNA was a major risk factor not only for overall and disease-free survival in RCC but also for disease-free survival in clear cell RCC.
Conclusions
Our results indicated that AP-2α expression was deregulated in RCC and associated with overall patient survival and disease-free survival. Such findings suggest that AP-2α might play an important role in the pathogenesis of RCC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Renal cell carcinoma (RCC) is the most common kidney cancer that occurs in adulthood [1, 2]. RCC is classified into different subtypes based on morphology and histology, including clear cell, papillary cell carcinoma, chromophobe, oncocytoma, collecting duct, and unclassified. Clear cell RCC is the most common subtype, accounting for 75% of RCCs. As the cancer itself is rather resistant to chemotherapy or radiotherapy, surgical removal is the only curative treatment available to date [1, 3]. However, approximately 40% of patients undergoing treatment for primary tumors will relapse with local or metastatic RCC with a 5-year survival rate of 60–70% in organ-confined tumors versus 10% for metastatic tumors [3,4,5,6]. Reports indicated that the incidence of subsequence malignancies varies by population level, ranging from 4.5 to 26.9%, with the most common subsequent malignancies following an RCC diagnosis being prostate, bladder, lung, breast, and colon cancers [7,8,9]. Analyses from The Cancer Genome Atlas (TCGA) indicated that RCC histological subtypes were associated with distinct genetic and epigenetic changes that contributed to the pathogenesis of RCC. The most common genetic alteration associated with clear cell RCC is the Von Hippel‒Lindau (VHL) gene, which is known to alter the half-life of hypoxia-induced factor (HIF) alpha and consequently promotes a prominent angiogenesis process that supplies blood to the tumor [10, 11]. Additionally, VHL hypermethylation is characteristic of most clear cell RCC [12]. It has been proposed that increased DNA hypermethylation was associated with disease at a higher stage and lower survival in all RCC histopathological subtypes [12]. The discovery of particular molecular biomarker associated with survival will enhance patient treatment strategies and offer potential targets for precision therapy designed for specific subtypes. Over the last two decades, medical treatment for RCC has transitioned from the use of cytokines to targeted therapies against vascular endothelial growth factor (VEGF) or tyrosine kinase to combination therapies involving immune checkpoint inhibitors and tyrosine kinase inhibitors [3, 13]. However, there are still many unknown mechanisms that might be involved in RCC pathogenesis that should be further investigated.
The transcription factor (TF) activator protein (AP)-2α, also known as TFAP2A, represents the best-characterized member of the AP-2 TF family that plays important roles in several biological processes, such as cell growth, apoptosis, and tissue differentiation during early development [14, 15]. Oya et al. showed that distinct expression patterns of AP-2 isoforms in RCC, with AP-2α predominantly expressed in clear cell RCC [16]. Aberrant AP-2α expression has been observed in various cancers, acting as a tumor suppressor or oncogene depending on the cancer type [17,18,19,20,21,22,23]. Despite its recognized involvement in different cancers, the role of AP-2α in RCC remains unexplored. While epigenetic alterations of the AP-2α gene, where it function as a tumor suppressor, have been reported in breast cancer and melanoma [17, 24], no study to date has demonstrated similar DNA methylation regulatory mechanisms in the context of RCC. Moreover, there has been a lack of comprehensive exploration into the biological role, prognostic value, and clinical significance of the AP-2α gene in RCC. Consequently, this study aimed to investigate the clinical significance of the expression of AP-2α in RCC and determine whether epigenetic regulation of the AP-2α gene plays an important role in the carcinogenesis of RCC.
Methods
Patients and specimens
A total of 107 RCC samples were collected from the tissue bank between 2004 and 2010, and this study was approved by the Institutional Review Board of Chang Gung Memorial Hospital, LinKou (IRB no. 100-4590B). Among them, 27 RCC samples were used for the Methylated-CpG island recovery assay (MIRA) combined with microarray analysis to identify methylated genes. 80 RCC samples were used for the other experiments, including combined bisulfite restriction analysis (COBRA) and reverse transcription-quantitative polymerase chain reaction (RT‒qPCR). After surgical resection, tissue specimens were washed with PBS, snap-frozen in liquid nitrogen and stored at -80 °C. Tumors were staged according to the American Joint Committee on Cancer (AJCC) TNM guidelines, and the nuclear grade of the tumors was determined according to the Fuhrman grading system [25, 26]. All clinicopathological features are listed in Table 1. Follow-up data were obtained from patient health records and the cancer registration unit at Chang Gung Memorial Hospital.
DNA and RNA preparation
DNA from tissues was extracted using a standard proteinase K/phenol/chloroform procedure. DNA was quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE, USA) and then further analyzed by 1% agarose gel electrophoresis (Invitrogen Life Technologies, Carlsbad, CA).
Total RNA from tissues was extracted using TRIzol reagent (Invitrogen Life Technologies) and stored at -80 °C according to the manufacturer’s instructions. Total RNA was quantified and its quality was assessed using ND-1000 spectrophotometry.
Methylated-CpG island recovery assay and microarray analysis
To detect the DNA methylation pattern among different types of RCC, MIRA combined with microarray analysis was employed following the protocol described by Rauch et al. [27, 28]. Nimblegen’s Signalmap program (version 1.9, Roche-NimbleGen, Madison, WI) was used to visualize DNA methylation data and generate profiling snapshots.
Combined bisulfite restriction analysis
A total of 67 RCCs from 80 patients were included in the COBRA experiment. Of these, 13 cases had limited amounts of tissue and could only be used for RNA extraction. The bisulfite conversion of 500 ng DNA was performed using the EZ DNA methylation Gold kit (Zymo Research, Orange, CA, USA) according to the manufacturer’s instructions. The methyl primers obtained from Methyl Primer Express® Software v1.0 (Applied Biosystems Inc., Foster City, CA, USA) in COBRA were AP-2α methyl-1 forward primer (5’-GGTATTTTTTTTGGGGTAGGTA-3’) and reverse primer (5’-TACAACCTAAACCCCCTACAC-3’). The PCR products were digested with BstUI (New England Biolabs, Ipswich, MA, USA) at 60 °C for 90 min and separated on a 10% nondenaturing polyacrylamide gel. CpGenome universal methylated DNA (Merck Millipore, Bedford, MA, USA) was used as a positive control.
Reverse transcription-quantitative polymerase chain reaction
Complementary DNA (cDNA) synthesis was performed using a SuperScript™ III First-Strand Synthesis SuperMix kit (Invitrogen Life Technologies) according to the manufacturer’s instructions, and the product was stored at -20°C. qPCRs were performed using iQ™ SYBR® Green Supermix (Bio-Rad, Hercules, CA, USA) in a total volume of 20 µl with a Bio-Rad iQ5 iCycler Real-Time PCR Detection System following the manufacturer’s instructions. The primers used for amplification of AP-2α were as follows: forward primer (5’-GATCCCAATGAGCAAGTGAC-3’) and reverse primer (5’-ATGAGGTTGAAGTGGGTCAA-3’). In addition, a β-actin forward primer (5’-TCAGCAAGCAGGAGTATG-3’) and reverse primer (5’-GTCAAGAAAGGGTGTAACG-3’) were used. The -ΔCT value of relative AP-2α mRNA expression was calculated according to the formula: -ΔCT = -(CTTarget –CTInternal). CTTarget is the CT value of the target gene AP-2α in RCC tumor tissue samples, and CTInternal is the CT value of the internal control gene β-actin in RCC tumor tissue samples.
Bioinformatic analysis of the NCBI database
Data on AP-2α expression in TCGA/GTEx dataset and its association with overall survival and disease-free survival were derived from the GEPIA2 database (http://gepia2.cancer-pku.cn) [29].
Statistics
Statistical analysis was accomplished using the SPSS statistical software package (version 26.0; SPSS). One-way ANOVA was employed to compare AP-2α mRNA expression between different subtypes of RCC. Spearman rank correlation analysis was performed to examine the correlation between AP-2α mRNA expression levels and methylation status. The correlation coefficient (r) was calculated to measure the correlation degree between AP-2α mRNA expression level and methylation status. The association between AP-2α mRNA expression and clinicopathologic variables was estimated using Pearson’s chi-square test or Fisher’s exact test. We used the median -ΔCT value of -12.8 and − 13.1 as the cutoff value of AP-2α mRNA expression in patients with RCC and clear cell RCC respectively. This value was used to divide the samples into two groups: low expression (-ΔCT ≤-12.8 or -ΔCT ≤-13.1 in patients with RCC and clear cell RCC respectively) and high expression (-ΔCT >-12.8 or ΔCT >-13.1 in patients with RCC and clear cell RCC respectively). Survival curves were calibrated from the date of surgery until death related to RCC or the last follow-up finding. Disease-free survival curves were calibrated from the date of surgery until recurrence or metastasis associated with RCC or the last follow-up finding. Kaplan‒Meier curves were calibrated for each relevant clinicopathological variable and AP-2α mRNA expression; differences in survival time between subgroups of patients were analyzed by the log-rank test. Cox regression analysis was conducted at both univariate and multivariate levels. Differences were considered to be statistically significant at p < 0.05.
Results
Methylation status of AP-2α in RCC
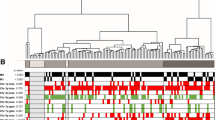
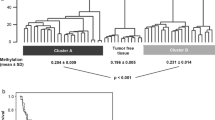
From our methylated CpG island recovery assay in various RCC histological types (19 clear cell RCCs, 4 papillary RCCs, and 4 chromophobe RCCs), we identified 2806 genes with altered methylation, with 120 genes, including AP-2α, overlapping among the types (Fig. 1A). NimbleScan (version 2.3.78, NimbleGen Systems Inc.) analysis highlighted a frequently methylated region near exon 2 of the AP-2α gene (Fig. 1B). We verified this with the COBRA assay on 67 RCC clinical samples, revealing 22 CpG dinucleotides and 3 BstUI cutting sites in the methyl-primer amplified region (Fig. 1C). BstUI digested products appeared in the representative methylated #11 tumor samples but not in the unmethylated #8 tumor samples (Fig. 1D). The AP-2α gene was methylated in most clear cell (78.7%, 37/47) and papillary (50.0%, 4/8) RCC clinical tumor samples, but not in chromophobe (0%, 0/9) or other RCC (0%, 0/3) samples (Fig. 1E).
COBRA methylation analysis of the AP-2α gene in different subtypes of RCC. A, Venn diagram depicted the number of genes identified as methylated for 19 clear cell RCC, 4 papillary RCC and 4 chromophobe RCC. B, Snapshot views from Signalmap of AP-2α gene showed the transcription start site (TSS, position:10,523,256), CpG islands (gray column) and methylation area (black column) of these regions from the RCC samples. The data showed the methylation status of the representative 2 clear cell RCC, 2 chromophobe RCC, 2 papillary RCC, and 2 oncocytoma RCC. The arrow indicated the transcription direction of AP-2α gene. C, Schematic representation of CpG distribution in the exon 2 region of the human AP-2α gene. A total of 22 CpG dinucleotides were analyzed in the indicated region (vertical tick mark). Bisulfite PCR primers (arrows) were designed with Methyl Primer Express® Software v1.0 and used to amplify DNA from different subtypes of RCC tumor specimens. 3 BstUI cutting sites were included in the amplified fragment. D, Methylation analysis by COBRA for different paired RCC tumor (T) and adjacent nontumor tissue samples (N) was performed, and the representative results are shown. We used CpGenome universal methylated DNA as a positive control (C) for methylation studies. Arrows indicate unmethylated (U)/methylated (M) alleles. E, The methylation status of different subtypes of RCC was analyzed using the COBRA approach. 47 clear cell, 8 papillary, 9 chromophobes, and 3 other (2 granular and 1 unclassified) RCC clinical tumor samples were included in this experiment
AP-2α gene expression in RCC
RT‒qPCR assays were performed to examine AP-2α mRNA expression in 80 RCC clinical samples. AP-2α mRNA expression was significantly lower in clear cell and papillary RCC compared with chromophobe and other RCC (p < 0.0001, Fig. 2A). To further investigate whether AP-2α mRNA expression was associated with methylation status as determined by the COBRA assay, Spearman rank correlation analysis was performed. The results revealed a trend of a negative correlation between AP-2α mRNA expression levels and methylation status (r = -0.384, p = 0.001, Fig. 2B).
Expression of AP-2α in different RCC subtypes. A, Relative AP-2α expression levels in different subtypes of RCC were determined by RT‒qPCR. The -ΔCT value of relative AP-2α expression was calculated according to the following formula: -ΔCT = -(CTTarget – CTInternal). B, The correlation between AP-2α mRNA expression levels and methylation status (p < 0.05 was considered to indicate significant)
Association of AP-2α expression with clinicopathologic characteristics in RCC
Next, we investigated the association between AP-2α mRNA expression and clinicopathologic characteristics in RCC patients. The median -ΔCT value of -12.8 was used as the cutoff value. No significant association between the AP-2α mRNA expression level and clinicopathologic characteristics was found (Table 1), except for the pathological T stage (p = 0.026).
Association between AP-2α expression and RCC patient survival
We further analyzed the relationship between AP-2α mRNA expression and RCC patient prognosis. Kaplan‒Meier survival curves showed that higher AP-2α mRNA expression correlated with poor overall survival (p = 0.005) and disease-free survival (p = 0.005) (Fig. 3A, C). Similarly, patients with unmethylated AP-2α had poorer overall survival (p = 0.021) and disease-free survival (p = 0.028) compared to those with methylated AP-2α (Fig. 3B, D).
Kaplan‒meier survival curve based on AP-2α expression in patients with RCC. Kaplan‒meier analysis of survival probability (A, B) and disease-free survival (C, D) were performed based on AP-2α mRNA expression in RT‒qPCR assay (A, C) and COBRA assay (B, D). The -ΔCT value of relative AP-2α mRNA expression was calculated according to the following formula: -ΔCT = -(CTTarget – CTInternal). The median -ΔCT value was -12.8. Low expression was defined as -ΔCT ≤ -12.8, whereas high expression was defined as -ΔCT > -12.8 (p < 0.05 was considered to indicate significance)
Univariate analysis revealed that higher AP-2α mRNA expression was associated with poor overall survival and disease-free survival [hazard ratio (HR): 5.101, 95% confidence interval (CI): 1.438–18.100, p = 0.012; HR: 5.160, 95% CI: 1.455–18.301, p = 0.011; respectively] (Table 2). After controlling for the clinical confounders that were statistically significant in the univariate analysis, multivariate analysis confirmed these findings, showing an increased risk for overall survival and disease-free survival (HR: 28.155, 95% CI: 3.798–208.698, p = 0.001; HR: 38.767, 95% CI: 4.822–311.671, p = 0.001; respectively) (Table 2).
Considering the negative correlation between AP-2α mRNA expression and methylation status, we further explored the prognostic significance of AP-2α methylation. Multivariate analysis indicated a significantly increased risk for overall survival and disease-free survival in patients with unmethylated AP-2α (HR: 0.239, 95% CI: 0.065–0.871, p = 0.030; HR: 0.186, 95% CI: 0.041–0.832, p = 0.028; respectively) (Table 3).
Association between AP-2α expression and clear cell RCC patient survival
We investigated the relationship between AP-2α mRNA expression and clear cell RCC prognosis due to the significant differences among different histological types (Table 1). Kaplan‒Meier survival curves showed that patients with higher AP-2α mRNA expression had significantly poorer overall survival (p = 0.009) and disease-free survival (p = 0.007) (Fig. 4A, E). Similarly, patients with unmethylated AP-2α had poorer overall survival (p = 0.039) and disease-free survival (p = 0.043) than those with methylated AP-2α (Fig. 4B, F). Using the TCGA database, we confirmed that higher AP-2α mRNA expression was associated with poorer overall and disease-free survival in both clear cell (Fig. 4C, G) and papillary RCC (Fig. 4D, H) (overall survival, p < 0.001 and p < 0.001, respectively; disease-free survival, p < 0.001 and p < 0.01, respectively).
Kaplan‒Meier survival curve based on AP-2α expression in patients with different RCC subtypes. Kaplan‒Meier analysis of survival probability (A, B) and disease-free survival (E, F) were performed based on AP-2α mRNA expression in patients with clear cell RCC in RT‒qPCR assay (A, E) and COBRA assay (B, F). The -ΔCT value of relative AP-2α mRNA expression was calculated according to the following formula: -ΔCT = -(CTTarget – CTInternal). The median -ΔCT value was − 13.1. Low expression was defined as -ΔCT ≤ -13.1, whereas high expression was defined as -ΔCT > -13.1 (p < 0.05 was considered to indicate significance). The expression pattern of AP-2α in clear cell RCC (C, G) and papillary RCC (D, H) was predicted by the GEPIA2 database
Univariate analysis identified AP-2α mRNA expression as a major risk factor for overall and disease-free survival in clear cell RCC (HR: 9.558, 95% CI: 1.195–76.484, p = 0.033; HR: 10.151, 95% CI: 1.268–81.246, p = 0.029, Table 4). Multivariate analysis further revealed that AP-2α mRNA expression was an independent risk factor for disease-free survival (HR: 21.421, 95% CI: 1.777–258.210, p = 0.016) but not overall survival in clear cell RCC (Table 4).
Discussion
The AP-2 family consists of different TFs that bind to the consensus DNA-binding sequence GCCNNNGGC [30, 31]. Among these, AP-2α, encoded by the TFAP2A gene, plays a significant role. Genetic and epigenetic alterations, such as DNA copy number variation and CpG island methylation, could influence gene expression during tumor pathogenesis [32, 33]. Our RT‒qPCR and COBRA assay results revealed that variable AP-2α mRNA expression across different RCC subtypes. We hypothesize that this deregulation in clear cell RCC is partly due to DNA methylation, as indicated by high methylation status of AP-2α detected by the COBRA assay. However, NimbleScan only predicted the methylation status of selective site, and not all methylation sites of AP-2α were examined. This suggests that other methylated sites may contribute to the observed variability in AP-2α mRNA expression in clear cell RCC.
To date, the exact role of AP-2α in RCC remains unclear. Different RCC subtypes are known to have distinct genetic bases and precursor cells [34, 35]. Previous studies, such as by Oya et al., demonstrated differential expression of AP-2 isoforms in RCC and normal adult kidneys, with clear cell RCC showing higher nuclear AP-2α expression, suggesting a functional role in this subtype [16]. However, their study found no significant difference in AP-2α expression based on tumor stage and grade. Our current results show that higher AP-2α mRNA expression is associated with high pathological T stage, poor overall survival, and poor disease-free survival in RCC (Table 1; Fig. 3). Both univariate and multivariate analyses indicated that AP-2α mRNA expression is a major risk factors for overall and disease-free survival in RCC patients (Table 2). We observed significant variability in AP-2α mRNA expression among RCC subtypes, with a similar impact on survival noted in clear cell RCC (Table 4; Fig. 4). We propose differing precursor cells may influence AP-2α function. Additionally, our results suggest that AP-2α functions differently in aggressive versus less aggressive tumors. Higher AP-2α mRNA expression levels were found in oncocytoma and chromophobe RCC compared to clear cell and papillary RCC. However, higher expression was significantly associated with poor survival in clear cell and papillary RCC patients (Fig. 4). We hypothesize that AP-2α plays an oncogenic role in aggressive RCC subtypes like clear cell RCC, but not in less aggressive subtypes. The inconsistency with Oya’s study might be due to different experimental approaches or populations used in assessing AP-2α expression in RCC [16]. AP-2 isoforms can form homo- or heterodimer complexes and bind differently to target DNA [14, 36], suggesting that their mechanisms and roles may differ in a tissue-specific manner.
Although AP-2α is known to play important roles in biological processes and is often associated with cancer progression and metastasis as a tumor suppressor gene [17,18,19], our results showed that higher AP-2α expression is linked to poor overall and disease-free survival in clear cell RCC. This suggests that AP-2α may play an oncogenic role in RCC, aligning with findings in other studies [20, 37, 38]. Studies have shown similar associations of AP-2α with poor prognosis in various cancers. For instance, Shi et al. found that AP-2α overexpression correlates with advanced tumor stage and poor prognosis in nasopharyngeal carcinoma, and it regulates cell growth and survival via the HIF-1α-mediated VEGF/PDGF pathway [20]. Yamashita et al. reported that AP-2α overexpression is linked to metastasis and recurrence in basal-squamous bladder cancer [37]. Liao et al. found that higher AP-2α expression predicts worsen prognosis in lung adenocarcinoma [38]. Furthermore, specific long non-coding RNAs or microRNAs (miRNAs) have been found to modulate AP-2α levels, influencing tumor biology. For example, Qin et al. identified 8 prognostic miRNAs in clear cell RCC and proposed a TF-miRNA signature involving AP-2α that could improve prognosis predictions [39]. Liang et al. discovered that Linc00467 enhances invasion and inhibit apoptosis in head and neck squamous cell carcinoma through the miRNA-1285-3p/AP-2α axis [40]. Yang et al. found that AP-2α acts as an oncogene in oral squamous cell carcinoma via the AP-2α-AS1/miRNA-1297/AP-2α axis [41]. Our study’s findings raise the possibility that AP-2α may function similarly in RCC, but further investigation is needed. Limitations of our study include the small number of cases for different RCC subtypes and the incomplete methylation status prediction by NimbleScan software, which restricted the verification of AP-2α methylation comprehensively.
In conclusion, our results showed that AP-2α was deregulated in RCC and that higher AP-2α expression was associated with poor overall and disease-free survival in RCC patients. These findings strongly suggest that AP-2α plays a crucial role in the pathophysiology of RCC. Further studies are needed to confirm its specific function in RCC and its potential as a prognostic marker.
Data availability
All data generated or analyzed during the current study are included in this published article.
Abbreviations
- RCC:
-
Renal cell carcinoma
- AP-2α:
-
Transcription factor activator protein (AP)-2α
- TCGA:
-
The Cancer Genome Atlas
- VHL:
-
Von Hippel‒Lindau
- HIF:
-
Hypoxia-induced factor
- VEGF:
-
Vascular endothelial growth factor
- MIRA:
-
Methylated-CpG island recovery assay
- COBRA:
-
Combined bisulfite restriction analysis
- RT‒qPCR:
-
Reverse transcription-quantitative polymerase chain reaction
- AJCC:
-
American Joint Committee on Cancer
- cDNA:
-
Complementary DNA
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
References
Chowdhury N, Drake CG. Kidney Cancer: an overview of current therapeutic approaches. Urol Clin North Am. 2020;47(4):419–31.
Jindong C. Perspective Chapter: An Update on Renal Cell Carcinoma. In: Renal Cell Carcinoma - Recent Advances, New Perspectives and Applications edn. Edited by Prof. Jindong C. Rijeka: IntechOpen; 2023: Ch. 1.
Kathuria-Prakash N, Drolen C, Hannigan CA, Drakaki A. Immunotherapy and metastatic renal cell carcinoma: a review of New Treatment approaches. Life (Basel) 2021, 12(1).
Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med. 2017;376(4):354–66.
Gu L, Ma X, Li H, Chen L, Xie Y, Li X, et al. Comparison of oncologic outcomes between partial and radical nephrectomy for localized renal cell carcinoma: a systematic review and meta-analysis. Surg Oncol. 2016;25(4):385–93.
Dovalova D, Rybar L, El Falougy H, Kubikova E, Mifkovic A. Renal cell carcinoma - summarizing overview, biomarkers, metastases and new perspectives. Bratisl Lek Listy. 2022;123(10):697–704.
Lu YC, Tsai YC, Chow PH, Weng WC, Huang WY, Chueh SJ, et al. The risk of subsequent malignancies in patients with renal cell carcinoma: a nationwide, population-based study. Urol Sci. 2022;33(3):124.
Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97(4):698–702.
Sato S, Shinohara N, Suzuki S, Harabayashi T, Koyanagi T. Multiple primary malignancies in Japanese patients with renal cell carcinoma. Int J Urol. 2004;11(5):269–75.
Clifford SC, Prowse AH, Affara NA, Buys CH, Maher ER. Inactivation of the Von Hippel-Lindau (VHL) tumour suppressor gene and allelic losses at chromosome arm 3p in primary renal cell carcinoma: evidence for a VHL-independent pathway in clear cell renal tumourigenesis. Genes Chromosomes Cancer. 1998;22(3):200–9.
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, et al. Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A. 1994;91(21):9700–4.
Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019;16(9):539–52.
Barata PC, Rini BI. Treatment of renal cell carcinoma: current status and future directions. CA Cancer J Clin. 2017;67(6):507–24.
Hilger-Eversheim K, Moser M, Schorle H, Buettner R. Regulatory roles of AP-2 transcription factors in vertebrate development, apoptosis and cell-cycle control. Gene. 2000;260(1–2):1–12.
Wenke AK, Bosserhoff AK. Roles of AP-2 transcription factors in the regulation of cartilage and skeletal development. FEBS J. 2010;277(4):894–902.
Oya M, Mikami S, Mizuno R, Miyajima A, Horiguchi Y, Nakashima J, et al. Differential expression of activator protein-2 isoforms in renal cell carcinoma. Urology. 2004;64(1):162–7.
Douglas DB, Akiyama Y, Carraway H, Belinsky SA, Esteller M, Gabrielson E, et al. Hypermethylation of a small CpGuanine-rich region correlates with loss of activator protein-2alpha expression during progression of breast cancer. Cancer Res. 2004;64(5):1611–20.
Heimberger AB, McGary EC, Suki D, Ruiz M, Wang H, Fuller GN, et al. Loss of the AP-2alpha transcription factor is associated with the grade of human gliomas. Clin Cancer Res. 2005;11(1):267–72.
Schwartz B, Melnikova VO, Tellez C, Mourad-Zeidan A, Blehm K, Zhao YJ, et al. Loss of AP-2alpha results in deregulation of E-cadherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene. 2007;26(28):4049–58.
Shi D, Xie F, Zhang Y, Tian Y, Chen W, Fu L, et al. TFAP2A regulates nasopharyngeal carcinoma growth and survival by targeting HIF-1alpha signaling pathway. Cancer Prev Res (Phila). 2014;7(2):266–77.
Kolat D, Kaluzinska Z, Bednarek AK, Pluciennik E. The biological characteristics of transcription factors AP-2alpha and AP-2gamma and their importance in various types of cancers. Biosci Rep 2019, 39(3).
Yang J, Gao Y, Yao S, Wan S, Cai H. TFAP2A promotes cervical cancer via a positive feedback pathway with PD–L1. Oncol Rep 2023, 49(6).
Zhao J, Lan G. TFAP2A activates HMGA1 to promote glycolysis and lung adenocarcinoma progression. Pathol Res Pract. 2023;249:154759.
Hallberg AR, Vorrink SU, Hudachek DR, Cramer-Morales K, Milhem MM, Cornell RA, et al. Aberrant CpG methylation of the TFAP2A gene constitutes a mechanism for loss of TFAP2A expression in human metastatic melanoma. Epigenetics. 2014;9(12):1641–7.
Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–63.
Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz A, Greene F. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
Rauch TA, Pfeifer GP. The MIRA method for DNA methylation analysis. Methods Mol Biol. 2009;507:65–75.
Rauch TA, Pfeifer GP. DNA methylation profiling using the methylated-CpG island recovery assay (MIRA). Methods. 2010;52(3):213–7.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47(W1):W556–60.
Williams T, Tjian R. Analysis of the DNA-binding and activation properties of the human transcription factor AP-2. Genes Dev. 1991;5(4):670–82.
Eckert D, Buhl S, Weber S, Jager R, Schorle H. The AP-2 family of transcription factors. Genome Biol. 2005;6(13):246.
Setty M, Helmy K, Khan AA, Silber J, Arvey A, Neezen F, et al. Inferring transcriptional and microRNA-mediated regulatory programs in glioblastoma. Mol Syst Biol. 2012;8(1):605.
Liu Q, Su PF, Zhao S, Shyr Y. Transcriptome-wide signatures of tumor stage in kidney renal clear cell carcinoma: connecting copy number variation, methylation and transcription factor activity. Genome Med. 2014;6(12):1.
Kuroda N, Tanaka A. Recent classification of renal epithelial tumors. Med Mol Morphol. 2014;47(2):68–75.
Muglia VF, Prando A. Renal cell carcinoma: histological classification and correlation with imaging findings. Radiol Bras. 2015;48(3):166–74.
Bosher JM, Totty NF, Hsuan JJ, Williams T, Hurst HC. A family of AP-2 proteins regulates c-erbB-2 expression in mammary carcinoma. Oncogene. 1996;13(8):1701–7.
Yamashita H, Kawasawa YI, Shuman L, Zheng Z, Tran T, Walter V, et al. Repression of transcription factor AP-2 alpha by PPARgamma reveals a novel transcriptional circuit in basal-squamous bladder cancer. Oncogenesis. 2019;8(12):69.
Liao H, Lin P. The comprehensive investigation of transcription factor AP-2 alpha in lung adenocarcinoma. Transl Cancer Res. 2020;9(3):1547–57.
Qin S, Shi X, Wang C, Jin P, Ma F. Transcription factor and miRNA interplays can manifest the survival of ccRCC patients. Cancers. 2019;11(11):1668.
Liang Y, Cheng G, Huang D, Yuan F. Linc00467 promotes invasion and inhibits apoptosis of head and neck squamous cell carcinoma by regulating miR-1285-3p/TFAP2A. Am J Transl Res. 2021;13(6):6248–59.
Yang K, Niu Y, Cui Z, Jin L, Peng S, Dong Z. Long noncoding RNA TFAP2A-AS1 promotes oral squamous cell carcinoma cell growth and movement via competitively binding miR-1297 and regulating TFAP2A expression. Mol Carcinog. 2022;61(9):865–75.
Acknowledgements
The authors want to thank You-Shin Chang for the excellent experimental help. The authors also thank Ping-Hsuan Huang for the statistical assistance and wish to acknowledge the support of the Maintenance Project of the Center for Big Data Analytics and Statistics (Grant CLRPG3N0011) at Chang Gung Memorial Hospital for study design and monitor, data analysis and interpretation. The authors also express their gratitude to the entire team at the Cancer Center in Chang Gung Memorial Hospital for their indispensable assistance.
Funding
This work was supported by grants from the Chang Gung Medical Research Fund (CORPG3G0051, CGMH-NTUT-106-No.06) and the National Science and Technology Council of Taiwan (MOST 109-2314-B-182 A-132-MY3, NMRPG3K6243).
Author information
Authors and Affiliations
Contributions
KJY, IHS, CKC, WHW performed data curation; PHL, KJY, IHS, CKC, WHW performed data curation and methodology; CHH performed experiments and analyzed data; PHL, CHH designed experiments and wrote the manuscript; CKC, STP provided funding acquisition; TH, STP supervised and planned research. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Chang Gung Memorial Hospital, LinKou Branch, Taiwan with IRB no. 100-4590B. Informed consent requirement was waived by the Institutional Review Board of Chang Gung Memorial Hospital, LinKou Branch, Taiwan.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, PH., Hsieh, CH., Yu, KJ. et al. AP-2α gene deregulation is associated with renal cell carcinoma patient survival. BMC Cancer 24, 966 (2024). https://doi.org/10.1186/s12885-024-12526-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12526-w