Abstract
Purpose
This report aims to present a case of corneal keloid caused by chronic corneal insult after trauma and Descemet stripping automated endothelial keratoplasty (DSAEK).
Case presentation
A 35-year-old male with a history of vision loss in the right eye was referred to our hospital. The patient underwent Ahmed Glaucoma Valve Implantation to alleviate elevated intraocular pressure after ocular trauma to the same eye. One year following the procedure, the eye developed endothelial failure, leading to the performance of Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) with repositioning of the shunt tube. Upon initial examination, a well-circumscribed elevated white opaque lesion involving the central corneal surface of the RE was observed. Based on the patient’s clinical history, slit lamp examination, and UBM findings, the diagnosis of corneal keloid was established. Superficial keratectomy was performed. Histopathological analysis confirmed the diagnosis of corneal keloid. Following the procedure, BCVA improved slightly. However, 3 months later, the patient underwent a penetrating keratoplasty for visual rehabilitation.
Conclusion
Corneal keloids should be considered following any form of ocular trauma, particularly in cases involving ocular surgery. Diagnosing corneal keloids can sometimes be challenging due to the variety of potential differentials; however, by carefully evaluating the patient’s medical history and clinical presentation, we can effectively narrow down the differential diagnosis of corneal conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Corneal keloids were initially described by Szokalski in 1865 [1, 2], as an uncommon ocular condition. These lesions are characterized by single, elevated, white growths on the cornea, often exhibiting a glossy appearance, resulting from abnormal wound healing processes [3]. They may vary in vascularity and thickness and typically exhibit well-defined borders. Keloids can manifest at any age, ranging from congenital cases to those occurring later in life. While most cases are associated with corneal trauma or disease [4,5,6], there are documented instances of primary corneal keloids in individuals without such antecedents, often appearing months to years after an inciting event.
Diagnosis is confirmed through biopsy and histopathological examination, revealing hyperplastic changes in the corneal epithelium and abundant, irregularly arranged collagen bundles along with activated fibroblasts in the stroma [1, 3, 7]. Surgical intervention is often required for clinically significant cases affecting vision; however, the outcomes of such procedures vary considerably from case to case [3]. We present a comprehensive analysis of the clinical characteristics, surgical management outcomes, and histopathological findings related to a corneal keloid developed in a patient with a history of ocular trauma, corneal decompensation, and an endothelial transplant.
Case presentation
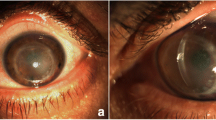
A 35-year-old male with a five-year history of vision loss in the right eye (RE) was referred to the Cornea Department at Instituto de Oftalmología Conde de Valenciana. The patient had a prior ocular trauma to the same eye that required an Ahmed glaucoma valve (AGV) implantation for intraocular pressure (IOP) management. Subsequently, the eye experienced corneal decompensation one year after valve implantation, leading to the performance of Descemet’s Stripping Automated Endothelial Keratoplasty (DSAEK) with repositioning of the shunt tube. Initially, the patient reported good visual acuity (VA) for two years post-procedure; however, he then began to develop a small central opacity accompanied with loss of vision, which progressively worsened over the subsequent two years. Ophthalmological examination revealed a best-corrected visual acuity (BCVA) of Hand movement in the RE and 20/20 in the left eye (LE). Furthermore, there was a well-circumscribed elevated white opaque lesion involving the central corneal surface of the RE (Fig. 1). Anterior segment optical coherence tomography (AS-OCT) was performed, revealing a lesion on the cornea that was separated from the corneal epithelium by a distinct plane. However, detailed visualization was limited due to posterior shadowing of the lesion. Ultrasound biomicroscopy (UBM) showed a hyperechoic lesion extending from the corneal surface to the superficial stromal layer, measuring 1300 μm in thickness (Fig. 1). Additionally, an increased central corneal thickness of 770 μm was noted. While the UBM lacked detail due to posterior shadowing, the DSAEK graft was discernible with poor definition. No abnormal findings were identified in the posterior segment by ocular B-mode ultrasonography (US). Based on the patient’s clinical history, slit lamp examination, and UBM findings, the presumptive diagnosis of corneal keloid was established.
A, B. Clinical photographs showing a well-circumscribed elevated white opaque lesion involving the central corneal surface on the RE. C, D UBM showed a hyperechoic lesion extending from the corneal surface to the superficial stromal layer, measuring 1300 μm with an increased central corneal thickness of 770 μm. DSAEK graft was discernible with poor definition
Superficial keratectomy was performed on the RE. Major portion of the lesion was detached from Bowman’s layer; however, a small portion infiltrated the stroma, resulting in corneal scarring and fibrosis (Fig. 2). The excised mass was immersed in formalin and subsequently submitted for a comprehensive pathological examination. After the procedure, the BCVA improved to 20/1600. However, 3 months later, the patient underwent a penetrating keratoplasty (PKP) to rehabilitate this eye.
Histopathological examination of the excised corneal sample revealed variations in the thickness of the surface epithelium, alternating areas of hyperplasia or irregular acanthosis with areas of decreased number of layers. Numerous cells in the basal layer showed intracytoplasmic edema with the formation of small epithelial bullae. The Bowman layer showed discontinuity throughout much of its extent. The stroma presented variations in thickness, irregularity in the arrangement of the lamellae, numerous reactive keratocytes alternating with thick bands of collagen arranged in an irregular and disorganized manner, more evident with Masson’s trichrome staining (Fig. 3). Congo red staining was negative, ruling out the presence of amyloid. Descemet’s membrane and endothelial cell layer were not identified. There was no adhesion of the uveal tissue to the posterior surface of the cornea.
Discussion
Corneal keloids exhibit similarities with skin keloids, likely stemming from excessive production of collagen by activated corneal fibroblasts [8, 9]. Unlike hypertrophic scars, which are confined to the area of the original injury, keloids have the potential to extend beyond the initial lesion, spreading across significant portions of the corneal surface, and they are frequently noted to recur [9]. While existing reports do not suggest a direct correlation between a predisposition for corneal keloid development and skin keloids [10], recent cases have reported corneal scarring after laser in situ keratomileusis (LASIK) and photorefractive keratectomy (PRK) in Caucasian patients with a history of skin keloid formation [11, 12]. Moreover, keloids have been documented following superficial keratectomy (SK) in patients without known risk factors [13]. However, they are significantly less common than skin keloids and are often associated with ocular trauma, a history of penetrating or non-penetrating surgery, or, in some cases, rare diseases such as Lowe’s syndrome and Rubinstein–Taybi syndrome [1, 14,15,16]. Corneal keloids have been reported more frequently in men [7]. In our case, the patient, a male, had a history of trauma necessitating a valve implant procedure, which led to endothelial failure, requiring endothelial transplantation.
Diagnosing corneal keloids can be challenging due to various terms used interchangeably, including corneal fibroma, myofibroma, and hypertrophic scars. Additionally, it can be easily mistaken for other conditions such as corneal dermoid tumors, ocular surface squamous neoplasia, Salzmann’s nodular degeneration, limbal dermoid, sclerocornea, Peters anomaly, myxomas, congenital glaucoma with corneal edema, metabolic diseases like mucopolysaccharidosis and mucolipidoses, and corneal inclusion cysts [4, 8, 10, 17, 18]. A definitive diagnosis of corneal keloids fundamentally requires a biopsy and histopathological examination, revealing dysplasia of the corneal epithelium and hyperplasia of collagen fibers [4,5,6, 13]. Bowman’s layer is usually disrupted or absent [7].
Pathogenesis of this uncommon ophthalmic disorder remains unclear. Trauma and intraocular surgeries are well-established predisposing factors for keloid development. It is well-established that keloids can manifest several months or even years following these surgeries. Additionally, any of these events, individually or in combination, might serve as the source of the keloid. There have been reports of congenital corneal keloids without a history of ocular injury or trauma, no history of chronic inflammation, or a family history of fibrovascular proliferative disease [1, 10]. This proliferation may induce an inflammatory response in the corneal stroma, serving as a stimulus for the expansion of the fibrotic process beyond the limits of the corneal injury, a characteristic feature of corneal keloids [13, 19]. In our case, a male patient had a history of trauma that required a valve implantation, leading to corneal decompensation necessitating endothelial transplantation. Although the keloid didn’t appear until 2 years after the endothelial transplant, and it is difficult to determine the exact cause. We hypothesize that fibroblasts were activated following the DSAEK and tube valve repositioning. However, the keloid might also result from the initial trauma and subsequent surgeries.
Successful management of corneal keloids involves various surgical techniques, including superficial keratectomy, lamellar excision, lamellar keratoplasty, or penetrating keratoplasty, with favorable outcomes reported in several studies [3]. Recurrence of corneal keloids post-superficial keratectomy, photorefractive keratectomy, and penetrating keratoplasty has been observed [3, 13, 20]. Recently, successful management of corneal keloids with deep anterior lamellar keratoplasty was reported, highlighting the importance of removing the entire stroma to prevent recurrent corneal stromal opacification. Additional treatments post-surgery may include the use of amniotic membrane, corticosteroids, mast cell stabilizers, cyclosporine, laser therapy, and cryotherapy [12, 21]. The choice of treatment depends on factors such as lesion depth, surface area, and surrounding corneal conditions, with penetrating keratoplasty being recommended in cases with extensive corneal damage and endothelial dysfunction. Additionally, sclerokeratoplasty has emerged as a successful alternative procedure, particularly in cases where conventional keratoplasty is not feasible due to extensive corneal damage. In our patient, we observed endothelial dysfunction following the SK, leading us to perform a PKP.
In summary, corneal keloids should be considered in cases of corneal lesions characterized by a dense, white, glistening, and bulging appearance, which may or may not be vascularized. These lesions often arise following surgical procedures, corneal insults, or trauma and can manifest months or even years after the initial injury. While surgical intervention is a common treatment approach, it carries an inherent risk of recurrence.
Data availability
No datasets were generated or analysed during the current study.
References
Jung JJ, Wojno TH, Grossniklaus HE. Giant corneal keloid: case report and review of the literature. Cornea. 2010;29(12):1455–8.
Smith HC. Keloid of the cornea. Trans Am Ophthalmol Soc. 1940;38:519–38. [cited 2024 Mar 18].
Lee HK, Choi HJ, Kim MK, Wee WR, Oh JY. Corneal keloid: four case reports of clinicopathological features and surgical outcome. BMC Ophthalmol. 2016;16(1):198.
Minamidate R, Toyono T, Asahina Y, Yamazawa S, Miyai T. Corneal keloid caused by persistent atopic eye disease and chronic eyelid closure. Am J Ophthalmol Case Rep. 2023;30:101819.
Fukuda K, Chikama T, Takahashi M, Nishida T. Long-term follow-up after lamellar keratoplasty in a patient with bilateral idiopathic corneal keloid. Cornea. 2011;30(12):1491–4.
Vanathi M, Panda A, Kai S, Sen S. Corneal keloid. Ocul Surf. 2008;6(4):186–97.
Mejía LF, Acosta C, Santamaría JP. Clinical, surgical, and histopathologic characteristics of corneal keloid. Cornea. 2001;20(4):421–4.
Miyamoto R, Sakimoto T, Homma T, Tanaka Y, Sugiura T, Yamagami S. A rare case of corneal keloid occurred 30 years after pterygium surgery and 3 years after cataract surgery. Am J Ophthalmol Case Rep. 2020;20:100901.
Bukowiecki A, Hos D, Cursiefen C, Eming SA. Wound-healing studies in cornea and skin: parallels, differences and opportunities. Int J Mol Sci. 2017;18(6):1257.
Palko JR, Arfeen S, Farooq AV, Reppa C, Harocopos GJ. Corneal keloid presenting forty years after penetrating injury: case report and literature review. Surv Ophthalmol. 2019;64(5):700–6.
Holbach LM, Font RL, Shivitz IA, Jones DB. Bilateral keloid-like myofibroblastic proliferations of the cornea in children. Ophthalmology. 1990;97(9):1188–93.
Gupta J, Gantyala SP, Kashyap S, Tandon R. Diagnosis, management, and histopathological characteristics of corneal keloid: a case series and literature review. Asia-Pac J Ophthalmol. 2016;5(5):354–9.
Bakhtiari P, Agarwal DR, Fernandez AA, Milman T, Glasgow B, Starr CE, et al. Corneal keloid: report of natural history and outcome of surgical management in two cases. Cornea. 2013;32(12):1621–4.
Shilpashree P, Jaiswal AK, Kharge PM. Keloids: an unwanted spontaneity in rubinstein-taybi syndrome. Indian J Dermatol. 2015;60(2):214.
Esquenazi S, Eustis HS, Bazan HE, Leon A, He J. Corneal keloid in Lowe syndrome. J Pediatr Ophthalmol Strabismus. 2005;42(5):308–10.
Martin GC, Putterman M, Dureau P. Corneal keloid in Lowe syndrome. Ophthalmology. 2022;129(6):625.
Nair AG, Kenia H, Gopinathan I, Mehta SV, Mehta VC. Corneal fibroma: an uncommon stromal tumor. Indian J Ophthalmol. 2018;66(5):699–701.
Zimmermann L, Reinhard T, Lange C, Heegaard S, Auw-Haedrich C. Corneal myofibroma (keloid) in a young patient with neurofibromatosis type 2. Ocul Oncol Pathol. 2017;3(4):247–9.
Lee JY-Y, Yang C-C, Chao S-C, Wong T-W. Histopathological differential diagnosis of keloid and hypertrophic scar. Am J Dermatopathol. 2004;26(5):379–84.
Rao SK, Fan DSP, Pang CP, Li WWY, Ng JSK, Good WV, et al. Bilateral congenital corneal keloids and anterior segment mesenchymal dysgenesis in a case of Rubinstein-Taybi syndrome. Cornea. 2002;21(1):126–30.
Chawla B, Agarwal A, Kashyap S, Tandon R. Diagnosis and management of corneal keloid. Clin Exp Ophthalmol. 2007;35(9):855–7.
Acknowledgements
Not applicable.
Funding
No funding or grant support.
Author information
Authors and Affiliations
Contributions
GRVD, REB, LGP: Follow the patient, collected data, writing, preapred Figs. 1 and 2. AARR: Collected data, validation, visualization, prepared Fig. 3. AN, EOGH, ARM: Planning, supervision, validation, visualization. All authors discussed the results and contributed to the final manuscript.
Corresponding author
Ethics declarations
Ethics
Being a case report, ethical approval was not required according to local or national guidelines. However, written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.
Patient consent
The patient provided a written consent regarding the publication of the present case report.
Competing interests
The authors declare no competing interests.
All authors have no financial disclosure.
All authors attest that they meet the current ICMJE criteria for Authorship.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Vera-Duarte, G.R., Eskenazi-Betech, R., Garcia-Padilla, L. et al. Giant corneal keloid following Descemet stripping automated endothelial keratoplasty for the treatment of corneal decompensation secondary to trauma. BMC Ophthalmol 24, 404 (2024). https://doi.org/10.1186/s12886-024-03667-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12886-024-03667-4