Abstract
Background
The dietary environment promoting adiposity keeps evolving and of interest is the significance of dietary branched-chain amino acids (BCAA). This study assessed the association between dietary BCAA intakes and odds of obesity among immigrant Filipino women in Korea.
Method
We included 423 immigrant Filipino women enrolled in the Filipino Women’s diet and health study in the Republic of Korea. Dietary BCAA intakes were estimated from 24 hour recalls and adjusted for energy intake using the residual method. General obesity was derived from direct anthropometric measurements (height, weight and waist circumference – WC) and defined as body mass index (BMI) ≥25 kg/m2 and abdominal obesity as WC ≥80 cm. Odds ratios (OR) and 95% confidence intervals (CI) by tertile distribution of energy-adjusted BCAA intakes were estimated using multivariable logistic regression with a two-sided P < 0.05.
Results
Median (interquartile range) for BCAA intakes in g/day were; 7.9 (5.8, 10.3) g/day for total BCAA; 2.0 (1.5, 2.6) g/day for isoleucine, 3.5(2.5, 4.6) g/day for leucine and 2.4 (1.8, 3.1) g/day for valine. Mean BMI and WC were 23.6 ± 3.8 kg/m2 and 79.8 ± 9.3 cm, respectively. Also, 30.2% (128) had BMI ≥25 kg/m2 and 42.0% (178) had WC ≥80 cm. ORs (95%CIs) of general obesity across tertile distribution of energy-adjusted total BCAA intakes were 1.00, 0.81 (0.47, 1.37) and 0.62 (0.36, 1.07; P for trend = 0.08). A similar trend was observed across tertile distribution of energy-adjusted isoleucine, leucine and valine intakes. However, there was a statistically significant inverse association between total BCAA intake and odds of general obesity in a subset of non-smokers; 1.00, 0.68 (0.39, 1.20) and 0.55 (0.31, 0.98; P for trend = 0.04).
Conclusion
We found a suggestive inverse association between higher dietary BCAA intake and odds of obesity in this sample of immigrant Filipino women, particularly among non-smokers. Prospective cohort studies among the immigrant population will be necessary to verity these findings.
Similar content being viewed by others
Background
Obesity is the excessive accretion of fats in the subcutaneous tissues and internal organs [1]. It is a complex and leading health concern involving the multifaceted interaction of lifestyle, genetic and environmental factors [2,3,4]. Dietary environment promoting obesity in populations are still evolving, and of recent is the significance of branched-chain amino acids (BCAA). Elevated serum concentrations of BCAA has been established to be associated with obesity risk [5, 6] with little information on the significance of dietary BCAA exposure.
BCAA are vital constituents of metabolism [7, 8] with several functions (including regulating glucose and protein metabolism) in the body [9]. They are primarily derived from diets [9] and accounts for more than half of essential amino acids in the mammalian food supply [10]. They are substrates of energy balance and key transducers in nutrient signalling [11]. Epidemiological reports have revealed the significance of dietary BCAA in obesity events with limited data accounting for population difference(s), dietary exposure and potential interaction with other metabolic risk factors.
Most previous epidemiological reports on dietary BCAA intake and obesity have been conducted in country-specific indigenous populations [12,13,14,15]; however, there are limited studies on this subject among immigrant populations. Migration is a growing phenomenon that plays a critical role in nutritional transitions and health outcomes. It is unclear if the association between dietary BCAA and obesity differs in an immigrant population. The hypothesis of the dietary BCAA and obesity odds is yet to be tested among the immigrant population. Similarly, only a few studies have assessed the relationship between dietary BCAA and odds of obesity by subgroup analyses of traditional lifestyle factors including age, smoking, alcohol use and history of diseases. For example, some studies have reported that higher dietary BCAA intake was directly associated with the risk of diabetes mellitus [16, 17]. However, few studies have tested the relationship between dietary BCAA intakes and odds of obesity according to the history of chronic diseases. Understanding the diet-disease relationship in immigrant populations is important for discerning the significance of shifting dietary exposures’ in disease outcomes. Also, whether these differences could be attributed to the diversity of the study population(s) is yet to be clearly understood.
In this study, we explored the association between dietary intake of BCAA (isoleucine, leucine and valine) and odds of obesity (by subgroup analyses of age, smoking, current alcohol use and history of chronic diseases) among a sample of immigrant Filipino women in Korea.
Methods
Study population
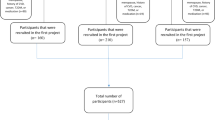
The Filipino women’s diet and health study (FiLWHEL) is an ongoing study among immigrant Filipino women ≥19 years in Korea. The study started in 2014 and was designed to assess the significance of health-related behaviour(s), lifestyle and acculturation on the progression of cardiovascular diseases among Filipino women in Korea. The Institutional Review Board of Sookmyung Women’s University (SMWU-1311-BR-012) approved the study, and all participants provided written informed consent. Participants were sampled by convenience from several cities and provinces in Korea from 2014 to 2016. A total of 504 Filipino women were recruited into the study. Details of the protocol, recruitment of participants [18] and preliminary observations [19,20,21] in the FiLWHEL study has been reported elsewhere [18,19,20,21].
Briefly, interviewer-administered questionnaires were deployed to collect demographic, health-related behaviour, medical history, quality of life and acculturation information. Also, anthropometric assessment and 24-h recalls were carried out in person. For the 24-h recall, portion sizes were estimated using food miniatures, photographs, household measures, weight/volume, and standard units and portions. Filipino staff fluent in the Filipino language conducted all interviews using the same protocol across all sites under the supervision of the principal investigators. Also, all information on the questionnaire were inspected on-site, questionnaires were checked (to clarify any inconsistencies), and double-checked for data reliability before data entry. Of the 504 women enumerated in the study, 81 were excluded for the following reasons; pregnant and lactating (n = 68) or missing information (diet, n = 07 and anthropometry, n = 06). The final analysis of the report was based on 423 Filipino women with complete information on dietary and anthropometric data.
Dietary BCAA intake assessment (exposure)
Dietary BCAA; isoleucine, leucine and valine were derived from a one-day 24-h recall. Participants recollected information on all food items, portion sizes or amount consumed in the previous day preceding the survey. Nutrient data (including BCAA and total energy intakes) were computed using the computer-aided analysis program (Can-Pro 4.0, The Korean Nutrition Society, Seoul, Korea) for professionals [22]. Where food information was unavailable, the food composition tables of the Food and Nutrition Research Institute of the Philippines (for Filipino diets) [23], Korean Rural Development Administration [24], US Department of Agriculture [25] or manufacturers’ information were used to derive nutrition information.
Anthropometric measurements and ascertainment of obesity (outcome)
Anthropometric measures were collected in keeping with standard protocol. Height and waist circumference (WC) were collected to the nearest 0.1 cm (in a standing position without shoes) using a stretch-resistant tape rule. WC was measured at the midpoint between the lowest border of the rib cage and the uppermost lateral border of the right iliac crest. Also, weight (in kg) was measured using bioelectric impedance equipment (In Body 620, Biospace Company Limited, Seoul, Korea). Body mass index (BMI) was estimated as weight (kg) divided by height (m) square. General obesity was defined as BMI ≥ 25 kg/m2, and abdominal obesity was defined as WC ≥80 cm according to cut-offs for populations in the Asia-Pacific region by the World Health Organization (Western Pacific Regional Office), the International Association for the Study of Obesity, and the International Obesity task force [26].
Demographic and lifestyle characteristics (covariates)
Participants provided information on age (in years), length of stay in Korea (in years), highest education completed (and classified as ‘elementary to high school’ or ‘college education and above’), current employment status (no, yes), ever smoked (no, yes) and current alcohol use (no, yes). The average number of hours and the number of days spent on physical activity (moderate, vigorous or walking) were provided and vigorous physical activity was defined as having spent at least an hour daily of vigorous physical activity. Also, participants were asked if they have been diagnosed with diabetes or hypertension by a certified clinician or are currently taking medications to lower blood glucose or pressure, respectively.
Statistical analysis
Isoleucine, leucine, valine and total BCAA (the summation of isoleucine, leucine and valine) were adjusted for energy intake using the residual method [27] and categorized into tertiles to include a reasonable number of participants in each category. Characteristics of participants (mean ± SD or n(%)) were presented across tertile distribution of energy-adjusted BCAA intakes. Logistic regression was used to estimate the odds ratio (OR)s and 95% confidence interval (CI)s of obesity odds by tertile distribution of energy-adjusted BCAA intakes. We assessed changes in ORs of exposure when deciding variables to be included in the final model. First, we adjusted for age (in years, continuous). In Model 1, we adjusted for age (in year, continuous), years of stay in Korea (≤4, 5-9, ≥10 years), education (elementary to high school, college education and above), employment (unemployed, employed), ever smoked a cigarette (no, yes), current alcohol use (no, yes) and energy intake (in kcal/d, continuous), Model 2 was additionally adjusted for history of chronic disease such as; diabetes (no, yes) and hypertension (no, yes). Test for trend was carried out by assigning the median value of tertile distribution as a continuous variable in the model. Furthermore, we conducted subgroup analyses to examine if the association between energy-adjusted total BCAA and odds of obesity varied by age (< 35 or ≥ 35 years), ever smoked (no, yes), current alcohol use (no, yes) or self-reported history of diabetes (no, yes) and hypertension (no, yes). Test of interaction was conducted using likelihood ratio test. All statistical analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA) at a two-sided P < 0.05.
Results
A total of 295 controls, 128 cases of overweight/obesity (BMI ≥ 25 kg/m2) and 178 cases of abdominal obesity (WC ≥80 cm) were identified in this sample. Mean BMI and WC were 23.6 ± 3.8 kg/m2 and 79.3 ± 9.3 cm, respectively. The characteristics of the participants by tertile distribution of total BCAA intakes are presented in Table 1. Women in the third tertile of total BCAA intakes were older, presented a higher proportion of participants with a history of diabetes, had higher energy and protein intakes than those in the first tertile of total BCAA intakes. The median values (interquartile range) of total BCAA intakes across increasing tertile distribution of energy-adjusted total BCAA were 4.8 (3.5, 5.8) g/d, 7.9 (7.2, 8.7) g/d and 11.4 (10.3, 13.6) g/d.
The multivariable-adjusted ORs (95% CIs) for the general and abdominal obesity across tertile distribution of dietary BCAA intake are presented in Table 2. Total BCAA intake was not statistically significantly associated with the odds of having general obesity, but there was a suggestive inverse association – 1.00, 0.81 (0.47, 1.37) and 0.62 (0.36, 1.07; P for trend = 0.08). These findings were similar for isoleucine intake – 1.00, 0.91 (0.53, 1.55) and 0.64 (0.37, 1.07; P for trend = 0.09), leucine intake – 1.00, 0.67 (0.39, 1.16) and 0.63 (0.37, 1.07; P for trend = 0.09), and valine intake – 1.00, 0.95 (0.55, 1.61) and 0.73 (0.43, 1.25; P for trend = 0.25). Similarly, higher dietary BCAA intake was not associated with the odds of having abdominal obesity across tertile distribution of BCAA intakes; total BCAA – 1.00, 1.00 (0.61, 1.66) and 0.76 (0.46, 1.26; P for trend = 0.29); isoleucine – 1.00, 1.00 (0.60, 1.66) and 0.74 (0.45, 1.23; P for trend = 0.24); leucine – 1.00, 0.68 (0.41, 1.14) and 0.69 (0.41, 1.13; P for trend = 0.15); and valine – 1.00, 1.16 (0.70, 1.92) and 0.88 (0.53, 1.46 P for trend = 0.60).
The associations between dietary BCAA intakes and odds of general obesity (Table 3) did not vary by age or current alcohol use (P for Interaction = 0.87 and 0.95, respectively). We found a similar association when we limited the analysis to participants without a history of diabetes or hypertension. However, higher energy-adjusted total BCAA intake was associated with lower odds of obesity in a subset of non-smoking Filipino women only; ORs (95%CI) were 1.00, 0.68 (0.39, 1.20) and 0.55 (0.31, 0.98; P for trend = 0.04). The associations between dietary BCAA intakes and odds of abdominal obesity were different by smoking status (P for Interaction = 0.04), but not by age or current alcohol use (P for Interaction = 0.30 and 0.35, respectively) (Table 4). Although the association between dietary BCAA intake and abdominal obesity was not statistically significant, there was a suggestive inverse association in a subset of non-smoking Filipino women; ORs (95% CIs) were 1.00, 0.85 (0.50, 1.43) and 0.64 (0.38, 1.09; P for trend = 0.10).
Discussion
In this study, we found an inverse relationship between higher dietary BCAA intake and odds of obesity among non-smokers, but a suggestive inverse association in the overall sample of this study. Our findings add to the body of literature on the interaction of smoking status in the relationship of dietary BCAA intake and adiposity, accounting for evidence in the context of populations likely experiencing changes in diet pattern and eating behaviour due to migration.
The significance of dietary BCAA intake and odds of adiposity has been tested in some epidemiological reports [12,13,14,15, 28, 29], but not in a sample of a population experiencing a change in diet patterns [30,31,32]. For example, higher dietary BCAA intakes were associated with lower odds of obesity in the INTERMAP population-based survey from China [15]. Similarly, higher dietary BCAA intake was inversely related to the prevalence of overweight and adiposity-related metabolites independent of genetic differences in another population-based survey from the United Kingdom [12]. Because BCAA are derived from diets, the overall dietary exposure should be considered in the relationship of dietary BCAA with obesity. In tandem with this observation, some reports have established that the relationship between dietary BCAA intake and diabetes mellitus was primarily within the context of dietary patterns [16, 33]. To date, few studies have tested the interaction effect of history of disease on the relationship between dietary BCAA intake and obesity. Our study did not have a sufficient number of participants with a history of diabetes or hypertension in subgroup analyses. A meta-analysis has demonstrated that the odds of dietary BCAA relationship differ with obesity and diabetes mellitus. In that report, higher dietary BCAA intake was associated with lower and higher odds of obesity and diabetes mellitus, respectively [34].
There are several plausible mechanisms for the inverse association between higher dietary BCAA intakes and odds of obesity. First, some intervention trials have observed a modest reduction in body weight and fat after BCAA supplementation in a clinical trial [35, 36]. BCAA intake may have impacted body weight through the down-regulation of lipogenic factors and improved insulin sensitivities. Poor insulin sensitivities have been linked with obesity, but BCAA intake/supplementation has been linked with improved insulin sensitivities [12], maintenance of lean body [37] and in some cases, modest weight loss [36]. Also, animal trials have demonstrated that increased dietary BCAA improved glucose and lipid metabolism [38, 39] by upregulating the expression of peroxisome proliferator-activated receptor-alpha to prevent diet-induced obesity in mice models [39]. Second, higher BCAA intake may likely promote lower body weight by increasing circulating leptin concentration to reduce appetite and regulate energy metabolism. Rats fed with leucine-enriched diet were found to have 40% decrease in leptin levels [40]. Exogenous leptin administration has been reported to down-regulate appetite, and consequently decrease body weight in animal models [41].
Furthermore, levels of dietary BCAA intake in this sample of immigrant Filipino women were lower than the indigenous Korean population [42, 43] and other populations [17, 33, 44]. There are currently no local studies among indigenous Filipinos in the Philippines to compare our findings. Our study has several strengths; it is the first epidemiological report on dietary BCAA and obesity among an immigrant population likely to be experiencing changes in diet pattern and eating behaviour. Our report observed a potential interaction of smoking status in the relationship between dietary BCAA intake and obesity. The multivariate adjustment for potential confounding alludes to the reliability of our findings. However, a causal relationship cannot be inferred because of the cross-sectional nature of the study design. The generalizability of our findings is likely to be limited, given that convenient sampling was adopted for participant recruitment. Dietary information using 24-h dietary recall in a single time point may not represent overall dietary exposure, and residual confounding or unmeasured factors are potential factors to consider in examining our results. Longitudinal studies from diverse ethnic backgrounds are necessary to clarify the association of dietary BCAA intake with obesity. Future studies should consider the moderating effects of lifestyle factors and disease history to broaden the scientific understanding of this subject. Also, discerning the contributions of the whole spectrum of dietary exposure could fill gaps on the significance of dietary BCAA in the aetiology of obesity and disease outcomes.
Conclusions
Higher dietary BCAA intakes appeared to exert a suggestive inverse association with odds of obesity in this sample of immigrant Filipino women, and smoking status may modify the observed inverse relationship.
Availability of data and materials
The data for this study cannot be made publicly available because FiLWHEL is an ongoing study, and during the signing of consent, the participants were not informed that their information would be stored in a publicly accessible database. However, other researchers are welcome to collaborate with the study team under approval procedures posted on the study website (www.filwhel.org). Requests to access the data may be sent to the data access committee (nutepid@gmail.com). Professors Jung Eun Lee (jungelee@snu.ac.kr) and Chang Beom Lee (lekang@hanyang.ac.kr) have access to the entire dataset.
Abbreviations
- BCAA:
-
Branched-chain amino acids
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- WC:
-
Waist circumference
- BMI:
-
Body mass index
References
Obesity: its causes and treatment. Hospital (Lond 1886). 1891;11(265):46.
van Vliet-Ostaptchouk JV, Snieder H, Lagou V. Gene–Lifestyle Interactions in Obesity. Curr Nutr Rep. 2012;1(3):184–96.
Heymsfield SB, Wadden TA. Mechanisms, pathophysiology, and Management of Obesity. N Engl J Med. 2017;376(3):254–66.
Collaborators GBDO, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
Okekunle AP, Li Y, Liu L, Du S, Wu X, Chen Y, et al. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res Clin Pract. 2017;132:45–58.
Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26.
Shimomura Y, Kitaura Y, Kadota Y, Ishikawa T, Kondo Y, Xu M, et al. Novel physiological functions of branched-chain amino acids. J Nutr Sci Vitaminol (Tokyo). 2015;61(Suppl):S112–4.
Jung MK, Okekunle AP, Lee JE, Sung MK, Lim YJ. Role of branched-chain amino acid metabolism in tumor development and progression. J Cancer Prev. 2021;26(4):237–43.
Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17.
Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54.
Green CR, Wallace M, Divakaruni AS, Phillips SA, Murphy AN, Ciaraldi TP, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15–21.
Jennings A, MacGregor A, Pallister T, Spector T, Cassidy A. Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: a twin study. Int J Cardiol. 2016;223:992–8.
Cogate PG, Natali AJ, de Oliveira A, Alfenas RC, Hermsdorff HH. Consumption of branched-chain amino acids is inversely associated with central obesity and Cardiometabolic features in a population of Brazilian middle-aged men: potential role of leucine intake. J Nutr Health Aging. 2015;19(7):771-7.
Li Y-C, Li Y, Liu L-Y, Chen Y, Zi T-Q, Du S-S, et al. The ratio of dietary branched-chain amino acids is associated with a lower prevalence of obesity in young northern Chinese adults: An internet-based cross-sectional study. Nutrients. 2015;7(11):9573–89.
Qin LQ, Xun P, Bujnowski D, Daviglus ML, Van Horn L, Stamler J, et al. Higher branched-chain amino acid intake is associated with a lower prevalence of being overweight or obese in middle-aged east Asian and Western adults. J Nutr. 2011;141(2):249–54.
Isanejad M, LaCroix AZ, Thomson CA, Tinker L, Larson JC, Qi Q, et al. Branched-chain amino acid, meat intake and risk of type 2 diabetes in the Women's Health Initiative. Br J Nutr. 2017;117(11):1523–30.
Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. 2016.
Abris GP, Hong S, Provido SMP, Lee JE, Lee CB. Filipino women's diet and health study (FiLWHEL): design and methods. Nutr Res Pract. 2017;11(1):70–5.
Abris GP, Kim N-H, Provido SMP, Hong S, Yu SH, Lee CB, et al. Dietary diversity and nutritional adequacy among married Filipino immigrant women: the Filipino Women’s diet and health study (FiLWHEL). BMC Public Health. 2018;18(1):359.
Abris GP, Provido SMP, Hong S, Yu SH, Lee CB, Lee JE. Association between dietary diversity and obesity in the Filipino Women’s diet and health study (FiLWHEL): a cross-sectional study. PLoS One. 2018;13(11):e0206490.
Provido SMP, Abris GP, Hong S, Yu SH, Lee CB, Lee JE. Association of fried food intake with prehypertension and hypertension: the Filipino women's diet and health study. Nutr Res Pract. 2020;14(1):76–84.
Korean Nutrition Society (KNS). Computer-Aided Nutrition Analysis Program 4.0 (CAN-Pro 4.0). Release Date: November 2011. KNS, Seoul 2011. http://kns.or.kr/Center/CanPro.asp.
Food and Nutrition Research Institute (FNRI). The Philippine food composition tables. Philippines: FNRI; 1997.
Lim S-H, Kim J-B, Cho Y-S, Choi Y, Park H-J, Kim S-N. National Standard Food Composition Tables Provide the Infrastructure for Food and Nutrition Research According to Policy and Industry. Korean J Food & Nutr. 2013;26(4):886–94. https://doi.org/10.9799/ksfan.2013.26.4.886.
U.S. Department of Agriculture (USDA). FoodData Central: USDA; 2019 [updated October 2020. Available from: fdc.nal.usda.gov.
World Health Organization. The Asia-Pacific perspective: redefining obesity and its treatment: regional Office of the Western Pacific (WPRO). World Health Organization. (ISBN: 0-9577082-1-1) http://apps.who.int/iris/bitstream/10665/206936/1/0957708211_eng.pdf.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65(4):1220S–8S.
Lu J, Gu Y, Liu H, Wang L, Li W, Li W, et al. Daily branched-chain amino acid intake and risks of obesity and insulin resistance in children: a cross-sectional study. Obesity. 2020;28(7):1310–6.
Okekunle AP, Wu X, Feng R, Li Y, Sun C. Higher intakes of energy-adjusted dietary amino acids are inversely associated with obesity risk. Amino Acids. 2018.
Chen C, Chaudhary A, Mathys A. Dietary change scenarios and implications for environmental, nutrition, human health and economic dimensions of food sustainability. Nutrients. 2019;11(4):856.
Kris-Etherton PM, Petersen KS, Velarde G, Barnard ND, Miller M, Ros E, et al. Barriers, Opportunities, and Challenges in Addressing Disparities in Diet‐Related Cardiovascular Disease in the United States. J Am Heart Assoc. 2020;9(7):e014433.
Zhang J, Wang Z, Du W, Huang F, Jiang H, Bai J, et al. Twenty-five-year trends in dietary patterns among Chinese adults from 1991 to 2015. Nutrients. 2021;13(4):1327.
Okekunle AP, Wu X, Duan W, Feng R, Li Y, Sun C. Dietary intakes of branched-chained amino acid and risk for type 2 diabetes in adults: the Harbin cohort study on diet, nutrition and chronic non-communicable diseases study. Can J Diabetes. 2018;42(5):484–92.e7.
Okekunle AP, Zhang M, Wang Z, Onwuka JU, Wu X, Feng R, et al. Dietary branched-chain amino acids intake exhibited a different relationship with type 2 diabetes and obesity risk: a meta-analysis. Acta Diabetol. 2019;56(2):187–95.
Layman DK. The role of leucine in weight loss diets and glucose homeostasis. J Nutr. 2003;133(1):261S–7S.
Piccolo BD, Comerford KB, Karakas SE, Knotts TA, Fiehn O, Adams SH. Whey protein supplementation does not alter plasma branched-chained amino acid profiles but results in unique metabolomics patterns in obese women enrolled in an 8-week weight loss trial. J Nutr. 2015;145(4):691–700.
Dudgeon WD, Kelley EP, Scheett TP. In a single-blind, matched group design: branched-chain amino acid supplementation and resistance training maintains lean body mass during a caloric restricted diet. J Int Soc Sports Nutr. 2016;13:1.
Zhang Y, Guo K, LeBlanc RE, Loh D, Schwartz GJ, Yu YH. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes. 2007;56(6):1647–54.
Nishimura J, Masaki T, Arakawa M, Seike M, Yoshimatsu H. Isoleucine prevents the accumulation of tissue triglycerides and upregulates the expression of PPARalpha and uncoupling protein in diet-induced obese mice. J Nutr. 2010;140(3):496–500.
Lynch CJ, Gern B, Lloyd C, Hutson SM, Eicher R, Vary TC. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am J Physiol-Endocrinol Metab. 2006;291(3):E621–E30.
Dhillon H, Kalra SP, Kalra PS. Dose-dependent effects of central leptin gene therapy on genes that regulate body weight and appetite in the hypothalamus. Mol Ther. 2001;4(2):139–45.
Chae M, Park HS, Park K. Association between dietary branched-chain amino acid intake and skeletal muscle mass index among Korean adults: interaction with obesity. Nutr Res Pract. 2021;15.
Chae M, Park H, Park K. Estimation of dietary amino acid intake and independent correlates of skeletal muscle mass index among Korean adults. Nutrients. 2020;12(4):1043.
Nagata C, Nakamura K, Wada K, Tsuji M, Tamai Y, Kawachi T. Branched-chain amino acid intake and the risk of diabetes in a Japanese community: the Takayama study. Am J Epidemiol. 2013;178(8):1226–32.
Acknowledgements
The authors are grateful to all volunteers for participating in this study.
Funding
This research work was supported by the Brain Pool Program through the National Research Foundation of Korea, funded by the Ministry of Science and ICT (2020H1D3A1A04081265), the Hanmi Pharmaceutical Co., Ltd., (No. 201300000001270) and Chong Kun Dang Pharm. Seoul, Korea, (No. 201600000000225). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
APO conceptualized and designed the study; HJ and SMPP conducted the data acquisition and curation; APO conducted analysis; SH, GHC, SHY contributed to the interpretation; HJ contributed to the data analysis and interpretation; APO drafted the manuscript; CBL and JEL critically revised the manuscript for important intellectual content. All authors read and approved the final version to be published and agreed to be accountable for the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Institutional Review Board (IRB) of Sookmyung Women’s University, Korea, approved this study (SMWU-1311-BR-012), and all participants provided written informed consent before participating in the study. All procedures performed in this study were in accordance with the ethical standards of the IRB of Sookmyung Women’s University, Korea and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
All participants provided consent to publish their data, and all authors approved the final manuscript for publication.
Competing interests
The authors declare they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Okekunle, A.P., Lee, H., Provido, S.M.P. et al. Dietary branched-chain amino acids and odds of obesity among immigrant Filipino women: the Filipino women’s diet and health study (FiLWHEL). BMC Public Health 22, 654 (2022). https://doi.org/10.1186/s12889-022-12863-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-022-12863-0