Abstract
Background
People with epilepsy (PWE) frequently experience sleep disturbances that can severely affect their quality of life. Depression is also a common symptom in the PWE population and can aggravate sleep problems. However, the interplay between epilepsy, depression, and sleep disturbances is not yet fully understood. Our study was designed to investigate the association between epilepsy and sleep disturbances in US adults and to determine whether depressive symptoms play a mediating role in this relationship.
Methods
We examined data from the National Health and Nutrition Examination Survey (NHANES) spanning January 1, 2015, to March 2020, before the pandemic.A total of 10,093 participants aged ≥ 20 years with complete data on epilepsy and sleep disturbance were included. Weighted multiple logistic regression and mediation analysis were used to explore the associations among depression, epilepsy, and sleep disturbance. Interaction effects of epilepsy with various covariates were also investigated.
Results
Epilepsy was associated with depression and sleep disturbances. Weighted logistic regression analysis revealed a significant association between epilepsy and sleep disturbances (OR = 3.67, 95% CI = 1.68–8.04). Depression partially mediated this relationship, demonstrating a mediation effect of 23.0% (indirect effect = 0.037, P < 0.001). Subgroup analyses revealed variations in the relationship between epilepsy and sleep disturbances among different groups. Furthermore, interaction analyses revealed significant interactions between epilepsy and age (P = 0.049) and hypertension (P = 0.045).
Conclusions
Our study utilizing NHANES data confirmed that depression partially mediated the association between epilepsy and sleep disturbance. Additionally, we observed differences in this association across demographic groups. Addressing depressive symptoms in PWE may improve their sleep quality, but further research is needed to explore the underlying mechanisms.
Similar content being viewed by others
Introduction
Sleep disturbances frequently present serious challenges for individuals diagnosed with epilepsy. Earlier studies indicate that people with epilepsy (PWE) experience twice the frequency of sleep disturbances compared to their healthy counterparts [1, 2]. These disruptions primarily encompass difficulties in the onset and maintenance of sleep [2], regular nighttime awakenings, and episodes of obstructive sleep apnea [3]. Consequently, PWE often experience fatigue, cognitive impairments, and increased daytime sleepiness, compounding the difficulties they encounter in managing their health [4, 5].
Numerous studies have investigated the possible pathophysiological mechanisms contributing to sleep disturbances in this population. It is widely recognized that multiple contributors may influence the onset and development of sleep disturbances, including alterations in neurotransmitter systems, neuroendocrine dysfunction, and changes in sleep architecture [6]. Additionally, the adverse effects of antiepileptic medications [7], frequent nocturnal seizures [8], poor sleep hygiene habits, and psychological factors may worsen sleep issues in PWE.
Depression frequently coexists with epilepsy, affecting more than one-third of PWE [9]. A study conducted in China reported that 28.8% of PWE suffer from depression [10]. Fiest et al., in a meta-analysis, found that depression rates in PWE are 23.1% [11]. Hospitalized PWE exhibit an even higher depression rate, exceeding 50%, compared to approximately 8% among patients in remission [12]. The combination of social isolation, dysfunction, and inappropriate use of antiepileptic medications has the potential to trigger depressive symptoms [13]. Population-based studies suggest that individuals with prior depression have an elevated likelihood of developing epilepsy, ranging from four to seven times the risk compared to the broader population [14]. The comorbidity of epilepsy and depression is often caused or induced by a combination of pathological mechanisms, such as neurotransmitter imbalance, hippocampal dysfunction, and endocrine dysregulation [15, 16].
Notably, sleep disturbances and symptoms of depression often co-occur and exhibit patterns of interaction and influence [17]. Depressed individuals commonly suffer from a variety of sleep issues, including insomnia, excessive daytime sleepiness, and disrupted sleep-wake patterns [18]. Sleep disturbances can also serve as precipitating factors in the onset and progression of depression, exerting adverse effects on emotional regulation, cognitive function, and neuroendocrine homeostasis.
Although depression and sleep disturbances have been extensively discussed in the context of epilepsy, existing studies often isolate the relationship between epilepsy and sleep or between epilepsy and depression. However, this segmented perspective may overlook the potential complex interactions among these three factors. Understanding these interactions is crucial for enhancing management strategies and improving treatment outcomes. Thus, our study aimed to evaluate the association between epilepsy and sleep disturbances among adults in the United States, specifically investigating the potential role of depression as a mediator.
Methods
Study design and participants
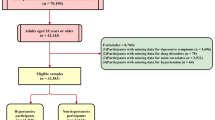
Our study drew on data from the National Health and Nutrition Examination Survey (NHANES), a comprehensive database initiated in 1999, which evaluates the health and nutritional status of the U.S. population [19].To ensure national representativeness, the NHANES employs a continuous multistage probability sampling method. The NHANES study was granted ethical approval by the Research Ethics Review Board of the National Center for Health Statistics, and all participants provided informed consent prior to their involvement. Furthermore, this database is publicly accessible, and no additional ethical or administrative permissions are required. We adhered to NHANES guidelines and regulations in all analyses conducted. We included participants from the NHANES data collected between January 1, 2015, and March 2020 (prepandemic), as this period encompassed the comprehensive set of variables essential for this investigation, including the utilization of prescription medications, sleep assessment, and the Patient Health Questionnaire 9 (PHQ-9). Figure 1 illustrates the diagram of the screening procedure.
Antiepileptic drug use and epilepsy diagnosis
The NHANES survey assessed participants to determine if they had taken any prescription drugs in the past 30 days, querying them on the generic names of the medications, the primary reasons for their use, and any corresponding ICD-10-CM codes provided. Detailed codes and names of antiepileptic drugs are listed in Table S1. PWE were identified by their use of antiepileptic medications and the relevant ICD-10-CM codes [20, 21].
Assessment of sleep disturbance
Sleep parameter data were obtained from the “Sleep Disorders” dataset of the NHANES questionnaire [22]. Participants reported their typical nightly sleep hours on weekdays or workdays. Sleep duration was categorized as short (< 7 h), normal (7–9 h), or long (> 9 h) based on these reports [23]. Participants were also asked whether they had informed a doctor about their sleep difficulties, which was used to assess the presence of trouble sleeping. Table 1 outlines the calculation of a sleep quality score, incorporating sleep duration, trouble sleeping, snoring, snorting, or stopping breathing, and excessive daytime sleepiness as metrics. Scores ranged from 0 to 5, with lower scores indicating poorer sleep quality [24]. A sleep quality score below 2 points indicated the presence of sleep disturbances [25].
Assessment of depression
Depression data were derived from the “Mental Health - Depression Screener” dataset within the NHANES questionnaire. Depression severity was assessed using the PHQ-9 questionnaire, a reliable and validated diagnostic tool designed to evaluate mood disorders based on diagnostic criteria for depression [26]. The questionnaire consists of nine questions, each scored on a scale of 0 to 3, resulting in a total score ranging from 0 to 27. A higher cumulative score signifies increased severity of depression [27, 28].A PHQ-9 total score ≥ 10 is considered indicative of major depressive disorder (MDD) [29].
Potential covariates
Demographic covariates were gathered through household interviews, while health-related covariates were assessed at Mobile Examination Centers (MEC). Age was stratified as < 40, 40–60, and > 60 years. Race categories were defined as Mexican American, non-Hispanic White, non-Hispanic Black, other Hispanic, and other races. Educational attainment was categorized into three groups: less than high school, high school graduate, and beyond high school. Marital status was classified as never married, married or living with a partner, and widowed, divorced, or separated. Poverty income ratio (PIR) was categorized as < 2 (low-income) and ≥ 2 (moderate to high-income).Participants reporting having smoked at least 100 cigarettes in their lifetime were classified as smokers. Hypertension was determined by clinical diagnosis, ongoing treatment with hypertension medication, or blood pressure readings above 140 mmHg for systolic or 90 mmHg for diastolic pressure measured during the physical assessment. Average blood pressure was calculated from up to three readings taken at different times. The presence of diabetes was confirmed using any of the following criteria: (1) hemoglobin A1C levels at or above 6.5%, or fasting blood glucose levels equal to or exceeding 126 mg/dL [30]; (2) physician-diagnosed diabetes, or use of anti-hyperglycemic medication or insulin. Diagnosis of coronary heart disease (CHD) and asthma was determined based on participants’ responses to whether they had ever been diagnosed with these conditions.
Statistical analysis
Given the complex multistage sampling strategy of NHANES, the data were combined and adjusted using the wtmec2 year weighting factor. Baseline characteristics were compared using t-tests for continuous variables and chi-square tests for categorical variables.Continuous data were presented as mean (standard deviation), and categorical data as weighted numbers (weighted percentages). To investigate the association between epilepsy and sleep disturbances, we utilized weighted univariate and multivariate logistic regression analyses. Three models were employed: Model 1, with no covariate adjustments; Model 2, adjusted for age, sex, race, education, marital status, and PIR; and Model 3, which included adjustments for all covariates. Mediation analyses were conducted to explore whether depression mediates the link between epilepsy and sleep disturbance. Subgroup and interaction analyses were employed to investigate associations between epilepsy and various covariates.Findings were presented as odds ratios (ORs) with 95% confidence intervals (CIs). All statistical procedures were conducted using R Studio (version 4.3.2), with a significance level set at P < 0.05.
Results
Population characteristics
Descriptive statistics of the study are detailed in Table 2. Following a sequence of screening processes, 10,093 individuals were selected from the NHANES database (January 1, 2015 - March 2020), representing a sample of 187,353,423 individuals in the general population. Among these individuals, 73 (weighted count 1,002,936) were diagnosed with epilepsy, while 10,020 (weighted count 186,350,487) were not. Compared to the control group, PWE significantly more frequently experienced MDD (18.84% vs. 7.57%, P = 0.003) and sleep disturbances (47.17% vs. 15.48%, P < 0.001). Additionally, there were statistically significant differences in terms of PIR, prevalence of hypertension, diabetes, and asthma, as well as PHQ-9 scores between individuals with and without epilepsy (P < 0.05).
Weighted logistic regression
We performed weighted univariate and multivariate logistic regression analyses to examine the relationship between epilepsy and sleep disturbance. As shown in Table 3; Fig. 2, the fully adjusted model (Model 3) revealed a robust and statistically significant association between epilepsy and sleep disturbance (OR = 3.67, 95% CI = 1.68–8.04). Model 3 also indicated a significant relationship between MDD and the occurrence of sleep disturbances (OR = 3.32, 95% CI = 2.68–4.11).
Mediation analyses
The study evaluated the intermediary role of depression in the relationship between epilepsy and sleep disturbance through a mediation analysis. As depicted in Table 4, mediation effects were observed across all models. Specifically, in Model 1, the mediating impact represented 26.5% of the overall relationship between epilepsy and sleep disturbances (indirect effect = 0.053, 95% CI = 0.007–0.020, P < 0.001). In Model 2, with additional adjustments for age, sex, race, education level, marital status, and PIR, the mediation effect constituted 25.4% of the relationship (indirect effect = 0.049, 95% CI = 0.006–0.020, P < 0.001). With all covariates adjusted, the mediation effect comprised 23.0% of the total relationship (indirect effect = 0.037, 95% CI = 0.004–0.015, P < 0.001).
Stratified analyses and interaction analyses
Subgroup analysis elucidated the heterogeneity in the association between epilepsy and sleep disturbances across various demographic strata (Fig. 3). Notably, substantial disparities were identified among subgroups delineated by sex, age, race, educational level, marital status, PIR, smoking status, and the presence of hypertension, diabetes, CHD, and asthma (all P < 0.05). Specifically, significant differences were detected among males, females, those younger than 40 years, individuals aged 40–60 years, Mexican Americans, non-Hispanic whites, non-Hispanic blacks, individuals with education beyond high school and those with education lower than high school, those who were married or living with a partner, those with a PIR lower than 2, smokers, and those not suffering from hypertension, diabetes, CHD, or asthma. In the analysis of the interaction effects on subgroups, age (P = 0.049) and hypertension (P = 0.045) were found to interact with epilepsy. Participants under 40 years of age (OR = 3.50, 95% CI = 1.17–10.47, P = 0.025) and those aged 40–60 years (OR = 4.22, 95% CI = 1.90–9.34, P < 0.001) had a higher risk of sleep disturbance compared to those over 60 years old. Participants without hypertension (OR = 4.45, 95% CI = 2.05–9.68, P < 0.001) showed a higher risk of sleep disturbance compared to those with hypertension.
Subgroup analysis of the association between epilepsy and sleep disturbance. Eachstratification was adjusted for sex, age, race, education level, marital status, PIR, smoking status, hypertension, diabetes, CHD and asthmaexcept the stratification factor itself. Abbreviations: PIR, poverty income ratio; CHD, coronary heart disease
Discussion
Our research investigated the connection between epilepsy and sleep disturbances using NHANES data. After adjusting for all confounders, we revealed a higher prevalence of sleep disturbance in PWE, which was consistent with prior research [31, 32]. Furthermore, mediation models highlighted that depression was significantly correlated with sleep disturbances in the adult American population.
A multitude of studies have documented a significant link between depressive states and disruptions in sleep [33]. Lin et al. identified several shared genetic variations, including MEIS1, OLFM4, and HEXIM1, that link MDD with insomnia [34]. Zhao et al. conducted research on 3,275 MDD patients at 32 different sub-centers, uncovering that more than 70% suffered from sleep disturbances [35]. Our data reaffirmed this association. In a retrospective and prospective collaborative study, it was found that higher depression scores could predict sleep disturbances in PWE [36]. Some researchers believe that depression, rather than epilepsy itself, is the main cause of sleep disturbances [37], which contradicts our findings. We identified epilepsy as an important contributing factor to sleep disturbances, with depression mediating approximately 23.0–26.5% of the relationship between epilepsy and sleep disturbances across various mediation models.
Epilepsy patients are at an elevated risk of experiencing sleep disturbances. Due to the considerable risks posed by comorbid conditions, extensive research has been conducted into the causes of the concurrent presence of epilepsy and sleep disorders, as well as the exploration of preventive measures [38]. Our study revealed that the prevalence of sleep disturbances among epilepsy patients was strikingly high at 47.17%, significantly exceeding that observed in the control group. A cross-sectional study indicated that 50.4% of epilepsy patients reported suffering from insomnia [39]. In a meta-analysis encompassing 25 original studies, Bergmann et al. revealed that epilepsy patients had notably higher Pittsburgh Sleep Quality Index scores than their counterparts [40]. Epileptic seizures often manifest during sleep, and recurrent episodes can disrupt sleep microstructure, hindering the progression from light to deep sleep stages, thus impacting both the quality and duration of sleep [41]. Certain antiepileptic drugs such as barbiturates and phenytoin may directly alter sleep patterns, induce drowsiness, reduce sleep quality, or trigger other related issues [42, 43].
Through subgroup analysis and interaction tests, we identified significant interactions between age, hypertension, and epilepsy regarding sleep disturbances. Our study findings indicate that epilepsy significantly increases the risk of sleep disturbance in individuals aged ≤ 60 years, whereas the association is not evident in individuals aged > 60 years. Possible explanations include age-related alterations in sleep architecture, coupled with the influence of physiological changes and comorbid chronic conditions [44, 45].It is important to recognize that although our findings reveal that the likelihood of sleep disturbances in epilepsy patients without hypertension is 4.45 times higher than in patients without epilepsy, this association is weakened among patients with hypertension. One must be cautious in interpreting these observations. Certain antihypertensive medications, including Alpha-2 agonists, can regulate the sleep structure of patients [46]. Additionally, hypertension often coexists with cardiovascular and cerebrovascular diseases [47, 48], obesity [49], and multiple comorbidities [50], potentially contributing to an elevated prevalence of sleep disturbances among hypertensive individuals, irrespective of epilepsy diagnosis. Moreover, elevated blood pressure can result in compromised regulation of cerebral blood flow [51] and perturb the equilibrium of neurotransmitters in the brain, thereby disrupting typical sleep patterns. Hence, the link between epilepsy, hypertension, and sleep disturbances may be attributed to a shared pathophysiological mechanism rather than a direct causal link.
Our study utilized a large dataset representing a national population sample to confirm that depression is a significant factor contributing to sleep disturbances in PWE. Additionally, we investigated the effects of multiple factors such as age, racial background, and the presence of hypertension. Disturbances in sleep can profoundly affect the well-being of individuals with epilepsy and have been associated with a wide range of health concerns, as indicated by prior research [52, 53]. Addressing depressive symptoms may lead to improvements in sleep quality and, consequently, promote the overall health and well-being of individuals in this patient population.
Despite the findings, our research is subject to constraints. Primarily, the cross-sectional design prevents the determination of cause-and-effect relationships between epilepsy, depressive symptoms, and sleep disturbances. Furthermore, our selection criteria, based on pre-existing data, may not fully represent the entire epilepsy patient population. Some individuals may not have undergone standardized antiepileptic medication therapy, and some patients with a history of epilepsy did not take antiepileptic medications within the last 30 days of the survey. Additionally, the study did not consider the impact of antiepileptic drugs on sleep and depression, nor did it include key variables such as epilepsy duration, types of seizures, and seizure frequency, which were absent due to insufficient NHANES data. Another limitation arises from our reliance on self-reported assessments of sleep disturbances, lacking objective measures such as actigraphy or polysomnography, potentially introducing reporting bias. Furthermore, the use of the PHQ-9 for diagnosing depression lacks clinical confirmation. These limitations should be considered and addressed in future research.
Conclusion
This investigation reveals a strong correlation between epilepsy and the occurrence of sleep disturbances. The role of depression as a key factor in this correlation underscores the intricate links between epilepsy, psychological health, and sleep disturbances. Despite these insights, additional research is necessary to determine causal relationships and to explore the potential mechanisms. Furthermore, expanded prospective studies are required to confirm these observations and to guide the development of more precise strategies for enhancing sleep and overall health in the epileptic population.
Data availability
Additional details about the NHANES database are available on the official website at www.cdc.gov/nchs/nhanes/.
Abbreviations
- PWE:
-
People with epilepsy
- MDD:
-
Major depressive disorder
- PHQ-9:
-
Patient Health Questionnaire-9
- PIR:
-
Poverty income ratio
- CHD:
-
Coronary heart disease
- NHANES:
-
National health and nutrition examination survey
- MEC:
-
Mobile examination center
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ACME:
-
Average causal mediation effects
- ADE:
-
Average direct effects
- PM:
-
Proportion mediated
References
van Golde EG, Gutter T, de Weerd AW. Sleep disturbances in people with epilepsy; prevalence, impact and treatment. Sleep Med Rev. 2011;15(6):357–68.
Vendrame M, Yang B, Jackson S, Auerbach SH. Insomnia and epilepsy: a questionnaire-based study. J Clin Sleep Med. 2013;9(2):141–6.
Jain SV, Simakajornboon N, Glauser TA. Provider practices impact adequate diagnosis of sleep disorders in children with epilepsy. J Child Neurol. 2013;28(5):589–95.
Quigg M, Gharai S, Ruland J, Schroeder C, Hodges M, Ingersoll KS, Thorndike FP, Yan G, Ritterband LM. Insomnia in epilepsy is associated with continuing seizures and worse quality of life. Epilepsy Res. 2016;122:91–6.
Giorelli AS, Neves GS, Venturi M, Pontes IM, Valois A, Gomes Mda M. Excessive daytime sleepiness in patients with epilepsy: a subjective evaluation. Epilepsy Behav. 2011;21(4):449–52.
Bazil CW. Epilepsy and sleep disturbance. Epilepsy Behav. 2003;4(Suppl 2):S39–45.
Toledo M, Gonzalez-Cuevas M, Miró-Lladó J, Molins-Albanell A, Falip M, Martinez AB, Fernandez S, Quintana M, Cambrodi R, Santamarina E, et al. Sleep quality and daytime sleepiness in patients treated with adjunctive perampanel for focal seizures. Epilepsy Behav. 2016;63:57–62.
Tezer FI, Rémi J, Erbil N, Noachtar S, Saygi S. A reduction of sleep spindles heralds seizures in focal epilepsy. Clin Neurophysiol. 2014;125(11):2207–11.
Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, Caplan R, et al. Depression and Epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav. 2012;24(2):156–68.
Song H, Zhao Y, Hu C, Zhao C, Wang X, Xiao Z. Relationships among anxiety, depression, and health-related quality of life in adult epilepsy: a network analysis. Epilepsy Behav. 2024;154:109748.
Fiest KM, Dykeman J, Patten SB, Wiebe S, Kaplan GG, Maxwell CJ, Bulloch AG, Jette N. Depression in Epilepsy: a systematic review and meta-analysis. Neurology. 2013;80(6):590–9.
Piedad J, Rickards H, Besag FM, Cavanna AE. Beneficial and adverse psychotropic effects of antiepileptic drugs in patients with epilepsy: a summary of prevalence, underlying mechanisms and data limitations. CNS Drugs. 2012;26(4):319–35.
Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016;12(2):106–16.
Hesdorffer DC, Lúdvígsson P, Hauser WA, Olafsson E, Kjartansson O. Co-occurrence of major depression or suicide attempt with migraine with aura and risk for unprovoked seizure. Epilepsy Res. 2007;75(2–3):220–3.
Singh T, Goel RK. Epilepsy Associated Depression: an update on current scenario, suggested mechanisms, and opportunities. Neurochem Res. 2021;46(6):1305–21.
Kanner AM. Psychiatric comorbidities and epilepsy: is it the old story of the chicken and the egg? Ann Neurol. 2012;72(2):153–5.
Wen W, Zhou J, Zhan C, Wang J. Microglia as a Game Changer in Epilepsy Comorbid Depression. Mol Neurobiol 2023.
Yan T, Qiu Y, Yu X, Yang L. Glymphatic dysfunction: a Bridge between Sleep Disturbance and Mood disorders. Front Psychiatry. 2021;12:658340.
The National Health and Nutrition Examination Survey. https://www.cdc.gov/nchs/nhanes/index. htm.
Terman SW, Hill CE, Burke JF. Disability in people with epilepsy: a nationally representative cross-sectional study. Epilepsy Behav. 2020;112:107429.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2017–2018 Prescription Medications Data. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/RXQ_RX_J.htm
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2017–2018 Sleep Disorder Data.https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/SLQ_J.htm
Li C, Shang S. Relationship between Sleep and Hypertension: findings from the NHANES (2007–2014). Int J Environ Res Public Health. 2021;18(15):7867.
Zhu F, Liu B, Kuang D, Zhu X, Bi X, Song Y, Quan T, Yang Y, Ren Y. The association between physical activity and sleep in adult ADHD patients with stimulant medication use. Front Psychiatry. 2023;14:1236636.
Li Y, Tan S. Sleep factors were associated with a higher risk of MAFLD and significant fibrosis. Sleep Breath. 2024;28(3):1381–91.
Artom M, Czuber-Dochan W, Sturt J, Norton C. Cognitive behavioural therapy for the management of inflammatory bowel disease-fatigue with a nested qualitative element: study protocol for a randomised controlled trial. Trials. 2017;18(1):213.
Yuan L, Zhu L, Chen F, Cheng Q, Yang Q, Zhou ZZ, Zhu Y, Wu Y, Zhou Y, Zha X. A survey of psychological responses during the Coronavirus Disease 2019 (COVID-19) epidemic among Chinese police officers in Wuhu. Risk Manag Healthc Policy. 2020;13:2689–97.
Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey. 2017–2018 Depression Data. https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DPQ_J.htm
Manea L, Gilbody S, McMillan D. Optimal cut-off score for diagnosing depression with the Patient Health Questionnaire (PHQ-9): a meta-analysis. CMAJ. 2012;184(3):E191–196.
Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988–2012. JAMA. 2015;314(10):1021–9.
Winsor AA, Richards C, Bissell S, Seri S, Liew A, Bagshaw AP. Sleep disruption in children and adolescents with epilepsy: a systematic review and meta-analysis. Sleep Med Rev. 2021;57:101416.
Fonseca E, Campos Blanco DM, Castro Vilanova MD, Garamendi Í, Gómez-Eguilaz M, Pérez Díaz H, Poza JJ, Querol-Pascual MR, Quiroga-Subirana P, Rodríguez-Osorio X, et al. Relationship between sleep quality and cognitive performance in patients with epilepsy. Epilepsy Behav. 2021;122:108127.
Khachatryan SG, Vardanyan LV, Stepanyan TA, Tunyan YS. [Impact of sleep disorders on quality of life in epilepsy]. Volume 120. Zh Nevrol Psikhiatr Im S S Korsakova; 2020. pp. 24–8. 5.
Lin YS, Wang CC, Chen CY. GWAS Meta-Analysis reveals Shared genes and Biological pathways between Major Depressive Disorder and Insomnia. Genes (Basel) 2021, 12(10).
Zhao J, Liu H, Wu Z, Wang Y, Cao T, Lyu D, Huang Q, Wu Z, Zhu Y, Wu X, et al. Clinical features of the patients with major depressive disorder co-occurring insomnia and hypersomnia symptoms: a report of NSSD study. Sleep Med. 2021;81:375–81.
Moser D, Pablik E, Aull-Watschinger S, Pataraia E, Wöber C, Seidel S. Depressive symptoms predict the quality of sleep in patients with partial epilepsy–A combined retrospective and prospective study. Epilepsy Behav. 2015;47:104–10.
Karapinar E, YunusoĞlu C, Tekin B, Dede H, Bebek N, Baykan B, GÜrses C. Depression is a major determinant of sleep abnormalities in patients with epilepsy. Arq Neuropsiquiatr. 2020;78(12):772–7.
Nobili L, Beniczky S, Eriksson SH, Romigi A, Ryvlin P, Toledo M, Rosenzweig I. Expert Opinion: managing sleep disturbances in people with epilepsy. Epilepsy Behav. 2021;124:108341.
Planas-Ballvé A, Grau-López L, Jiménez M, Ciurans J, Fumanal A, Becerra JL. Insomnia and poor sleep quality are associated with poor seizure control in patients with epilepsy. Neurologia (Engl Ed). 2022;37(8):639–46.
Bergmann M, Tschiderer L, Stefani A, Heidbreder A, Willeit P, Högl B. Sleep quality and daytime sleepiness in epilepsy: systematic review and meta-analysis of 25 studies including 8,196 individuals. Sleep Med Rev. 2021;57:101466.
Adiga D, Gupta A, Khanna M, Taly AB, Thennarasu K. Sleep disorders in children with cerebral palsy and its correlation with sleep disturbance in primary caregivers and other associated factors. Ann Indian Acad Neurol. 2014;17(4):473–6.
Jain SV, Glauser TA. Effects of Epilepsy treatments on sleep architecture and daytime sleepiness: an evidence-based review of objective sleep metrics. Epilepsia. 2014;55(1):26–37.
Nayak CS, Sinha S, Nagappa M, Thennarasu K, Taly AB. Effect of carbamazepine on the sleep microstructure of temporal lobe epilepsy patients: a cyclic alternating pattern-based study. Sleep Med. 2016;27–28:80–5.
Ryden AM, Martin JL, Matsuwaka S, Fung CH, Dzierzewski JM, Song Y, Mitchell MN, Fiorentino L, Josephson KR, Jouldjian S, et al. Insomnia disorder among older veterans: results of a Postal Survey. J Clin Sleep Med. 2019;15(4):543–51.
Jaqua EE, Hanna M, Labib W, Moore C, Matossian V. Common Sleep disorders affecting older adults. Perm J. 2023;27(1):122–32.
Hernández-Aceituno A, Guallar-Castillón P, García-Esquinas E, Rodríguez-Artalejo F, Banegas JR. Association between sleep characteristics and antihypertensive treatment in older adults. Geriatr Gerontol Int. 2019;19(6):537–40.
He L, Fan C, Li G. The relationship between serum C-reactive protein and senile hypertension. BMC Cardiovasc Disord. 2022;22(1):500.
Cai N, Li C, Gu X, Zeng W, Zhong J, Liu J, Zeng G, Zhu J, Hong H. CYP2C19 loss-of-function is associated with increased risk of hypertension in a Hakka population: a case-control study. BMC Cardiovasc Disord. 2023;23(1):185.
Weng C, Shen Z, Li X, Jiang W, Peng L, Yuan H, Yang K, Wang J. Effects of chemerin/CMKLR1 in obesity-induced hypertension and potential mechanism. Am J Transl Res. 2017;9(6):3096–104.
Li N, Lin M, Heizhati M, Wang L, Luo Q, Li Y, Yili J, Hong J, Yao X, Zhu Q. Effect of spironolactone on cardiovascular morbidity and mortality in patients with hypertension and glucose metabolism disorders (ESCAM): a study protocol for a pragmatic randomised controlled trial. BMJ Open. 2020;10(11):e038694.
Gewirtz AN, Gao V, Parauda SC, Robbins MS. Posterior reversible Encephalopathy Syndrome. Curr Pain Headache Rep. 2021;25(3):19.
Goyal M, Mishra P, Jaseja H. Obstructive sleep apnea and epilepsy: understanding the pathophysiology of the comorbidity. Int J Physiol Pathophysiol Pharmacol. 2023;15(4):105–14.
Gutter T, Callenbach PMC, Brouwer OF, de Weerd AW. Prevalence of sleep disturbances in people with epilepsy and the impact on quality of life: a survey in secondary care. Seizure. 2019;69:298–303.
Acknowledgements
We extend our appreciation to the team at the National Center for Health Statistics for their efforts in planning, gathering, and organizing the NHANES data, as well as for establishing the public database.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Qianhui Wen conceptualized the research, conducted the preliminary analysis, and drafted the initial manuscript. Qian Wang reviewed and revised the manuscript. Hua Yang was responsible for the study supervision and data collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance for this study was obtained from the National Center for Health Statistics Ethics Review Committee, and all methods were conducted in line with the principles outlined in the Declaration of Helsinki. Prior to their involvement, participants gave their voluntary and informed consent in written form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, Q., Wang, Q. & Yang, H. The association between epilepsy and sleep disturbance in US adults: the mediating effect of depression. BMC Public Health 24, 2412 (2024). https://doi.org/10.1186/s12889-024-19898-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-024-19898-5