Abstract
Background
This study aimed to investigate the radiological changes in patients with nontuberculous mycobacterial pulmonary disease (NTM-PD) having bronchiolitis patterns on computed tomography (CT).
Methods
We retrospectively reviewed the final diagnosis and radiologic changes of patients suspected of having NTM-PD without cavity or bronchiectasis on CT image, between January 1, 2005 and March 31, 2021. NTM-PD was diagnosed based on the American Thoracic Society and Infectious Diseases Society of America criteria. The initial and final CT findings (bronchiectasis, cellular bronchiolitis, cavity formation, nodules, and consolidation) were compared between patients diagnosed with and without NTM-PD.
Results
This study included 96 patients and 515 CT images. The median CT follow-up duration was 1510.5 (interquartile range: 862.2–3005) days. NTM-PD was recognized in 43 patients. The clinical variables were not significantly different between patients with and without NTM-PD, except for underlying chronic airway disease (P < 0.001). Nodule and consolidation were more frequently observed on the initial CT scans of patients with NTM-PD compared with those without (P < 0.05). On the final follow-up CT scan, bronchiectasis (P < 0.001), cavity (P < 0.05), nodule (P < 0.05), and consolidation (P < 0.05) were more frequently observed in patients with NTM-PD. Among the 43 patients with NTM-PD, 30 showed a radiological progression on CT, with bronchiectasis (n = 22) being the most common finding. The incidence of bronchiectasis increased over time.
Conclusion
The bronchiolitis pattern on CT images of patients with NTM-PD showed frequent radiological progression during the follow-up period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Nontuberculous mycobacteria (NTM) are ubiquitous organisms found in water, soil, and soil dust [1]. NTM can cause infections of the lungs, sinuses, lymph nodes, and central nervous system in immunocompetent patients, and disseminated disease in patients with innate or acquired immunodeficiency [2, 3]. Among the many organs in which NTM can cause infection, the lungs are the most commonly affected [4]. Recent studies have shown that the incidence and prevalence of nontuberculous mycobacterial pulmonary disease (NTM-PD) are increasing globally [5,6,7,8,9,10].
Two radiological patterns of NTM-PD have been well recognized: fibrocavitary (FC) and nodular bronchiectatic (NB) [2, 11, 12]. The FC pattern features cavitary lesions mainly in the upper lobes and usually develops in older males with underlying lung diseases [11]. The NB pattern is characterized by bronchiectasis with multiple nodules and tree-in-bud opacities on computed tomography (CT) scans and occurs predominantly in postmenopausal nonsmoking female patients [11]. Consolidative and infiltrative patterns have also been reported [13, 14]; however, most previous studies have focused on FC and NB patterns.
The radiological appearance of NTM-PD is a combination of several findings, including cavity formation, bronchiectasis, consolidation, nodules varying in size, and bronchiolitis (centrilobular nodules with branching opacities) [15,16,17,18]. Among these findings, the bronchiolitis pattern is the most common, and was observed in 98% of patients with NTM-PD in a study [15]. In routine CT interpretation practice, it is difficult to specifically suspect NTM-PD when only a mild bronchiolitis pattern is observed without information on respiratory symptoms or a previous CT image. We theorized that a bronchiolitis pattern on CT may indicate an early finding of NTM-PD and may progress to a severe form of NTM-PD. This study aimed to investigate the clinical characteristics and radiological changes in patients with NTM-PD who exhibited a bronchiolitis pattern on CT.
Methods
Study population
We retrospectively examined the electronic medical records of patients referred to the outpatient respiratory clinic of a tertiary hospital between January 1, 2005 and March 31, 2021. We followed a stepwise approach to include patients who were referred under suspicion of NTM-PD and whose CT findings showed bronchiolitis without bronchiectasis or cavity formation.
Among the patients referred to the respiratory clinic, we first searched for those whose CT reports had keywords describing bronchiolitis (e.g., bronchiolitis, centrilobular nodule, branching opacity, tree-in-bud, and micronodule) and mentioned NTM infection as a differential diagnosis. Patients with chest CT reports mentioning a cavity or bronchiectasis, and those diagnosed with diffuse panbronchiolitis, pulmonary tuberculosis, tuberculous pleurisy, or sarcoidosis were excluded. Patients with a CT follow-up of < 1 year were excluded. Immunocompromised patients were excluded because pulmonary infections commonly occur in patients with overlapping radiological findings. Any patient suffering from a primary immunodeficiency or hematological malignancy, being treated with steroids or other immunosuppressive drugs for over 4 weeks, or having a history of solid organ or bone marrow transplantation were regarded as immunocompromised. Furthermore, we excluded patients whose CT findings were not likely to be NTM but other diseases with a similar CT appearance (e.g., diffuse panbronchiolitis, bronchopneumonia, or sarcoidosis) after closely reviewing the CT images and final clinical information. A board-certified chest radiologist reviewed all the CT images. The patients were categorized into those diagnosed and not diagnosed with NTM-PD. The diagnosis of NTM-PD was made based on the criteria proposed by the American Thoracic Society/Infectious Diseases Society of America [19]. This retrospective study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2309-851-105), and the requirement for a written informed consent was waived.
Data collection and assessment
Baseline demographics at the time of the first visit were collected from the electronic medical records, including age, sex, body mass index, comorbidities, smoking history, and symptoms. Microbiological test results (NTM species and acid-fast bacilli [AFB] culture) and chest CT images obtained during the follow-up period were also collected.
AFB cultures from respiratory specimens were obtained using standard methods [20]. The microbiological criteria for NTM-PD were met when NTM was isolated from the sputum ≥ 2 times or from the bronchial washing after the first visit. NTM species and subspecies were identified by polymerase chain reaction restriction fragment length polymorphism analysis of the rpoB gene.
CT image review
Chest CT scans were conducted at our institution using multi-detector CTs, with a slice thickness of 3 mm at 2.5 mm intervals. CT images from external hospitals were considered acceptable if they were reconstructed with a slice thickness of < 3 mm and a slice interval smaller than the slice thickness, employing overlapping reconstruction.
The presence of five NTM-related CT findings on the initial and final CT scans was assessed [15]: bronchiectasis, cellular bronchiolitis, cavity formation, nodules, and consolidation. Bronchiectasis was defined as a condition in which the bronchial lumen diameter was greater than that of the adjacent pulmonary artery, without normal bronchial tapering. Cellular bronchiolitis was defined as the presence of centrilobular nodules (< 10 mm in diameter) and branching opacities (i.e., the tree-in-bud sign) observed on CT scans. Nodules were defined as solid oval or round lesions 10–30 mm in diameter. Consolidation was defined as a polygonal (not oval or round) homogeneous opacity that replaced the airspace and obscured the underlying pulmonary structures.
A previously published CT scoring system [15] was slightly modified and used to assess the radiological progression between the initial and final CT images (Table 1): bronchiectasis (maximum score, 6), cellular bronchiolitis (maximum score, 6), cavity (maximum score, 9), nodules (maximum score, 3), and consolidation (maximum score, 3). Since the aim of our study is to compare long-term changes in CT images, we removed the mucus plugging item from the bronchiectasis section of the original scoring system. This adjustment ensures that our scores more accurately reflect chronic irreversible changes rather than fluctuations in inflammation. Radiological progression was defined as present when the final CT score of any finding was greater than the initial CT score. A scoring system was not used to compare the CT images between the groups because the initial CT scores were generally expected to be small. The distribution of radiological abnormalities was analyzed based on the lobe and multilobe involvements and bilaterality. The lingular division of the left lung was considered the middle lobe.
During the image analysis, the original CT images were downloaded and reviewed without radiology report or clinical information, such as NTM diagnosis. The initial and final CT images of patients were independently assessed using a scoring system. After assessment by a board-certified radiologist (S.H.Y.), the results were compared with the original radiology reports written by board-certified radiologists. The time point of progression was evaluated in the patients with NTM-PD who exhibited radiologic progression.
Statistical analysis
Data are presented as numbers (percentages) for categorical variables and medians (interquartile ranges) for continuous variables. Fisher’s exact test was used to compare the categorical variables. The Wilcoxon rank-sum test was used to compare the continuous variables. P-values < 0.05 were considered statistically significant. The statistical calculations were performed using the R 4.1.2 (R Foundation for Statistical Computing, Vienna, Austria) software.
Results
Patient characteristics
Among the 57,734 patients who visited the outpatient respiratory clinic and underwent a chest CT scan during the study period, 96 were suspected to have NTM-PD without bronchiectasis or cavity formation on CT. These patients underwent a total of 515 chest CT scans during the follow-up period.(Fig. 1).
The median CT follow-up duration for 96 patients was 1510.5 days (interquartile range [IQR]: 862.2–3005 days). The median number of CT scans per patient was 4 (IQR: 3–6), and the median CT interval was 221 days (IQR: 61.5–419.5 days). All CT images were considered acceptable for the analysis. During the study period, 43 patients were subsequently diagnosed with NTM-PD and 53 were not, despite repetitive AFB culture tests (Table 2). Among the patients with NTM-PD, 26 patients were diagnosed by having ≥ 2 separate positive sputum culture results, and 17 were diagnosed by having ≥ 1 positive culture result from bronchial washings. None of the patients without NTM-PD met the microbiological criteria for NTM-PD. Among these, 7 patients had only one positive sputum culture result despite undergoing two or more sputum cultures. The remaining 46 patients consistently had negative sputum cultures. Patients with NTM-PD underwent fewer sputum or bronchial washing culture tests before the final diagnosis (P < 0.05), although the CT follow-up period was not significantly different. Other clinical variables were not significantly different between the two groups, except for underlying chronic airway diseases (chronic obstructive pulmonary disease (COPD) and asthma) (P < 0.001). There was 1 case of COPD and 1 case of asthma in the patients with NTM-PD, and 6 cases of COPD and 12 cases of asthma in the patients without NTM-PD. It took at least five negative AFB culture tests over 3 years until 90% of the NTM patients were diagnosed (Fig. 2).
Comparison of the CT findings
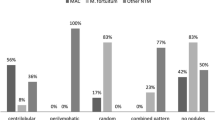
There was no disparity between the scoring results of CT images and the original radiology reports. Regarding the initial CT findings, nodules and consolidations were present in different proportions, which were more frequently observed in patients with NTM-PD than in those without NTM-PD (P < 0.05) (Table 3). The presence of other findings was not significantly different between the two groups. The distribution of radiological abnormalities was not significantly different, except for the upper lobe involvement, which was more frequently observed in patients with NTM-PD.
The frequency of radiological abnormalities on the final CT image showed a significant difference between the groups in four of the five findings (Table 4). Bronchiectasis (P < 0.001), cavities (P < 0.05), nodules (P < 0.05), and consolidations (P < 0.05) were more frequently observed in patients with NTM-PD. The distribution of radiological abnormalities did not differ significantly between the two groups.
The progression of each finding during the follow-up period was assessed using a scoring system and compared between the patients with and without NTM-PD (Fig. 3). Among the 43 patients with NTM-PD, the CT images of 30 patients (69.8%) showed a radiological progression. In comparison, 22 out of the 53 patients without NTM-PD (41.5%) exhibited radiological progression on their CT images. Additionally, of the 43 patients with NTM-PD, 11 (25.6%) received treatment, with 9 of these patients being in the radiologically progressive group. The mean increase in the total score for radiological abnormalities during the follow-up period was 2.05 in patients with NTM-PD and 0.08 in those without. Only the development of bronchiectasis and cavities differed significantly between the two groups (P < 0.01), with a higher frequency in patients with NTM-PD. Notably, the development of bronchiectasis was observed in 51.2% (22/43) of patients with NTM-PD and 73.3% (22/30) of those with radiological progression. The incidence of bronchiectasis increased over time in the NTM group (Fig. 4). The development and progression of bronchiectasis during the follow-up are shown in Figs. 5 and 6. Although the presence of nodules and consolidations was more common in patients with NTM-PD on both the initial and final CT scans, the progression of these two findings was not significantly different between patients with and without NTM-PD.
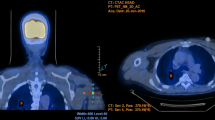
Chest CT images of a female patient. (A) The initial CT image showed no bronchiectasis. Centrilobular and small nodules were observed in the anterior segment of the left upper lobe. (B) Bronchiectasis became evident after 5 years and centrilobular nodules were still observed but to a lesser extent. CT, computed tomography
NTM species
The identification of NTM species was performed in 42 of the 43 patients (Table 5). M. avium (n = 18) was the most frequently encountered species, followed by M. intracellulare (n = 12) and M. abscessus (n = 7). The other three patients were infected with M. avium complex (n = 1), M. fortuitum (n = 1), and an unidentified NTM (n = 1). The radiological progression was observed in more than half of the patients with M. avium, M. intracellulare, and M. abscessus infections. Nearly half of the patients with M. avium, M. intracellulare, and M. abscessus infections developed bronchiectasis during the follow-up.
Discussion
In this study, 96 patients who presented with bronchiolitis on CT scans but without bronchiectasis or cavities were followed up for a median follow-up period of 1510.5 days. Among 43 patients who were confirmed to have NTM-PD, 30 exhibited a radiological progression, mostly due to bronchiectasis. The progression to bronchiectasis and cavities was significantly more common in patients with NTM-PD than in those without.
Although NTM-PD manifest in various forms on CT scans [18, 21], previous studies have primarily focused on two well-known patterns: FC and NB [2, 11, 12]. Consequently, other patterns have received relatively little attention [22]. Based on our clinical experience, we have observed a significant number of patients with NTM-PD who exhibit bronchiolitis without bronchiectasis or cavity on CT. However, only one study specifically investigated the bronchiolitis subtype of pulmonary NTM infections [22]. Additionally, there is a lack of research on the radiological changes that occur from the early to late stages of NTM-PD. Therefore, the early manifestations of NTM-PD have not been thoroughly investigated.
Our study demonstrated that among patients with NTM-PD having a bronchiolitis pattern, the radiological abnormalities progressed over time. Notably, bronchiectasis was the most frequent new finding in patients with NTM-PD with a radiological progression, suggesting that the bronchiolitis pattern may serve as an early indicator of NTM-PD. However, distinguishing between patients with and without NTM-PD using CT scans, when they present solely with bronchiolitis, remains challenging. The only discernible difference based on initial chest CT findings was the slightly higher prevalence of nodules and consolidations (14.0% vs. 1.9% and 30.2% vs. 7.5%, respectively; P < 0.05) in patients with NTM-PD. These findings may assist in identifying patients with suspected NTM-PD who require a meticulous observation with additional follow-up CT scans.
Although the correlation between bronchiectasis and NTM-PD is well-established, the exact cause remains uncertain. In a study involving 221 patients with non-NTM bronchiectasis, 31 were diagnosed with NTM-PD during a median observation period of 37 months [23]. Moreover, individuals with bronchiectasis are reportedly 50–75 times more likely to have NTM infections compared to those without [24]. In contrast, pulmonary NTM infections may lead to bronchiectasis. According to the U.S. Bronchiectasis Research Registry, approximately 60% of patients with bronchiectasis had a history of NTM-PD or NTM cultured from sputum samples, and patients with NTM were more likely to exhibit diffusely dilated airways [25]. Another study that focused on patients with bronchiectasis revealed that patients with NTM-PD had a higher number of pulmonary segments affected by bronchiectasis [26]. No study has yet directly shown bronchiectasis development in NTM-PD via serial CT scans. Our study closely reviewed the CT images of patients with NTM-PD who initially presented with bronchiolitis and observed the development of bronchiectasis. The incidence of bronchiectasis increased over time in patients with NTM-PD, strongly indicating pulmonary NTM infection as a direct cause of bronchiectasis.
Diagnosis and surveillance of patients with NTM-PD can be challenging due to nonspecific clinical signs and the lack of a pathognomonic test [11, 27]. If NTM-PD is highly suspected and the initial tests are nondiagnostic, additional sputum cultures should be obtained. However, repeated cultures often show negative results. In addition, no consensus has been reached regarding the number of additional cultures and the length of intervals between the cultures to exclude NTM-PD safely. Considering that it took a minimum of five AFB tests over 3 years to diagnose 90% of patients with NTM-PD (Fig. 2), we recommend considering at least five repetitive AFB culture tests within 3 years for patients exhibiting a bronchiolitis pattern and suspected NTM-PD.
This study had several limitations. First, as the study was conducted at a respiratory clinic in a single tertiary hospital, our results may not be generalizable to a broader population. Second, this study was retrospective and involved a relatively small sample size. We included patients based on CT reports indicating bronchiolitis and mentioning NTM. Some patients without explicit mentions of NTM in the initial CT report might have been excluded, though this is likely rare. As patients were referred to the respiratory clinic and NTM is prevalent in South Korea, diagnosing NTM is of significant interest to both clinicians and radiologists. Consequently, mentions of NTM often emerged in subsequent follow-up CT reports, and these cases were included in this study. Further research using larger cohorts and a prospective approach is necessary to validate and expand upon our observations. Third, the definitive diagnosis of patients without NTM-PD remains unclear. With repeated AFB testing and longer follow-up durations, it is possible that some individuals initially not diagnosed with NTM-PD may later be diagnosed with NTM-PD or other related conditions.
Conclusions
The CT images displaying bronchiolitis patterns in patients with NTM-PD exhibited frequent radiological progression during the follow-up period. The most common observation among patients with NTM-PD showing radiological progression was the development of bronchiectasis. These results strongly indicate that pulmonary NTM infection directly leads to bronchiectasis. NTM-PD manifesting as a bronchiolitis pattern on CT scans necessitates thorough follow-up, including additional CT scans.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- FC:
-
Fibrocavitary
- NB:
-
Nodular bronchiectatic
- NTM-PD:
-
Nontuberculous mycobacteria pulmonary disease
- NTM:
-
Nontuberculous mycobacteria
- CT:
-
Computed tomography
- AFB:
-
Acid-fast bacilli
- IQR:
-
Interquartile range
References
Cowman S, van Ingen J, Griffith DE, Loebinger MR. Non-tuberculous mycobacterial pulmonary disease. Eur Respir J 2019, 54(1).
Haworth CS, Banks J, Capstick T, Fisher AJ, Gorsuch T, Laurenson IF, Leitch A, Loebinger MR, Milburn HJ, Nightingale M, et al. British Thoracic Society guidelines for the management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). Thorax. 2017;72(Suppl 2):ii1–64.
Henkle E, Winthrop KL. Nontuberculous mycobacteria infections in immunosuppressed hosts. Clin Chest Med. 2015;36(1):91–9.
Tortoli E. Clinical manifestations of nontuberculous mycobacteria infections. Clin Microbiol Infect. 2009;15(10):906–10.
Shah NM, Davidson JA, Anderson LF, Lalor MK, Kim J, Thomas HL, Lipman M, Abubakar I. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis. 2016;16:195.
Ringshausen FC, Wagner D, de Roux A, Diel R, Hohmann D, Hickstein L, Welte T, Rademacher J. Prevalence of Nontuberculous Mycobacterial Pulmonary Disease, Germany, 2009–2014. Emerg Infect Dis. 2016;22(6):1102–5.
Prevots DR, Loddenkemper R, Sotgiu G, Migliori GB. Nontuberculous mycobacterial pulmonary disease: an increasing burden with substantial costs. Eur Respir J 2017, 49(4).
Izumi K, Morimoto K, Hasegawa N, Uchimura K, Kawatsu L, Ato M, Mitarai S. Epidemiology of adults and Children Treated for Nontuberculous Mycobacterial Pulmonary Disease in Japan. Ann Am Thorac Soc. 2019;16(3):341–7.
Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–34.
Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus nonpulmonary nontuberculous mycobacteria, Ontario, Canada. Emerg Infect Dis. 2017;23(11):1898–901.
Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416.
Ryu YJ, Koh WJ, Daley CL. Diagnosis and treatment of Nontuberculous Mycobacterial Lung Disease: clinicians’ perspectives. Tuberc Respir Dis (Seoul). 2016;79(2):74–84.
Gommans EP, Even P, Linssen CF, van Dessel H, van Haren E, de Vries GJ, Dingemans AM, Kotz D, Rohde GG. Risk factors for mortality in patients with pulmonary infections with non-tuberculous mycobacteria: a retrospective cohort study. Respir Med. 2015;109(1):137–45.
Shu CC, Lee CH, Hsu CL, Wang JT, Wang JY, Yu CJ, Lee LN. Clinical characteristics and prognosis of nontuberculous mycobacterial lung disease with different radiographic patterns. Lung. 2011;189(6):467–74.
Kim HS, Lee KS, Koh WJ, Jeon K, Lee EJ, Kang H, Ahn J. Serial CT findings of Mycobacterium massiliense pulmonary disease compared with Mycobacterium abscessus disease after treatment with antibiotic therapy. Radiology. 2012;263(1):260–70.
Kim SJ, Yoon SH, Choi SM, Lee J, Lee CH, Han SK, Yim JJ. Characteristics associated with progression in patients with of nontuberculous mycobacterial lung disease: a prospective cohort study. BMC Pulm Med. 2017;17(1):5.
Moon SM, Jhun BW, Baek SY, Kim S, Jeon K, Ko RE, Shin SH, Lee H, Kwon OJ, Huh HJ, et al. Long-term natural history of non-cavitary nodular bronchiectatic nontuberculous mycobacterial pulmonary disease. Respir Med. 2019;151:1–7.
Erasmus JJ, McAdams HP, Farrell MA, Patz EF Jr. Pulmonary nontuberculous mycobacterial infection: radiologic manifestations. Radiographics. 1999;19(6):1487–505.
Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr., Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020, 56(1).
Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376–95. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999.
Martinez S, McAdams HP, Batchu CS. The many faces of pulmonary nontuberculous mycobacterial infection. Am J Roentgenol. 2007;189(1):177–86.
Lee JH, Park YE, Chong YP, Lee HJ, Shim TS, Jo K-W. Radiologic subtypes and treatment outcome of unclassifiable type Mycobacterium avium Complex Pulmonary Disease. J Korean Med Sci 2023, 38(3).
Kwak N, Lee JH, Kim HJ, Kim SA, Yim JJ. New-onset nontuberculous mycobacterial pulmonary disease in bronchiectasis: tracking the clinical and radiographic changes. BMC Pulm Med. 2020;20(1):293.
Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432–9.
Aksamit TR, O’Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels MLA, Johnson M, Eden E, Griffith D, Knowles M, et al. Adult patients with bronchiectasis: a First look at the US Bronchiectasis Research Registry. Chest. 2017;151(5):982–92.
Eisenberg I, Yasin A, Fuks L, Stein N, Saliba W, Kramer MR, Adir Y, Shteinberg M. Radiologic characteristics of non-tuberculous Mycobacteria Infection in patients with Bronchiectasis. Lung. 2020;198(4):715–22.
Pennington KM, Vu A, Challener D, Rivera CG, Shweta FNU, Zeuli JD, Temesgen Z. Approach to the diagnosis and treatment of non-tuberculous mycobacterial disease. J Clin Tuberc Other Mycobact Dis. 2021;24:100244.
Acknowledgements
Not applicable.
Funding
No funding source.
Author information
Authors and Affiliations
Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, were fully responsible for all content, and were involved at all stages of manuscript development. Study conception and design: J.H.L. and S.H.Y.; Data collection: J.H.L., S.H.Y., and Jihang K.; Data Interpretation: J.H.L., S.H.Y., H.J.K., and Junghoon K.; and manuscript writing: J.H.L., S.H.Y., H.J.K., Jihang K., and Junghoon. K. As a corresponding author, J.H.L. has full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. This retrospective study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (IRB No. B-2309-851-105), and the requirement for a written informed consent was waived.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoon, S., Kim, HJ., Kim, J. et al. Nontuberculous mycobacterial pulmonary disease presenting as bronchiolitis pattern on CT without cavity or bronchiectasis. BMC Pulm Med 24, 432 (2024). https://doi.org/10.1186/s12890-024-03223-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-024-03223-2