Abstract
Background
Fournier’s Gangrene is a severe surgical infectious disease, and various risk factors can increase its mortality rate. The purpose of this study is to retrospectively analyze the clinical characteristics and laboratory data of Fournier’s Gangrene patients, followed by an analysis of mortality-related risk factors. This study has no secondary objectives.
Methods
This study included 46 hospitalized patients diagnosed with Fournier’s Gangrene at Suzhou Traditional Chinese Medicine Hospital from December 2013 to March 2024. Clinical data for all patients were extracted from the electronic medical records system. The collected data included gender, age, duration of illness, length of hospital stay, sites of infection involvement, comorbidities, white blood cell count, hematocrit, albumin, blood glucose, creatinine, serum sodium, serum potassium upon admission, microbial culture results, and patient outcomes (survival/death). The Simplified Fournier Gangrene Severe Index (SFGSI) was used to score all patients. Patients were categorized into survival and death groups based on clinical outcomes. Differences between categorical variables were compared using the χ² test or Fisher’s exact test. Differences between numerical variables were compared using Student’s t-test or the Mann-Whitney U test. Binary logistic regression was employed to analyze the risk factors for mortality in Fournier’s Gangrene.
Results
Among the 46 Fournier’s Gangrene patients, 39 were male (84.8%) and 7 were female (15.2%). The age ranged from 17 to 86 years, with a median age of 61 years. Fourteen cases (30.4%) were confined to the perianal area, 26 cases (56.5%) had fascial necrosis involving the perianal, perineal, and genital regions, while 6 cases (13.0%) extended to the abdominal wall. At a 3-month postoperative follow-up, 43 patients (93.5%) survived, while 3 patients (6.5%) died shortly after admission due to severe illness. Based on the outcome, patients were divided into survival and death groups with 43 and 3 cases, respectively. Significant differences were observed between the two groups in terms of age (P<0.05), extension to the abdominal wall (P<0.01), hematocrit (P<0.01), albumin (P<0.01), SFGSI (P<0.01), and SFGSI>2 (P<0.01). Binary logistic regression analysis indicated that decreased hematocrit was an independent risk factor for mortality in Fournier’s Gangrene patients.
Conclusion
This study provides a detailed analysis of the clinical characteristics and risk factors for mortality in Fournier’s Gangrene patients. The primary outcome of this study is that a decreased hematocrit is an independent risk factor for predicting mortality in FG patients. These findings offer valuable prognostic insights for clinicians, underscoring the importance of early identification and correction of reduced hematocrit to improve patient outcomes and survival rates.
Similar content being viewed by others
Introduction
Necrotizing fasciitis is a severe and rapidly progressing soft tissue infection, known for its highly invasive nature and high mortality rates [1, 2]. The hallmark of this disease is extensive fascial necrosis caused by microbial infection, rapidly leading to tissue destruction and toxic shock, posing a grave threat to the patient’s life [1,2,3]. Severe inflammation and the infectious process spread rapidly along the fascial plane, affecting adjacent soft tissues [2, 3]. The spread of inflammation and infection can lead to vascular thrombosis, subsequently causing ischemia and necrosis of adjacent soft tissues and fascia [4]. Therefore, the disease may initially go unnoticed or unrecognized, as there may be minimal or no skin manifestations in its early stages.
Necrotizing fasciitis occurring in the scrotum, perianal, and perineal regions is also known as Fournier’s Gangrene [1, 5]. Necrotizing fasciitis results from a synergistic infection of the fascia and subcutaneous tissues by both aerobic and anaerobic microorganisms [2, 6]. Literature reports that Gram-positive bacteria, such as Group A Streptococcus and Staphylococcus aureus, and Gram-negative bacteria, like Escherichia coli and Pseudomonas aeruginosa, are the most common bacteria isolated from wound cultures in Fournier’s Gangrene patients [7,8,9]. These bacteria can infect through various routes, including the urinary tract, gastrointestinal tract, or skin. The infectious and inflammatory processes spread rapidly along the Dartos, Colles, and Scarpa fasciae, early involving the abdominal wall [7, 10, 11]. Trauma, postoperative conditions, urinary tract infections, and other perineal infections like perianal abscesses often serve as the initial points of infection [1, 12]. Fournier’s Gangrene most commonly affects males over 50 years of age with diabetes mellitus [4, 7]. Fournier’s Gangrene is a surgical emergency often requiring urgent and multiple surgical debridements, antibiotic therapy, and supportive measures [5, 12].
Due to the rapid progression and high lethality of necrotizing fasciitis, prompt diagnosis and treatment are key to improving outcomes. Therefore, early identification and management of factors associated with mortality are crucial. Risk factors primarily involve host factors, pathogens, and environmental factors, including but not limited to diabetes, immunosuppressive states, history of trauma or surgery, and prolonged use of non-steroidal anti-inflammatory drugs [12, 13]. Understanding these risk factors aids in identifying high-risk populations and providing them with more targeted prevention and management strategies.
This study aims to conduct a retrospective analysis of clinical characteristics and laboratory data of Fournier’s Gangrene patients managed by the authors’ institution. We aim to analyze factors associated with mortality to enhance the clinical team’s understanding and comprehension of this disease. By doing so, we aim to provide robust evidence for the early identification of high-risk Fournier’s Gangrene patients, thereby reducing the harm to patients’ health and improving treatment success rates and survival rates.
Materials and methods
Study subjects
This is a retrospective, descriptive study that received approval from the Ethics Committee of Suzhou Traditional Chinese Medicine (TCM) Hospital (2024 Ethics Approval 004). We included 46 hospitalized patients diagnosed with Fournier’s Gangrene (FG) who were treated at Suzhou TCM Hospital from December 2013 to March 2024.
Inclusion criteria: Patients diagnosed with Fournier’s Gangrene who meet the following conditions: clinical presentation of localized cellulitis with significant systemic toxicity symptoms (high fever, significantly elevated white blood cell count, severe cases with altered mental status, rapid breathing, tachycardia); rapid progression of the condition, with the affected area showing quick swelling, crepitus, blackened and necrotic tissue, and significant pain; infection originating from the skin, urethra, or rectum [3].
Exclusion criteria: Patients with simple perianal, perineal, urethral, and scrotal abscesses without evidence of fascial necrosis; and patients with missing or incomplete data [1].
Among the included patients, 42 cases originated from the Department of Anorectal Surgery, indicating FG originating from perianal and perirectal abscesses. Four cases of FG originating from scrotal infections were from the Department of Urology.
Data collection
Clinical data for all patients, including medical history, physical examination, laboratory tests, imaging studies, and surgical records, were extracted from the electronic medical record system. The Fournier’s Gangrene Severity Index (FGSI) score is commonly used to evaluate the severity of FG [9, 11, 14]. This is one of the most widely used scoring systems and includes eight parameters: temperature, heart rate, respiratory rate, sodium, potassium, creatinine, white blood cell count, and hematocrit. The higher the score, the worse the prognosis. Scoring is done by assigning each parameter a score from 0 to 4 based on the degree of abnormality. The total score is the sum of all parameter scores. Patients with a total score greater than 9 typically have a severe condition and a poor prognosis. The simplified Fournier’s Gangrene Severity Index (SFGSI) is currently the most widely used [11, 14]. In addition, the age-adjusted Charlson Comorbidity Index (aCCI) is a widely used comorbidity scoring system. It quantifies comorbidities based on the number and severity of a patient’s disease and can be used to predict the risk of disease mortality [15]. The collected data included gender, age, duration of illness, length of hospital stay, sites of infection, comorbidities, white blood cell count, hematocrit, albumin, blood glucose, creatinine, serum sodium, serum potassium upon admission, microbial culture results, and patient outcomes (survival/death), among others. All patients were scored using the SFGSI and aCCI.
Treatment strategies
Based on the increasing understanding of FG, a high-level response was initiated for all FG patients upon admission to complete laboratory tests and CT scans as quickly as possible to assess the severity of the condition [16]. All FG patients underwent initial emergency surgical debridement and drainage under spinal or general anesthesia within 24 h of admission. The initial debridement and drainage were conducted under a multi-disciplinary team (MDT) approach. Depending on the affected areas, colorectal surgeons, urologists, and general surgeons performed debridement and drainage of the perianal, perineal, genital, and abdominal wall regions. Swab samples were collected from the wound edges for culture. Debridement was performed to remove necrotic tissue until fresh bleeding surfaces were exposed, typically requiring at least one debridement of necrotic tissue. Once diagnosed with FG, all patients received broad-spectrum antibiotic treatment. After microbial culture results were available, antibiotics were selected based on sensitivity testing [6]. Our experience suggests early and adequate use of imipenem/cilastatin. For patients with severe conditions, they were transferred to the intensive care unit (ICU) for mechanical ventilation and hemodynamic support after the initial debridement. None of the included patients underwent colostomy or cystostomy procedures.
After the initial thorough debridement, dressing changes were performed daily. The wounds were cleaned with povidone-iodine, saline, and 2% hydrogen peroxide, and then covered with povidone-iodine dressings [4]. During dressing changes, the progression of wound infection was observed. Depending on the progression of the infection, one or more additional debridements might be needed, which could typically be completed in the ward.
Statistical analysis
Statistical analysis was performed using IBM SPSS 26.0 software. Categorical variables were described using frequencies (n) and percentages (%). Numerical variables were expressed as mean ± standard deviation (SD) or median with interquartile range after the Kolmogorov-Smirnov normality test. Differences between categorical variables were compared using the χ² test or Fisher’s exact test. Differences between numerical variables were compared using the student’s t-test or Mann-Whitney U test. Binary logistic regression was used for analyzing risk factors associated with mortality [11, 17]. A p-value < 0.05 was considered statistically significant.
Results
Clinical characteristics of FG patients
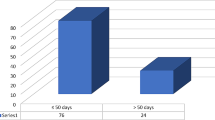
As shown in Table 1, among the 46 FG patients, there were 39 males (84.8%) and 7 females (15.2%). The age ranged from 17 to 86 years with a median age of 61 years. The shortest duration of illness was 3 days, the longest was 30 days, and the median duration was 7 days. The shortest length of hospital stay was 2 days, the longest was 30 days, and the median stay was 14 days. Regarding the sites of necrotic lesions, 14 cases (30.4%) were localized to the perianal area, 26 cases (56.5%) had fascial necrosis involving the perianal, perineal, and genital regions, and 6 cases (13.0%) had fascial necrosis extending to the abdominal wall. There were 17 patients (37.0%) with hypertension and 17 patients (37.0%) with diabetes. Nine patients (19.6%) had both hypertension and diabetes, while one patient (2.2%) had both a hematologic disorder and diabetes. Twenty patients (43.5%) had no comorbidities. During a 3-month follow-up after surgery, 43 patients (93.5%) survived, while 3 patients (6.5%) died shortly after admission due to the severity of their condition. It is noteworthy that the three deceased patients had very short hospital stays, lasting 2 days, 3 days, and 5 days respectively.
Analysis of laboratory data in FG patients
As shown in Table 2, after normality testing, the blood glucose and creatinine levels of the 46 included patients did not follow a normal distribution. The white blood cell count ranged from 6.63 × 10^9/L to 38.57 × 10^9/L, with an average of 16.5 ± 6.3 × 10^9/L. The hematocrit ranged from 20.3 to 47.8%, with an average of (37.0 ± 6.0) %. Albumin levels ranged from 21.0 g/L to 43.7 g/L, with an average of 32.5 ± 5.6 g/L. Serum sodium levels ranged from 126.6 mmol/L to 144.5 mmol/L, with an average of 135.8 ± 4.6 mmol/L. Serum potassium levels ranged from 2.96 mmol/L to 5.1 mmol/L, with an average of 3.8 ± 0.6 mmol/L. Blood glucose levels ranged from 4.48 mmol/L to 32.55 mmol/L, with a median of 7.91 mmol/L. Creatinine levels ranged from 47.0 umol/L to 294.3 umol/L, with a median of 80.1 umol/L. Bacterial cultures were performed on necrotic tissues from 38 patients (82.6%), with 28 cases (60.9%) yielding positive results. Among the cultured microorganisms, Escherichia coli was the most common, found in 19 cases (41.3%), followed by Klebsiella pneumoniae in 4 cases (8.7%).
Comparison of clinical and laboratory data based on patient outcomes
Based on outcomes (survival/death), the 46 patients were divided into two groups: the survival group with 43 cases and the death group with 3 cases. As shown in Table 3, after comparison, significant differences were found between the two groups in terms of age (P<0.05), length of hospital stay (P<0.001), extension to the abdominal wall (P<0.01), hematocrit (P<0.01), albumin (P<0.01), SFGSI (P<0.01), and SFGSI>2 (P<0.01). These results suggest that older age, extension to the abdominal wall, lower hematocrit, and higher SFGSI are risk factors for mortality in FG patients. Because the three patients in the death group died shortly after admission due to the severity of their condition, a short hospitalization duration cannot be proven as an independent risk factor associated with mortality. No significant differences were observed in the remaining variables.
Binary logistic regression analysis
For the variables showing differences in Table 3, binary logistic regression was employed to further analyze the risk factors related to FG mortality. Initially, a univariate regression analysis was conducted using age, length of hospital stay, involvement of the abdominal wall, hematocrit, albumin, and SFGSI>2 as independent variables, and mortality as the dependent variable. This was followed by a multivariate regression analysis where the variables showing statistical differences in the univariate analysis were used as independent variables to explore the independent risk factors affecting mortality after adjusting for confounding factors. As shown in Table 4, The binary logistic regression analysis showed that hematocrit was the variable with a significant difference. The univariate regression analysis found a 95% confidence interval (CI) for hematocrit of 0.597–0.953, with an odds ratio (OR) of 0.754 and a p-value of 0.018, which is less than 0.05. The multivariate regression analysis found a 95% CI for hematocrit of 0.556–0.987, with an adjusted odds ratio (AOR) of 0.740 and a p-value of 0.040, which is also less than 0.05. According to Table 4, for every unit increase in hematocrit, there was a significant 24.6% decrease in the likelihood of mortality in FG patients. None of the other factors showed statistical significance as predictors of mortality. Even after adjusting for age as a confounding factor, the relationship between hematocrit and mortality remained significant. Specifically, for every unit increase in hematocrit, there was a significant 26% decrease in the likelihood of mortality in FG patients.
We divided the 46 patients into two groups based on whether their hematocrit levels were below 30 (%) and compared the survival rates between the two groups. Statistical analysis indicated a significant difference in survival rates between the groups (P = 0.001). Similarly, we divided the 46 patients into two groups based on whether their hematocrit levels were below 35 (%) and compared their survival rates. The analysis showed a difference in survival rates between the groups (P = 0.037). These results further support the robustness of our conclusion that decreased hematocrit is an independent risk factor for mortality in FG patients (Supplementary Tables 1, 2).
Discussion
Fournier’s gangrene is a severe surgical infection that is relatively rare. Over an 11-year period, our hospital treated 46 cases of FG. The disease predominantly affects males, with our results showing that males accounted for 84.8% of FG patients, consistent with existing literature [10, 11]. Early diagnosis, proper management of predisposing factors, and aggressive surgical debridement can improve clinical outcomes [18]. Previous studies have reported varying mortality rates for this disease, ranging from 20 to 80% [18]. With increased attention to the disease and advancements in treatment, the reported mortality rate for FG now ranges between 7.5% and 16% [12]. Our study found a mortality rate of 6.5% for FG, consistent with the most recent literature.
Several studies have identified the main risk factors for the occurrence of FG, including diabetes, alcohol abuse, obesity, immunosuppression, hypertension, hematological diseases, perianal and rectal abscesses, perineal infections, and trauma [12, 13, 19]. Our study found that 37.0% of patients had hypertension, and 37.0% had diabetes. Additionally, 19.6% of the patients had both hypertension and diabetes. Among the 46 FG patients in our study, 91.3% originated from perianal abscesses, while 8.7% were from scrotal infections. These clinical data are consistent with what is described in the literature.
Studies have reported that risk factors associated with mortality in FG include advanced age, diabetes, infection extending to the abdominal wall, low hematocrit, abnormal serum potassium levels, and elevated creatinine levels [11, 17]. Due to the high mortality rate of FG, several assessment scales have been used to evaluate the severity of the disease and prognosis in patients. Currently, used clinical assessment scales include FGSI, ACCI, LRINEC, and UFGSI, among which FGSI is the most commonly used [11, 14, 20]. Research has shown that the sensitivity and specificity of FGSI in predicting mortality are both between 65% and 100% [14]. In 2014, Lin et al. developed the SFGSI based on the FGSI scale, incorporating three laboratory parameters: serum potassium, creatinine, and hematocrit. They compared it with other assessment scales and found that SFGSI is user-friendly with good sensitivity (87%) and specificity (77%) in predicting mortality, effectively identifying patients with poor prognosis [14]. Our results show that higher age, involvement of the abdominal wall, lower hematocrit, lower serum albumin, and higher SFGSI scores were observed in the non-survival group. Elderly patients experience a gradual decline in physiological function and a weakening of the immune system, which may increase the risk of infection and reduce their ability to fight off infections. Additionally, older patients often have multiple chronic conditions, such as diabetes and hypertension, which could exacerbate the severity and prognosis of Fournier’s gangrene. Abdominal wall involvement often indicates a large extent of the lesion that has spread to deeper tissues, resulting in more severe tissue necrosis and infection, and a poor prognosis. The involvement of the abdominal wall as a predictive factor for mortality has been described in related studies [13, 21]. Hypoalbuminemia was correlated with mortality in FG. Lin et al. reported significant differences in admission hematocrit and serum albumin levels between the survival and non-survival groups in FG patients [20]. The C-reactive protein/albumin ratio (CAR) has been shown to correlate with the severity of infection and serves as an effective inflammatory marker to predict prognosis [22]. Özgül et al.‘s study demonstrated that the CRP/albumin ratio can be used to predict FG-related mortality [23]. SFGSI is an important tool for assessing the severity and prognosis of patients with Fournier’s gangrene [11, 14]. In our study, SFGSI showed a higher trend in the mortality group, with all deceased patients having an SFGSI > 2, reflecting a severe decline in the patient’s overall condition. However, our results showed no significant differences in creatinine and serum potassium levels between the two groups. The mean and p-value (0.083) for serum potassium suggest a tendency toward statistical difference between the groups. Although the median creatinine level in the mortality group was noticeably higher than that in the survival group, the p-value (0.276) > 0.05. We believe that the lack of significant differences in serum potassium and creatinine between the two groups may be due to the small sample size and resulting bias in the study results.
The results of both univariate and multivariate regression analyses in this study showed that a decreased hematocrit level is a risk factor for mortality in FG patients. The statistical analysis demonstrated that lower hematocrit levels were significantly associated with increased mortality risk in Fournier’s Gangrene (univariate regression: OR 0.754, 95% CI: 0.597–0.953, p = 0.018; multivariate regression: AOR 0.740, 95% CI: 0.556–0.987, p = 0.040). Both univariate and multivariate regression analyses for the variable hematocrit showed p-values less than 0.05. This finding underscores the importance of monitoring and managing hematocrit levels in these patients to improve survival outcomes. Regardless of whether it’s FGSI or SFGSI, hematocrit remains an important laboratory parameter for predicting the prognosis of FG [12, 14]. Cen et al. conducted a retrospective analysis of 111 cases of necrotizing soft tissue infections to investigate the risk factors associated with mortality and amputation in these patients. Their study showed that high LRINEC scores, elevated WBC counts, low hematocrit (HCT), and multiple surgeries were associated with increased mortality rates [10]. Similar results have been corroborated in other studies [13, 17, 24, 25].
This study has several limitations. First, we recruited a relatively small sample of 46 FG patients over an 11-year period, which might introduce potential biases into our results. Second, this was a retrospective study conducted at a single center. The small sample size and single-center design limit the generalizability of our findings. The small sample size may affect the representativeness and accuracy of the results. Small sample data often fails to meet the traditional statistical test hypothesis, and special statistical methods are required for analysis, increasing the research’s complexity and uncertainty. In addition, single-center studies may have a single sample source and lack of diversity, further increasing the difficulty of statistical inference. Therefore, large-scale multicenter studies are needed to minimize potential biases as much as possible and validate these results in the future. Our findings are based on our clinical experience in managing FG patients, so we cannot generalize our findings to other populations. However, our experience and findings can serve as a valuable reference for other clinical teams tasked with managing FG.
Conclusions
This study provides a comprehensive analysis of the clinical characteristics and mortality risk factors in FG patients. The primary outcome of this study is that a decreased hematocrit level is an independent risk factor for predicting mortality in FG patients. These findings offer crucial prognostic guidance for clinicians, underscoring the importance of early identification and correction of decreased hematocrit levels. Timely correction of low hematocrit levels may improve patient outcomes and survival rates. Of course, this requires large-scale, multicenter studies to further validate the findings. Future research endeavors should delve deeper into investigating the correlation between hematocrit levels and prognosis in FG, while also striving to identify more efficacious interventions aimed at improving the quality of life and survival rates of affected individuals.
Data availability
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- FG:
-
Fournier’s Gangrene
- TCM:
-
Traditional Chinese medicine
- SFGSI:
-
Simplified Fournier Gangrene severe index
- FGSI:
-
Fournier Gangrene severe index
- aCCI/ACCI:
-
Age-adjusted Charlson comorbidity index
- MDT:
-
Multi-Disciplinary team
- ICU:
-
Intensive care unit
- CI:
-
Confidence interval
- OR:
-
Odds ratio
- AOR:
-
Adjusted odds ratio
- LRINEC:
-
Laboratory risk indicator for necrotizing fasciitis
- UFGSI:
-
Uludag Fournier’s Gangrene severity index
- CAR:
-
C-Reactive protein/albumin ratio
References
Ozkan OF, Koksal N, Altinli E, Celik A, Uzun MA, Cıkman O, et al. Fournier’s gangrene current approaches. Int Wound J. 2016;13:713–6.
Hua C, Urbina T, Bosc R, Parks T, Sriskandan S, De Prost N, et al. Necrotising soft-tissue infections. Lancet Infect Dis. 2023;23:e81–94.
Voelzke BB, Hagedorn JC. Presentation and diagnosis of Fournier Gangrene. Urology. 2018;114:8–13.
Taken K, Oncü MR, Ergun M, Eryılmaz R, Demir CY, Demir M et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32.
Tarasconi A, Perrone G, Davies J, Coimbra R, Moore E, Azzaroli F, et al. Anorectal emergencies: WSES-AAST guidelines. World J Emerg Surg. 2021;16:48.
Thwaini A, Khan A, Malik A, Cherian J, Barua J, Shergill I, et al. Fournier’s gangrene and its emergency management. Postgrad Med J. 2006;82:516–9.
Montrief T, Long B, Koyfman A, Auerbach J. Fournier Gangrene: a review for emergency clinicians. J Emerg Med. 2019;57:488–500.
Short B. Fournier gangrene: an historical reappraisal. Intern Med J. 2018;48:1157–60.
Warli SM, Pakpahan KA, Nasution R, Kadar DD, Adhyatma KP. Role of SFGSI, microbial culture and qSOFA as predictive factors in determining the survival rate in Fournier Gangrene patient. SMJ. 2024;45:230–4.
Cen H, Jin R, Yin J, Wang X. Risk Factors for Predicting Mortality and Amputation of Patients with Necrotizing Soft-Tissue Infections: Retrospective Analysis of 111 Cases from a Single Medical Center. Emerg Med Int. 2023; 2023:6316896.
Tenório CEL, Lima SVC, Albuquerque AVD, Cavalcanti MP, Teles F. Risk factors for mortality in fournier’s gangrene in a general hospital: use of simplified founier gangrene severe index score (SFGSI). Int braz j urol. 2018;44:95–101.
Hagedorn JC, Wessells H. A contemporary update on Fournier’s gangrene. Nat Rev Urol. 2017;14:205–14.
Tarchouli M, Bounaim A, Essarghini M, Ratbi MB, Belhamidi MS, Bensal A, et al. Analysis of prognostic factors affecting mortality in Fournier’s gangrene: a study of 72 cases. Can Urol Assoc J. 2015;9:E800–804.
Lin T, Ou C, Tzai T, Tong Y, Chang C, Cheng H, et al. Validation and simplification of F ournier’s gangrene severity index. Int J Urol. 2014;21:696–701.
Azmi YA, Alkaff FF, Renaldo J, Wirjopranoto S, Prasetiyanti R, Soetanto KM, et al. Comparison of different scoring systems for predicting in-hospital mortality for patients with Fournier gangrene. World J Urol. 2023;41:2751–7.
Levenson RB, Singh AK, Novelline RA. Fournier Gangrene: Role of Imaging. RadioGraphics. 2008; 28:519–28.
García Marín A, Turégano Fuentes F, Cuadrado Ayuso M, Andueza Lillo JA, Cano Ballesteros JC. Pérez López M. Predictive factors for mortality in Fournier’ gangrene: a series of 59 cases. Cir Esp. 2015;93:12–7.
Kuzaka B, Wróblewska MM, Borkowski T, Kawecki D, Kuzaka P, Młynarczyk G, et al. Fournier’s Gangrene: clinical presentation of 13 cases. Med Sci Monit. 2018;24:548–55.
El-Qushayri AE, Khalaf KM, Dahy A, Mahmoud AR, Benmelouka AY, Ghozy S, et al. Fournier’s gangrene mortality: a 17-year systematic review and meta-analysis. Int J Infect Dis. 2020;92:218–25.
Lin E, Yang S, Chiu AW, Chow Y-C, Chen M, Lin W-C, et al. Is Fournier’s gangrene severity index useful for predicting outcome of Fournier’s gangrene? Urol Int. 2005;75:119–22.
Tuncel A, Aydin O, Tekdogan U, Nalcacioglu V, Capar Y, Atan A. Fournier’s gangrene: three years of experience with 20 patients and validity of the Fournier’s Gangrene Severity Index score. Eur Urol. 2006;50:838–43.
Yılmaz EM, Kandemir A. Significance of red blood cell distribution width and C-reactive protein/albumin levels in predicting prognosis of acute pancreatitis. Ulus Travma Acil Cerrahi Derg. 2018;24:528–31.
Özgül H, Uzmay Y. The role of C-reactive protein albumin ratio for predicting mortality in patients with Fournier’s gangrene. Ulus Travma Acil Cerrahi Derg. 2023;29:1242–7.
Ioannidis O, Kitsikosta L, Tatsis D, Skandalos I, Cheva A, Gkioti A, et al. Fournier’s Gangrene: lessons learned from Multimodal and Multidisciplinary Management of Perineal necrotizing Fasciitis. Front Surg. 2017;4:36.
Ansari Djafari A, Rahavian A, Javanmard B, Montazeri S, Shahabi V, Hojjati SA et al. Factors related to mortality in patients with Fournier’s Gangrene or Necrotising Fasciitis; a 10-year cross-sectional study. Arch Acad Emerg Med. 2021;9: e33.
Acknowledgements
Not applicable.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
Zongqi He, Shuguang Zhen, and Bensheng Wu conceived and designed the study. Qingyun You, Jing Guan, Jun Du, and Yangyang Miao collected all clinical and laboratory data and were responsible for the literature review. Zongqi He, Xinxin Bai, and Yuhua Ma conducted the statistical analysis. All authors contributed significantly to drafting and revising the manuscript. All authors gave final approval for the manuscript to be published.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Suzhou Traditional Chinese Medicine Hospital (2024 Ethics Approval 004). This study was based on retrospective data collected by reviewing participants’ medical records. Informed consent from participants was waived due to the study’s retrospective nature. Additionally, some participants have passed away due to the severity of their illness, making it impossible to obtain informed consent from them.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
You, Q., Guan, J., Wu, B. et al. Fournier’s Gangrene: clinical case review and analysis of risk factors for mortality. BMC Surg 24, 251 (2024). https://doi.org/10.1186/s12893-024-02547-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12893-024-02547-4