Abstract
Background
Although lithium is considered the gold-standard treatment for bipolar disorder (BD), it is associated with a variety of major endocrine and metabolic side effects, including parathyroid hormone (PTH) dependent hypercalcemia. Aside from surgery and medication discontinuation, there are limited treatments for hypercalcemia. This paper will assess data from a randomized controlled trial (RCT).
Methods
This is a secondary analysis of an RCT that explored the effects of atorvastatin (n = 27) versus placebo (n = 33) on lithium-induced nephrogenic diabetes insipidus (NDI) in patients with BD and major depressive disorder (MDD) using lithium (n = 60), over a 12-week period. This secondary analysis will explore serum calcium levels and thyroid stimulating hormone (TSH) measured at baseline, week 4, and week 12.
Results
At 12-weeks follow-up while adjusting results for baseline, linear regression analyses found that corrected serum calcium levels were significantly lower in the treatment group (mean (M) = 2.30 mmol/L, standard deviation (SD) = 0.07) compared to the placebo group (M = 2.33 mmol/L, SD = 0.07) (β = − 0.03 (95% C.I.; − 0.0662, − 0.0035), p = 0.03) for lithium users. There were no significant changes in TSH.
Conclusion
In lithium users with relatively normal calcium levels, receiving atorvastatin was associated with a decrease in serum calcium levels. Although exciting, this is a preliminary finding that needs further investigation with hypercalcemic patients. Future RCTs could examine whether atorvastatin can treat PTH dependent hypercalcemia due to lithium and other causes.
Similar content being viewed by others
Background
Bipolar disorder (BD) affects over 800,000 Canadians at least once in their life [1]. Lithium is considered the gold standard treatment for BD and treatment-resistant major depressive disorder (MDD), with 30–40% of patients responding better to lithium than any other treatments [2, 3]. However, lithium’s use has declined in the last three decades due to the risk of chronic kidney disease and other adverse effects, with only 8% of BD patients currently receiving this drug in America [4,5,6,7]. There are complementary and alternative therapies that can be used in conjunction with medications for BD, but lithium remains as the gold standard [8].
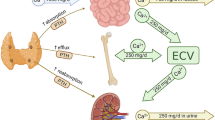
One of lithium’s important endocrine and metabolic adverse effects is hypercalcemia [9]. Health Canada and the U.S Food and Drug Administration (FDA) issued a black-box warning for lithium highlighting the risk of hypercalcemia and hyperparathyroidism [10, 11]. They have advised monitoring calcium levels throughout treatment and if hypercalcemia occurs, lithium withdrawal may be necessary as there are limited treatment options [11]. Approximately 2.0% of non-geriatric and 5.1% of geriatric lithium users have hypercalcemia and 23% of users have elevated PTH levels [12]. Lithium can alter calcium concentrations by increasing reabsorption of calcium within the loop of Henle and may also interfere with feedback mechanisms in the parathyroid, therefore preventing the suppression of parathyroid hormone (PTH) when calcium levels are high [13]. Hypercalcemia and hyperparathyroidism, can lead to acute kidney injury, chronic kidney disease, nervous system defects, and decreased skeletal health [4, 5, 14]. To date, surgery is the recommended treatment for hyperparathyroidism for patients with parathyroid adenomas and hyperplasia with symptoms caused by hypercalcemia [15]. The other unpalatable option to treat lithium-associated hypercalcemia is lithium discontinuation, which is associated with very high (> 33–50%) rates of mood disorder relapse [16, 17].
An additional metabolic side effect of lithium is hypothyroidism [4]. Lithium users have a two-fold increased risk of hypothyroidism in comparison to the general population [12], and 33% of chronic lithium users over 65 years of age developed hypothyroidism, usually within 1–7 years of initiation [18].
New treatments for lithium-associated adverse effects are needed. Observational and animal data suggest that statins may be helpful to improve lithium-induced nephrogenic diabetes insipidus (NDI) [19,20,21]. NDI is closely linked to other adverse effects associated with lithium use [22], and can have shared mechanisms (e.g. GSK3Beta inhibition with lithium use has been linked to effects on kidney function, diabetes, cancer) [23,24,25]. Thus, statins, given their pleiotropic effects, could be explored as a potential candidate for reducing serum calcium and other endocrine/metabolic adverse effects.
This secondary analysis of a randomized controlled trial (RCT) will assess whether atorvastatin use in lithium users can have an effect on serum calcium and thyroid stimulating hormone (TSH) [26,27,28].
Methods
Participants and study design
Data will be used from a pre-existing large double blind RCT conducted on lithium users to assess the effect of atorvastatin (20 mg/day) vs placebo on lithium induced NDI in patients with BD (n = 54) and MDD (n = 6). This double-blind RCT (Clinicaltrials.gov ID: NCT02967653) was conducted over a 12-week period [26,27,28]. For this secondary analysis, the RCT data will be used to assess whether atorvastatin versus placebo can reduce calcium levels in lithium users with BD and MDD. This study was approved by the local research ethics board at Douglas Mental Health University Institute (DMHUI), McGill University Health Centre (MUHC), and Jewish General Hospital (JGH) in Montreal, Canada and took place at these hospitals.
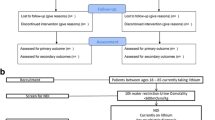
Participants were required to provide written informed consent prior to participating. Participants were considered eligible if they met the following inclusion criteria: 18–85 years old, psychiatric diagnosis of BD or MDD, currently using lithium for at least 2 months prior, and have partial NDI (urine osmolality of < 600 mOsm/Kg) or complete NDI (urine osmolality of < 300 mOsm/Kg). Participants not meeting this criteria were excluded from the study. A sample of n = 60 participants will allow an effect size of 0.34 at two-tailed alpha = 0.05 and Power (1-Beta) =0.8 [28]. For the detailed study methodology and Consort Diagram, refer to the study protocol and the original RCT [26,27,28].
Intervention and control group
Participants were randomly assigned 1:1 to the intervention group (20 mg/day atorvastatin for 12 weeks) or placebo control group. The placebo control group received a placebo pill that was similar to the atorvastatin pill for the duration of the 12 weeks. Over the 12-week period, participants were closely monitored and required to visit the data collection site (DMHUI, JGH or MUHC) at baseline, week 4, and week 12 for testing.
Outcomes
Upon each visit, blood and urine samples were collected after a mandatory 10-hour water restriction and a 12-hour fasting period. A variety of other tests took place to ensure safety of patients, including mood assessments and questionnaires (refer to study protocol and original RCT) [28].
This secondary analysis will assess serum calcium, thyroid stimulating hormone (TSH), and low-density lipoproteins (LDL) that were previously collected in the original RCT. LDL was previously reported in the original RCT [27]. Established literature and medical guidelines were used to determine which values were considered within normal ranges for all blood and urine tests [29,30,31].
The outcomes for this study are changes in serum calcium levels and TSH at 12-weeks follow-up adjusted for baseline in the atorvastatin compared to placebo group. LDL levels will be used as a proxy for atorvastatin treatment adherence (in addition to pill counts). All lab tests were collected at the same laboratory (DMHUI) throughout the study.
Data analysis
Demographic characteristics were described using descriptive statistics (e.g., mean and standard deviation or frequency and percentages). To assess adequacy of randomization, baseline clinical and demographic characteristics were compared in the treatment versus placebo group using Chi-square, Mann-Whitney U, and t-tests. Normality was assessed using the Shapiro-Wilk Test and a linear regression model was used to assess the relationship between continuous variables. For the study outcomes, linear regressions were used to assess the differences in the treatment versus placebo group between the adjusted baseline and week 12. For this analysis, a linear regression model is used where the change in calcium from baseline to 12 week is treated as a dependent variable while adjusting for possible confounders/covariates and baseline value. The current international standard used by biostatisticians recommends that when assessing continuous variables in RCTs that a linear regression of raw scores adjusted for baselines should be used [32]. All statistical analyses were computed on The R Project and Statistical Package for the Social Science (SPSS) software version 25.0. Statistical significance was set to a p-value of less than or equal to 0.05.
Results
Demographic characteristics
A total of 60 participants (35 females, 25 males) were recruited for this study, with four participants dropping out (Retention Rate = 93.3%). There were 27 participants (48.1% females, 51.9% males) who were randomly assigned to the treatment group and 33 (66.7% females, 33.3% males) assigned to the placebo group. There were no significant differences in demographics characteristics between the treatment and placebo group except that participants in the treatment group had: a higher degree of education (χ2 = 9.696, p = 0.021) and a lower prevalence of antidepressant use (χ2 = 4.42, p = 0.035). Table 1 shows additional demographic and clinical characteristics of the study.
Study outcome
After adjusting for baseline values, corrected calcium levels at 12-week follow-up were significantly lower in the treatment group (Mean (M) = 2.30 mmol/L, Standard Deviations (SD) = 0.07) compared to the placebo group (M = 2.33 mmol/L, SD = 0.07) (β = − 0.03 (95% C.I.; − 0.07, − 0.0035), p = 0.03) (Table 2, Fig. 1). There were no reports of patients with hypercalcemic or hypocalcemic serum calcium levels in this sample at baseline – the maximum serum calcium level was 2.62, with a minority of patients with a baseline serum calcium level ≥ 2.5 mmol/L, n = 2.
No statistical significance was found between the treatment and placebo group for TSH (p > 0.05) (Table 2).
As expected, LDL was significantly reduced in the atorvastatin treatment group (M = 1.69 mmol/L, SD = 0.79) compared to placebo (M = 3.07 mmol/L, SD = 0.98) at week 12 (β = − 1.07 (95% C.I.; − 1.38, − 0.77), p = 4.85 × 10− 9).
Discussion
This is the first randomized controlled trial, to our knowledge, that has demonstrated the potential of atorvastatin to lower serum calcium levels in lithium users. In fact, we are not aware of any human or animal studies that have evaluated the effect of statins on serum calcium levels. The main finding of our study was that serum calcium levels had significantly decreased in individuals using atorvastatin compared to individuals in the placebo group who were using lithium. These results provide helpful preliminary information that can lead to future large RCTs with hypercalcemic patients.
The mechanism by which atorvastatin could improve calcium levels remains unclear. The ability of statins to have pleiotropic mechanisms such as with cancer, testosterone, androgens, diabetes, and more, makes this a unique drug [33,34,35]. It appears that several lithium-associated adverse effects, including metabolic ones, occur concurrently with NDI [22]. This suggests a similar biological mechanism to NDI, such as effects on GSK3β or intracellular calcium signalling pathways. Lithium decreases the aquaporin 2 protein, leading to NDI, which can also affect calcium levels. Statins can reverse this effect by increasing aquaporin 2, which may play a role in affecting calcium levels [36]. The exact biological mechanisms could be explored further.
Since we processed all calcium and other lab tests at a single laboratory (DMHUI) and the standard deviations were very narrow, laboratory error is unlikely. However, a mean decrease of 0.03 mmol/L relative to placebo may be of limited clinical relevance. Considering this study had a relatively normo-calcemic sample of lithium users, it is possible that atorvastatin’s potential effects on serum calcium were less prominent. Future RCTs can investigate the effect of atorvastatin on participants with diagnosed hypercalcemia. The inclusion/exclusion criteria required participants to be taking lithium for at least two months prior to the start of the study, however the participants were mostly long-term lithium users with more than 5–10 years of lithium use (average age 50.7 years). Lithium adverse effects are generally more reversible when lithium use is < 5–10 years [37], therefore these effects of lowering serum calcium levels may be more prominent in other samples with patients who have more recently initiated lithium. To assess clinical significance, studies must assess the effect of statins on patients with hypercalcemia or recent initiation of lithium.
There were no significant effects of atorvastatin on TSH. According to the literature, statins could decrease TSH levels [38], or could have no significant effect on TSH levels [39]. Hypothyroidism has been described to occur during the first seven years of lithium treatment and it is often treated by lithium discontinuation or thyroid replacement therapy once hypothyroidism is diagnosed [18, 40]. The effect of statins may have been less compelling in our short-term treatment trial but could be tested in a longer-term lithium-associated hypothyroidism prevention study. Future RCTs can also monitor triiodothyronine (T3) and thyroxine (T4) to examine the mechanism by which atorvastatin affects the thyroid.
Statins are traditionally used to reduce cholesterol levels, specifically LDL. As the levels of LDL were significantly reduced in the treatment group after receiving atorvastatin, relative to the placebo group, it indicated that there was patient adherence in taking atorvastatin.
Strengths and limitations
A strength of this study was the use of a randomized double-blind placebo-controlled study design. This type of design allowed for the mitigation of potential biases in researchers and participants (e.g., demand characteristics). Additionally, the placebo-controlled and longitudinal aspect of this study was useful for baseline comparisons to measure the actual effect of the treatment group over time. One possible limitation is that this sample only included participants with partial or complete NDI (the parent RCT was designed to assess whether atorvastatin could treat NDI), which may theoretically limit generalizability to non-NDI lithium users. Another limitation was the modest sample size and small effect size. Since this was a secondary analysis of a parent RCT, we were unfortunately missing systematic data examining vitamin D, dietary calcium intake, food frequency questionnaire, 24-hour dietary recall, 3-day dietary record, serum PTH, serum proteins, serum albumin, urinary calcium, ACE inhibitors/ARBs, loop diuretics or NSAIDs [41, 42]. Our findings that serum calcium statistically decreased in atorvastatin users were nonetheless exciting, and the next steps would be examining the effect of atorvastatin while measuring for these variables in a sample with hyperparathyroidism and hypercalcemia. Hyperparathyroidism is relatively more common in older adults and females – our findings may be particularly relevant in that subpopulation of lithium users, which could be assessed in future studies.
Conclusion
We found that atorvastatin use may reduce serum calcium levels in lithium users. This study was the first to demonstrate a link between statin effects and calcium. This effect occurred rapidly within 12 weeks of treatment on a normo-calcemic sample, thus, future studies are needed to assess proper dosing and duration of atorvastatin use in a hypercalcemic sample. We propose that the calcium-reducing effects of statins may not solely be limited to lithium users and may be beneficial to other forms of hyperparathyroidism and PTH-dependent hypercalcemia. Future studies can assess clinical significance, explore mechanism behind statin pathway and calcium metabolism, and confirm whether statins can reduce calcium in patients with hypercalcemia.
Availability of data and materials
Restrictions apply to some or all the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Abbreviations
- BD:
-
Bipolar disorder
- PTH:
-
Parathyroid hormone
- RCT:
-
Randomized controlled trial
- TSH:
-
Thyroid stimulating hormone
- M:
-
Mean
- SD:
-
Standard deviation
- FDA:
-
Food and Drug Administration
- HbA1c:
-
Hemoglobin A1C
- NDI:
-
Nephrogenic diabetes insipidus
- DMHUI:
-
Douglas Mental Health University Institute
- JGH:
-
Jewish General Hospital
- MUHC:
-
McGill University Health Centre
- LDL:
-
low-density lipoproteins
- BMI:
-
Body mass index
- SPSS:
-
Statistical Package for the Social Science
- T3:
-
Triiodothyronine
- T4:
-
Thyroxine
References
Psychology works fact sheet: bipolar disorder. Canadian Psychology Association 2022. https://cpa.ca/psychology-works-fact-sheet-bipolar-disorder/
Oquendo MA, Galfalvy HC, Currier D, et al. Treatment of suicide attempters with bipolar disorder: a randomized clinical trial comparing lithium and valproate in the prevention of suicidal behavior. Am J Psychiatry. 2011;168(10):1050–6. https://doi.org/10.1176/appi.ajp.2011.11010163.
Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381(9878):1672–82. https://doi.org/10.1016/S0140-6736(13)60857-0.
Gitlin M. Lithium side effects and toxicity: prevalence and management strategies. Int J Bipolar Disord. 2016;4(1):27. https://doi.org/10.1186/s40345-016-0068-y.
Meehan AD, Udumyan R, Kardell M, Landén M, Järhult J, Wallin G. Lithium-Associated Hypercalcemia: Pathophysiology, Prevalence, Management. World J Surg. 2018;42(2):415–24. https://doi.org/10.1007/s00268-017-4328-5.
Deedwania PC. Statins in chronic kidney disease: cardiovascular risk and kidney function. Postgrad Med. 2014;126(1):29–36.
Shulman KI, Sykora K, Gill S, et al. Incidence of delirium in older adults newly prescribed lithium or valproate: a population-based cohort study. J Clin Psychiatry. 2005;66(4):424–7. https://doi.org/10.4088/jcp.v66n0403.
Wilson LAM, White KM. Integrating complementary and alternative therapies into psychological practice: a qualitative analysis. Aust J Psychol. 2011;63(4):232–42.
Lembke A. Optimal dosing of lithium, valproic acid, and lamotrigine in the treatment of mood disorders. Primary Psychiatry. 2009;16(10):37.
Lithium Risk of Hypercalcemia and Hyperparathyroidism: For health professionals. Health Canada. Accessed September, 2020. https://www.healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2014/37903a-eng.php.
Highlights of prescribing information. U.S. Food and Drug Administration. Accessed September, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/017812s033,018421s032,018558s027lbl.pdf.
Lehmann SW, Forester BP. Bipolar disorder in older age patients: Springer; 2017.
Leo RJ, Sharma M, Chrostowski DA. A case of lithium-induced symptomatic hypercalcemia. Prim Care Companion J Clin Psychiatry. 2010;12(4). https://doi.org/10.4088/PCC.09l00917yel.
Aksakal N, Erçetin C, Özçınar B, Aral F, Erbil Y. Lithium-associated primary hyperparathyroidism complicated by nephrogenic diabetes insipidus. Ulus Cerrahi Derg. 2015;31(3):166–9. https://doi.org/10.5152/UCD.2014.2859.
Farford B, Presutti RJ, Moraghan TJ. Nonsurgical management of primary hyperparathyroidism. Mayo Clin Proc. 2007;82(3):351–5. https://doi.org/10.4065/82.3.351.
Fahy S, Lawlor BA. Discontinuation of lithium augmentation in an elderly cohort. Intern J Geriatric Psychiatry. 2001;16(10):1004–9.
Hardy BG, Shulman KI, Zucchero C. Gradual discontinuation of lithium augmentation in elderly patients with unipolar depression. J Clin Psychopharmacol. 1997;17(1):22–6.
Lieber I, Ott M, Öhlund L, et al. Lithium-associated hypothyroidism and potential for reversibility after lithium discontinuation: findings from the LiSIE retrospective cohort study. J Psychopharmacol. 2020;34(3):293–303.
Li W, Zhang Y, Bouley R, et al. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of rho GTPase. Am J Physiol Renal Physiol. 2011;301(2):F309–18. https://doi.org/10.1152/ajprenal.00001.2011.
Procino G, Milano S, Carmosino M, et al. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014;86(1):127–38. https://doi.org/10.1038/ki.2014.10.
Elie D, Segal M, Low NC, et al. Statins in the prevention of lithium-associated diabetes insipidus: preliminary findings. Kidney Int. 2015;4:862.
Rej S, Segal M, Low NC, et al. In this correspondence, preliminary data in 100 lithium users found that urine osmolality correlated with chronic kidney disease, diabetes mellitus, and hypothyroidism. We hypothesize that GSK-3β inhibition is a potential mechanism for lithium-associated medical illness. Med Hypotheses. 2015;84(6):602. https://doi.org/10.1016/j.mehy.2015.03.002.
Rybakowski JK, Abramowicz M, Szczepankiewicz A, Michalak M, Hauser J, Czekalski S. The association of glycogen synthase kinase-3beta (GSK-3β) gene polymorphism with kidney function in long-term lithium-treated bipolar patients. Int J Bipolar Disord. 2013;1(1):8.
Kishore BK, Ecelbarger CM. Lithium: a versatile tool for understanding renal physiology. Am J Physiol Renal Physiol. 2013;304(9):F1139–49. https://doi.org/10.1152/ajprenal.00718.2012.
Mancinelli R, Carpino G, Petrungaro S, Mammola CL, Tomaipitinca L, Filippini A, Facchiano A, Ziparo E, Giampietri C. Multifaceted Roles of GSK-3 in Cancer and Autophagy-Related Diseases. Oxid Med Cell Longev. 2017;2017:4629495. https://doi.org/10.1155/2017/4629495. Epub 2017 Dec 12.
Fotso Soh J, Almadani A, Beaulieu S, et al. The effect of atorvastatin on cognition and mood in bipolar disorder and unipolar depression patients: a secondary analysis of a randomized controlled trial. J Affect Disord. 2020;262:149–54. https://doi.org/10.1016/j.jad.2019.11.013.
Fotso Soh J, Beaulieu S, Trepiccione F, et al. A double-blind, randomized, placebo-controlled pilot trial of atorvastatin for nephrogenic diabetes insipidus in lithium users. Bipolar Disord. 2021;23(1):66–75.
Fotso Soh J, Torres-Platas SG, Beaulieu S, et al. Atorvastatin in the treatment of Lithium-induced nephrogenic diabetes insipidus: the protocol of a randomized controlled trial. BMC Psychiatry. 2018;18(1):227. https://doi.org/10.1186/s12888-018-1793-9.
Biondi B. The Normal TSH reference range: what has changed in the last decade? The J Clin Endocrinol Metab. 2013;98(9):3584–7. https://doi.org/10.1210/jc.2013-2760.
Goldstein D. Serum Calcium. In: Walker HK, Hall WD, Hurst JW, editors. Clinical methods: the history, physical, and laboratory examinations. Boston: Butterworths; 1990.
Modan M, Meytes D, Rozeman P, et al. Significance of high HbA1 levels in normal glucose tolerance. Diabetes Care. 1988;11(5):422–8.
Colantuoni E, Rosenblum M. Leveraging prognostic baseline variables to gain precision in randomized trials. Stat Med. 2015;34(18):2602–17. https://doi.org/10.1002/sim.6507.
Shawish MI, Bagheri B, Musini VM, Adams SP, Wright JM. Effect of atorvastatin on testosterone levels. Cochrane Database Syst Rev. 2021;1:CD013211. https://doi.org/10.1002/14651858.CD013211.pub2.
Ahmadi M, Amiri S, Pecic S, et al. Pleiotropic effects of statins: A focus on cancer. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165968. https://doi.org/10.1016/j.bbadis.2020.165968.
Kavalipati N, Shah J, Ramakrishan A, Vasnawala H. Pleiotropic effects of statins. Indian. J Endocrinol Metab. 2015;19(5):554–62. https://doi.org/10.4103/2230-8210.163106.
Bonfrate L, Procino G, Wang DQ, Svelto M, Portincasa P. A novel therapeutic effect of statins on nephrogenic diabetes insipidus. J Cell Mol Med. 2015;19(2):265–82. https://doi.org/10.1111/jcmm.12422.
Boton R, Gaviria M, Batlle DC. Prevalence, pathogenesis, and treatment of renal dysfunction associated with chronic lithium therapy. Am J Kidney Dis. 1987;10(5):329–45.
Irving SA, Vadiveloo T, Leese GP. Drugs that interact with levothyroxine: an observational study from the thyroid epidemiology, audit and research study (TEARS). Clin Endocrinol. 2015;82(1):136–41. https://doi.org/10.1111/cen.12559.
Abbasinazari M, Nakhjavani M, Gogani S. The effects of simvastatin on the serum concentrations of thyroid stimulating hormone and free thyroxine in hypothyroid patients treated with levothyroxine. Iran J Med Sci. 2011;36(2):80–3.
Shulman KI, Sykora K, Gill SS, et al. New thyroxine treatment in older adults beginning lithium therapy: implications for clinical practice. Am J Geriatr Psychiatry. 2005;13(4):299–304. https://doi.org/10.1176/appi.ajgp.13.4.299.
Mansouri M, Pahlavani N, Sharifi F, et al. Dairy consumption in relation to hypertension among a large population of university students: the MEPHASOUS study. Diabetes Metab Syndr Obes. 2020;13:1633.
Pahlavani N, Khayyatzadeh SS, Banazadeh V, et al. Adherence to a dietary approach to stop hypertension (DASH)-style in relation to daytime sleepiness. Nat Sci Sleep. 2020;12:325.
Acknowledgements
Not applicable.
Disclosure statement
SR received an investigator-initiated research grant from Satellite Healthcare (Dialysis Company) (2017–2019), received salary support from the Fonds de Recherche Quebec-Santé, and has shares in Aifred Health (AI Healthcare company). OY has salary support from the Fonds de Recherche Quebec-Santé (Junior 1). HS has a CIHR fellowship award, MITACS fellowship award, and AGE-WELL award. The remaining authors report no conflict of interest.
Funding
The original study was funded by a grant from the Kidney Foundation of Canada (KFOC170016).
Author information
Authors and Affiliations
Contributions
J.S., O.L., S.R., I.M., B.M., N.H., T.R., S.B., S.R., had substantial contribution to the conception and design of the parent RCT; K.B., J.S., H.S., I.M., A.M., were involved in the conception of the secondary analysis; K.B., J.S., O.Y., C.S., H.S., S.R., were responsible for the statistical analyses of this manuscript; All authors were involved with the interpretation of the analysis; K.B., J.S., H.S., S.R., O.Y., revised the manuscript and all authors gave final approval of the version to be published and agree to be accountable for all aspects of the work. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Research Ethics Board at DMHUI, MUHC, and JGH approved the study protocol. Written informed consent was obtained from all participants in accordance with local institutional review boards.
Consent for publication
Not applicable.
Competing interests
SR received an investigator-initiated research grant from Satellite Healthcare (Dialysis Company) (2017–2019), received salary support from the Fonds de Recherche Quebec-Santé, and has shares in Aifred Health (AI Healthcare company). OY has salary support from the Fonds de Recherche Quebec-Santé (Junior 1). The remaining authors report no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Soh, J.F., Bodenstein, K., Yu, O.H.Y. et al. Atorvastatin lowers serum calcium levels in lithium-users: results from a randomized controlled trial. BMC Endocr Disord 22, 238 (2022). https://doi.org/10.1186/s12902-022-01145-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-022-01145-w