Abstract
Background
White-spot lesions are considered an initial carious stage characterized by an outer enamel layer with significantly reduced mineralization. This study was conducted to assess the combined effect of Biomin F toothpaste and Diode laser on remineralization of white spot lesions.
Materials and methods
An invitro study conducted on a total of 30 premolars divided into three groups; Group A (Biomin F Tooth paste), Group B (Biomin F with laser application for 30 sec), Group C (Negative control). The three groups were submitted to three stages; stage 1:Baseline,stage 2:After demineralization ,and stage 3:After remineralization. In each stage, elemental analysis(calcium, phosphorus, and fluoride)was measured quantitatively using Energy Dispersive X-ray (EDX) analysis and qualitatively by micrographs using scanning electron microscope. The data were tested to find significant difference between mineral changes during stages by using (ANOVA) test and Bonferroni test.
Results
Calcium, phosphorus and fluoride ions decreased in all groups after demineralization. In stage 3, after application of remineralizing agents, Calcium ions increased significantly in groups A and B where p<.05. As regards to the phosphorus ions, a significant increase was observed in all groups with group A showed the highest gain as phosphorus level percentage change (%mass) was 56.52±18.02 . Fluoride ions increased significantly in groups A and B (p<0.05) but decreased significantly in group C. There was no statistical significant difference between group A and B (p ≥.05) in calcium, phosphorus, and fluoride level after remineralization.
Conclusion
Within the limitation of the present study, we concluded that Biomin F toothpaste is promising in the repairing of white spot lesions on the surface of the demineralized enamel. Diode laser did not affect the remineralizing ability of Biomin F toothpaste.
Similar content being viewed by others
Introduction
Typically, patients seek orthodontic intervention to enhance their aesthetic appeal. Nonetheless, the use of fixed orthodontic devices could result in the development of white spot lesions (WSLs), posing an additional aesthetic challenge for the patient. Consequently, both the patient and orthodontist may experience disappointment upon the removal of the appliances [1].
White spot lesions are recognized as the initial stage of tooth decay, characterized by a surface enamel layer showing a significant reduction in mineral content [2]. This condition is prone to deteriorate, potentially necessitating invasive treatment [3]. Enamel demineralization and the development of WSLs advance swiftly [4], often developing within a few weeks [5].
Preventive approaches for WSLs during orthodontic treatment primarily involve the utilization of fluoride-releasing varnishes [6, 7], bonding materials, and cements [8, 9]. Additionally, the application of concentrated fluoride gels [10] and daily rinsing with mouthwash [11,12,13,14] are employed to reduce enamel demineralization.
While fluoride application is effective in halting WSLs, it comes with certain constraints. The consistent use of toothpaste containing fluoride necessitates a significant presence of bioavailable calcium and phosphate ions alongside fluoride ions [15]. Furthermore, fluoride exhibits diminished effectiveness when pH level drops below 4.5, a situation often instigated by bacterial actions [16].
When treating visible white spot lesions using concentrated fluoride agents (hypermineralization), the lesion is arrested at the surface instead of allowing saliva to promote remineralization in the deeper areas [16]. Presently, there are numerous methods available to halt or reverse the advancement of WSLs utilizing low levels of fluoride, such as casein phosphopeptides–amorphous calcium phosphate (CPP-ACP) [17], Nano-hydroxyapatite, Trimetaphosphate ion and Bioactive glasses [16, 18].
In recent times, bioactive glasses have emerged as a notable advancement in dental applications, extensively researched in various studies targeting the treatment of white spot lesions through remineralization. These materials have the potential to rejuvenate and regenerate dental tissues by triggering apatite formation upon exposure to saliva or any other physiological fluid [16, 19]. These apatites can either be hydroxyapatites or fluorapatites, depending on whether fluoride is integrated into the glass structure chemical composition. Glasses contain fluoride exhibit "smart" properties, demonstrating remineralization activity enhancement in low pH environments. Biomin F is recognized as a bioactive glass-based toothpaste that incorporates low concentration of fluoride (~600ppm) to aid in the remineralization of enamel.
Biomin F possesses the characteristic of prolonged fluoride delivery over a 12-hour period through gradual dissolution of the glass [16]. This characteristic is due to the polymer that enhances the bond between the calcium in the bioglass material and the calcium on the enamel. This bonding reduces the leaching of bioactive glass material [20]. Biomin F contains small bioglass particles that aid in the infiltration of remineralizing agents into subsurface lesions [21, 22]. With its high phosphate content, Biomin F facilitates rapid apatite formation (within 6 hours) and contains fewer carbon impurities, thereby rendering enamel less soluble in acid [21, 22].
Laser technology has been utilized to decrease the rate of subsurface demineralization of enamel by modifying its crystalline structure, acid solubility, and permeability. However, it is crucial to apply lasers at a low energy level to maintain enamel integrity [23].
Following laser irradiation, enamel undergoes chemical and structural changes, including reduction in carbonates fusion and re-crystallization of hydroxyapatite crystals. These alterations enhance enamel's resistance against the acid attacks. Moreover, studies have demonstrated synergistic effects between laser treatment and topical fluoride application, leading to a significant reduction in the rate of enamel decalcification [18, 21].
Hence, this study was conducted for the evaluation of the combined impact of Biomin F toothpaste and diode laser on the remineralization of WSLs.
The null hypothesis of this study assumed that no significant difference is expected between the effect of Biomin F toothpaste coupled with diode laser and Biomin F alone on remineralization of white spot lesions.
Materials and methods
This in-vitro study was conducted at the Department of Orthodontics, Faculty of Dentistry, Alexandria University and Scanning Electron Microscope unit, Faculty of Sciences, Alexandria University. The present study was approved by the Research Ethics Committee of the Faculty of Dentistry, Alexandria University (IRB:00010556– IORG:0008839).
Sample size calculation
The sample size estimate was calculated based on an invitro study by Aidaros et al. (2022) [15] that aimed to evaluate and compare the remineralizing potential of dentifrices containing fluoride and bioactive glass on enamel by assessing the enamel structure and elemental analysis through Energy Dispersive X-ray Analysis (EDX). During sample size calculation, a beta error of up to 20% is accepted, with a study power of 80%. The alpha level was established at 5%, corresponding to a significance level of 95%. Statistical significance was assessed at a p-value < 0.05 [24]. The minimum required sample size was determined to be 9 teeth per group (number of groups=3) (Total sample size=27 teeth). Any withdrawal for any reason will be compensated by replacement to control for attrition (loss of specimen) bias. Therefore sample size will be increased to 10 teeth per group (number of groups=3) (Total sample size=30 teeth).
A total of 30 human premolars were collected from patients requiring premolars extraction during their orthodontic treatment in the Orthodontic Department, Faculty of Dentistry, Alexandria University, Egypt. Informed consent was obtained from all subjects and/or their legal guardian(s).
All patients were born and lived in areas where the typical concentration of fluoride in the drinking water was 0.36 mg/L [25]. Any calculus or tissue remnants were removed from the teeth using a scaler. Subsequently, the teeth were stored in saline until the commencement of the study.
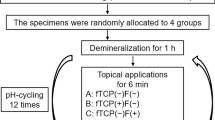
Procedures for each group are shown in the flowchart (Fig. 1).
Collection of teeth
Thirty human premolars that were extracted for orthodontic purposes were chosen for inclusion in this study. All teeth were examined macroscopically using a magnifying loupe and fulfilled the following selection criteria: Intact buccal enamel surface, with no decalcifications, cracks, or stains.Teeth previously bonded or received any chemical treatment or decayed premolars were excluded from this study. The teeth were preserved in saline until the start of the experiment.
Teeth preparation
After recruiting the appropriate teeth, all remnants were removed and teeth were cleaned with fluoride free pumice and running water. The roots of the teeth were cut 2mm under cemento-enamel junction using a diamond disk under water cooling. The crowns were embedded in self cured acrylic resin and the buccal surfaces were directed upward for easy manipulation [15] (Fig. 2a). Each sample was covered with acid resistant varnish (nail polish) at all tooth crown surfaces, leaving a window of 4mm X4mm in the middle third of the buccal surface of the premolar (Fig. 2b).
Randomization
Each tooth got a number from 1 to 30 typed at the base of the acrylic block using waterproof permanent marker. These numbers were used to randomly and equally assign the samples into 3 groups using computer generated random list.
Grouping of the teeth
Teeth were divided randomly into 3 groups
-
Group A: Biomin F tooth paste.
-
Group B: Biomin F with laser application for 30 sec .
-
Group C: Negative control group (no treatment).
Each group was placed in its own labeled beaker which contained 150 mL of artificial saliva solution at room temperature of 37°C and neutral PH to replicate the oral environment (Fig. 2c). The materials used, their specifications, compositions and manufactures are present in (Table 1).
Intervention
The study was divided into a number of stages with various procedures as the following:
-
First stage (Baseline) assessment
The assessment was conducted utilizing an environmental scanning electron microscope (JSM-IT 200-Japan) at Faculty of Sciences, Alexandria University. SEM attached with energy-dispersive X-ray (EDX) unit (Fig. 3).
The amount of remineralization was measured qualitatively by comparing the scanning electron microscope pictures and quantitatively by the Energy dispersive X-ray analysis that measured the average of three points selected in the area of concern. These values were taken at different stages of the study (before, after demineralization and after remineralization).
-
Second stage (Demineralization process)
The teeth were immersed in the demineralizing solution for 96 hours at 37°C until white spot lesions were obtained (Fig. 4 and Table 1). The samples were removed from the solution and rinsed with distilled water to stop the demineralization process and to remove any residuals of the solution [21, 26]. At this stage, evaluations were carried out using Scanning Electron Microscope (SEM) analysis alongside Energy Dispersive X-ray Analysis (EDX).
-
Third stage (Remineralization process)
In group A: Biomin F toothpaste was applied in circular motion on the demineralized region using microbrush twice daily, each lasted for two minutes and left undisturbed for 30 seconds (Fig. 5). Then the samples were rinsed carefully with distilled water to remove any excess paste. Then it was stored in artificial saliva to mimic oral environment. This procedure was repeated for two weeks (Table 1) [15, 21].
In group B: The toothpaste was applied twice daily each for two minutes in a circular motion using a microbrush and left undisturbed for 30 seconds which lasted for two weeks, then laser was applied at the 14th day (Fig. 6 and Table 2).
In group C: No treatment was received.
Laser application
Laser irradiation (Lastronix-Boland) has been done on the 14th day for 30 seconds after Laser beam activation with carbon particles and laser setting was done as shown in Table 2. The practitioner wore eye goggles for self protection from laser irradiation. The distance between the buccal surface of the tooth and laser fiber was kept 5mm using custom- made holder (Fig. 6 and Table 2) [15, 27].
The Scanning Electron Microscope assessment and Energy Dispersive X-ray Analysis (EDX) were repeated after remineralization process.
Blinding
Technician of Scanning Electron Microscope attached with EDX unit and the statistician were blinded.
Statistical analysis
-
The data were gathered and inputted into the computer using the Statistical Package for the Social Sciences (SPSS) software program for statistical analysis (ver 25) [28].
-
Kolmogorov-Smirnov test of normality revealed no significance in the distribution of the variables, so the parametric statistics were adopted [29].
-
Data were described using minimum, maximum, mean, standard deviation, standard error of the mean, and 95% CI of the mean, 25th to 75th percentile [30].
-
Comparisons were conducted among more than two independent normally distributed subgroups utilizing the one-way Analysis of Variance (ANOVA) test [31]. Post-hoc multiple comparisons [32] were performed using the Bonferroni method [33].
-
Repeated measures analysis of variance was used [34]. Model assumptions were tested and found to be satisfactory with the exception of Mauchly’s test of sphericity [35], and when it was statistically significant denoting the violation of the assumption of sphericity, Greenhouse-Geisser correction was used [36]. Pair-wise comparison was done with Bonferroni correction.
-
Linear trend analysis was used to test for within-subjects contrast [37].
Percentage change was calculated as follows:
Results
Tables 3, 4 and 5 shows the mean value (%mass) of mineral content (calcium –phosphate – fluoride) obtained from elemental analysis by using EDX for each tested group.
1) Mineral content (Ca,P,F) using EDX:
-
a.
Comparison between baseline and (After demineralization)
At baseline, there was no statistical significant difference between groups A,B and C regarding to mass% value of Ca, P and F.
After demineralization, it was shown that calcium level (% mass) significantly decreased in the three studied groups .Calcium level decreased from a mean of 31.34 ,29.98 and 30.91 (%mass) in groups A,B and C respectively at baseline to a mean of 22.64 ,20.86 and 20.16 % mass after demineralization (Fig. 7 and Table 3).
Phosphorus level % mass also significantly decreased from a mean of 16.38, 15.90 and 16.21 to 10.77, 11.34 and 10.76 %mass in groups A,B and C respectively (Fig. 8 and Table 4).
Regarding to fluoride level (%mass), there was significant decrease from 0.94, 0.87 and 0.78 to 0.62, 0.61 and 0.64 in groups A,B and C respectively (p<0.05) (Fig. 9 and Table 5).
There was no statistical significant difference among the three studied groups in Ca,P,F level after demineralization (p≥0.05).
-
b.
Comparison between (after demineralization) and (after remineralization):
After the application of the remineralizing agents, calcium ions gained a significant increase in groups A and B. Group B showed the highest gain of ions as percentage change (%) was 40.88±12.23. There was no statistical difference between group A and B after remineralization (p≥0.05) and both were significantly higher than the control group (p<0.05) (Table 3 and Fig. 7).
As regards the phosphorus ions, a significant increase was observed in all groups. Group A showed the highest gain as P level percentage change(%mass) was 56.52±18.02 and group C showed the least gain as P level percentage change was 32.60±23.26 .There was no significant difference between group A and group B but both were significantly higher than group C after remineralization (p<0.001) (Fig. 8 and Table 4).
Fluoride ions increased significantly in groups A and B (p<0.05) but decreased significantly in group C as shown in percentage change formula (-1.78±18.65). There was no statistical difference between group A and B (p≥.05) in fluoride level after remineralization (Fig. 9 and Table 5). Both groups A and B were significantly higher than group C (p=.006).
-
c.
Comparison between baseline and after remineralization
When comparing the mean values of minerals % mass between the baseline and the last stage after application of the remineralizing agents, it was obvious that there was no significant difference in minerals percentage (Ca,P,F) between baseline and after remineralization in groups A and B (p≥0.05).
While in the control group, there was significant decrease in the Ca,P and F %mass between baseline and after remineralization process.
2. Environmental scanning electron microscope (ESEM) analysis
The sample surface characteristics at each stage were described using ESEM at a magnification of x2000. At base line, micrographs showed smooth enamel surface. After demineralization, samples showed honey comb appearance which represent areas of minerals dissolution. After application of the remineralizing agent, samples showed partial restoration of enamel surface structure (Figs. 10, 11, 12, 13 and 14).
Discussion
This study was conducted to evaluate the impact of diode laser and Biomin F toothpaste on the remineralization of WSLs.
The null hypothesis was accepted as there was no significant difference between the effect of Biomin F toothpaste coupled with diode laser and Biomin F alone on the remineralization of white spot lesions.
The natural physiological processes of demineralization and remineralization in tooth structure may be disrupted when there is an imbalance between pathogenic and protective factors [16, 18]. From a scientific standpoint, it has been recognized that salivary dysfunction, fermentable carbohydrates, and cariogenic bacteria play significant roles as pathogenic factors [26]. Calcium and phosphate ions presence in a supersaturated state within human saliva enables it to potentially facilitate the remineralization of enamel [38]. However, if acid challenges exceed this physiological remineralization process, alternative therapeutic interventions are needed to strengthen remineralization [17, 26].
Therefore, Biomin F was utilized in this study as it is a bioactive glass material containing a low level of fluoride. Ali et al [39] noted in their study that Biomin F toothpastes exhibited lower total fluoride content compared to the values asserted by their respective companies (approximately 400 ppm).
The majority of studies conducted on Biomin F have explored its efficacy in occluding dentinal tubules and addressing hypersensitivity [40, 41]. A systematic review focused on investigating the role of bioactive glass in enamel remineralization highlighted the significant contribution of fluoride-containing bioactive glass dentifrice, specifically Biomin F, in enamel regeneration [18].
Aidaros et al [15] carried out an in-vitro investigation utilizing SEM and elemental analysis to assess the mineral composition of extracted permanent third molars prior to and following the application of remineralizing agents, which included Biomin F. Their study involved comparing these agents, employing a similar application regimen to that of the current study (two minutes, twice daily for two weeks). They utilized the materials in the form of toothpaste and concluded that the combination of fluoride with bioactive glass technology, as seen in Biomin F toothpaste, had the most significant impact on the demineralized enamel surface. This finding aligned with our study, which demonstrated that Biomin F possessed the ability to remineralize white spot lesions (WSLs) to restore them to their baseline mineral content.
In this current study, laser was examined in conjunction with Biomin F, given that numerous studies have evaluated various types of lasers, including CO2, Nd:YAG, Er:YAG [42], and diode lasers:, utilizing different parameters for caries prevention and enamel remineralization, either with [43, 44] or without [45] fluoride-containing agents.
The present study revealed that the mineral content following remineralization reached the baseline mineral level in both test groups. This finding contradicted the results reported by Omran et al. [21] as the mean calcium mass percentage after remineralization was significantly lower compared to the baseline calcium mass percentage in the Biomin group. Notably, both studies employed the same Biomin F application protocol (two minutes twice daily for two weeks). This variation might be explained by the fact that Omran-T applied Biomin F as toothpaste slurries and subjected the samples to a shorter demineralization period (72 hours), while the present study utilized it in the form of toothpaste, as commonly used by orthodontic patients. Nonetheless, both studies concurred on the high phosphate content of Biomin F.
An intriguing observation emerged from the study comparing laser treatment with a bioactive glass material (Novamin) using SEM: laser therapy did not provide additional benefits to Novamin in the process of remineralizing the enamel surface [26]. In the current study, where Biomin F demonstrated the capability to remineralize the enamel surface, the diode laser did not exhibit a synergistic effect in enamel remineralization, as indicated by the insignificant difference between group A and B. Similarly, comparable outcomes were observed when Novamin was utilized alongside laser therapy.
The potential rationale for the findings of this study could be that the efficacy of the bioactive glass material (Biomin F) relies on its interaction with physiological aqueous solutions, leading to the release of calcium, phosphorus, and fluoride. However, when a diode laser is employed, a certain degree of heat is generated within the treated surface, typically ranging from 1 to 6 degrees Celsius [46]. As a consequence, this leads to some dryness and removal of moisture from the paste, which is essential for mineral release.
The analysis of SEM micrographs enabled us to observe the notable regeneration of the enamel structure and the deposition of mineral crystals, a result that aligns with findings reported by Bakrey et al., who observed the deposition of mineral crystals blocking the dentinal tubules [40] after using Biomin F.
This study has a limitation that we must consider the dynamic complex system in oral environment which may differ from the in-vitro study employed in the present work.
Conclusion
Within the limitation of the present study, we concluded that Biomin F toothpaste is promising in repairing the white spot lesions on the surface of the demineralized enamel. Diode laser did not affect the remineralizing ability of Biomin F toothpaste.
Recommendation
Clinical studies are needed for more evaluation of the benefits of these approaches. It is recommended to evaluate the synergistic effect between laser and biomin F in decreasing the white spot lesions depth with a variation of laser protocols and exposure time. Microhadness testing is recommended to confirm the remineralization effect.
Availability of data and materials
The data and materials used to support the findings of this study are available from the corresponding authors upon request.
References
Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am J Orthod Dentofacial Orthop. 1989;96:423–7.
Palamara J, Phakey PP, Rachinger WA, Orams HJ. Ultrastructure of the intact surface zone of white spot and brown spot carious lesions in human enamel. J Oral Pathol. 1986;15:28–35.
Featherstone JD. The science and practice of caries prevention. J Am Dent Assoc. 2000;131:887–99.
Ogaard B, Rølla G, Arends J, ten Cate JM. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am J Orthod Dentofacial Orthop. 1988;94:123-8.
Melrose CA, Appleton J, Lovius BB. A scanning electron microscopic study of early enamel caries formed in vivo beneath orthodontic bands. Br J Orthod. 1996;23:43–7.
Demito CF, Vivaldi-Rodrigues G, Ramos AL, Bowman SJ. The efficacy of a fluoride varnish in reducing enamel demineralization adjacent to orthodontic brackets: an in vitro study. Orthod Craniofac Res. 2004;7:205–10.
Øgaard B, Larsson E, Henriksson T, Birkhed D, Bishara SE. Effects of combined application of antimicrobial and fluoride varnishes in orthodontic patients. Am J Orthod Dentofacial Orthop. 2001;120:28–35.
Donly KJ, Istre S, Istre T. In vitro enamel remineralization at orthodontic band margins cemented with glass ionomer cement. Am J Orthod Dentofacial Orthop. 1995;107:461–4.
Dubroc GC Jr, Mayo JA, Rankine CA. Reduction of caries and of demineralization around orthodontic brackets: effect of a fluoride-releasing resin in the rat model. Am J Orthod Dentofacial Orthop. 1994;106:583–7.
Tezel H, Ergücü Z, Onal B. Effects of topical fluoride agents on artificial enamel lesion formation in vitro. Quintessence Int. 2002;33:347–52.
Benson PE, Parkin N, Millett DT, Dyer FE, Vine S, Shah A. Fluorides for the prevention of white spots on teeth during fixed brace treatment. Cochrane Database Syst Rev. 2004:Cd003809.
Geiger AM, Gorelick L, Gwinnett AJ, Benson BJ. Reducing white spot lesions in orthodontic populations with fluoride rinsing. Am J Orthod Dentofacial Orthop. 1992;101:403–7.
Kalha A. Some evidence that fluoride during orthodontic treatment reduces occurrence and severity of white spot lesions. Evid Based Dent. 2004;5:98–9.
Linton JL. Quantitative measurements of remineralization of incipient caries. Am J Orthod Dentofacial Orthop. 1996;110:590–7.
Aidaros N, Eliwa ME, Kamh R. Remineralization Efficiency of Different Toothpastes on Human Enamel Subjected to Acid Challenge: in Vitro Study. Al-Azhar DJ Girls. 2022;9:61–72.
Goswami M, Saha S, Chaitra TR. Latest developments in non-fluoridated remineralizing technologies. J Indian Soc Pedod Prev Dent. 2012;30:2–6.
Adel SM, El-Harouni N, Vaid NR, editors. White Spot Lesions: Biomaterials, Workflows and Protocols. In: Seminars in Orthodontics; 2023: Elsevier.
Taha AA, Patel MP, Hill RG, Fleming PS. The effect of bioactive glasses on enamel remineralization: A systematic review. J Dent. 2017;67:9–17.
Elkabbany SMH, Mosleh AA, Metwally NI. Remineralization effect of diode laser, Nanoseal®, and Zamzam water on initial enamel carious lesions induced around orthodontic brackets. J Nat Sci Med. 2021;4:50–7.
Ramadoss R, Padmanaban R, Subramanian B. Role of bioglass in enamel remineralization: Existing strategies and future prospects-A narrative review. J Biomed Mater Res B Appl Biomater. 2022;110:45–66.
Omran TM, Mostafa MH, El-Raouf A, Eman M. Assessment of Remineralizing Effect of Bioactive Glass Based Toothpastes: an In-Vitro Comparative Study. Al-Azhar DJ Girls. 2021;8:491–9.
Bakry AS, Abbassy MA, Alharkan HF, Basuhail S, Al-Ghamdi K, Hill R. A Novel Fluoride Containing Bioactive Glass Paste is Capable of Re-Mineralizing Early Caries Lesions. Materials (Basel). 2018;11.
Farhadian N, Rezaei-Soufi L, Jamalian SF, Farhadian M, Tamasoki S, Malekshoar M, et al. Effect of CPP-ACP paste with and without CO2 laser irradiation on demineralized enamel microhardness and bracket shear bond strength. Dental Press J Orthod. 2017;22:53–60.
Curran-Everett D. Evolution in statistics: P values, statistical significance, kayaks, and walking trees. Adv Physiol Educ. 2020;44:221–4.
Adel SM, Marzouk ES, El-Harouni N. Combined effect of Er, Cr:YSGG laser and casein phosphopeptide amorphous calcium phosphate on the prevention of enamel demineralization. Angle Orthod. 2020;90:369–75.
Lara-Carrilloa E, Doroteo-Chimalb C, Lopez-Gonzaleza S, Morales-Luckiec RA, Olea-Mejiac OF, Kubodera-Itoa T, et al. Remineralization effect of low-level laser and amorphous sodium–calcium–phosphosilicate paste in teeth with fixed orthodontic appliances. Tanta Dent J. 2016;13:55–62.
Bahrololoomi Z, Zarebidoki F, Mostafalu N. The effect of different re-mineralizing agents and diode laser irradiation on the microhardness of primary molar enamel: An in vitro study. Laser Ther. 2019;28:187–92.
IBM Corp. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.; Released 2017.
Field A. Discovering Statistics Using IBM SPSS Statistics. 4th ed. London, California, New Delhi: SAGE Publications Ltd; 2013.
Greenland S, Senn SJ, Rothman KJ, Carlin JB, Poole C, Goodman SN, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337–50.
Montgomery D. Experiments with a Single Factor. In: The Analysis of Variance. Design and Analysis of Experiments. I. Ch 3: John Wiley & Sons; 2001.
Lowry R. One Way ANOVA–Independent Samples. Vassar. edu. Retrieved on December 4th. 2008 Retrieved on December 4th. 2008.
Ruxton GD, Beauchamp G. Time for some a priori thinking about post hoc testing. Behav Ecol. 2008;19:690–3.
Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61:310–7.
Mauchly JW. Significance test for sphericity of a normal n-variate distribution. Ann Math Stat. 1940;11:204–9.
Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112.
Gurevitch J, Chester S. Analysis of repeated measures experiments. Ecology. 1986;67:251–5.
Willmot DR. White lesions after orthodontic treatment: does low fluoride make a difference? J Orthod. 2004;31:235-42; discussion 02.
Ali S, Farooq I, Al-Thobity AM, Al-Khalifa KS, Alhooshani K, Sauro S. An in-vitro evaluation of fluoride content and enamel remineralization potential of two toothpastes containing different bioactive glasses. Biomed Mater Eng. 2020;30:487–96.
Bakry AS, Al-Harbi N, Al-Hadeethi Y, Abbassy MA, Katturi N, Xin B, et al. Invitro evaluation of new treatment for dentin hypersensitivity using BioMin F and BioMin C. J Non Cryst Solids. 2023;602: 122072.
Arshad S, Zaidi SJA, Farooqui WA. Comparative efficacy of BioMin-F, Colgate Sensitive Pro-relief and Sensodyne Rapid Action in relieving dentin hypersensitivity: a randomized controlled trial. BMC Oral Health. 2021;21:498.
Yavagal CM, Chavan VV, Yavagal PC. Laser induced enamel remineralization: A systematic. Int J Appl Dent Sci. 2020;6:168–73.
Vitale MC, Zaffe D, Botticell AR, Caprioglio C. Diode laser irradiation and fluoride uptake in human teeth. Eur Arch Paediatr Dent. 2011;12:90–2.
Alqahtani MA, Almosa NA, Alsaif KA, Alsaif NM, Aljaser YJ. Effect of topical fluoride application and diode laser-irradiation on white spot lesions of human enamel. Saudi Dent J. 2021;33:937–43.
Oliveira MRC, Kato IT, Oliveira LHC, Oliveira PHC, Alves CB, Benetti C, et al., editors. Dental Enamel Remineralization Following Diode Laser Irradiation. Latin American Conference on Biomedical Engineering; 2022: Springer.
Aoki A, Sasaki KM, Watanabe H, Ishikawa I. Lasers in nonsurgical periodontal therapy. Periodontol. 2000;2004(36):59–97.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This clinical research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Nazla O. Tamish and Ahmed M. Madian Guide the correct methodology for the trial and supervise the work . Help the student in interpreting results and revising the manuscript. Amira I. Eldeeb contributed to the concept, methodology and the design of the study.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was approved by the Research Ethics Committee of the Faculty of Dentistry, Alexandria University (IRB:00010556– IORG:0008839).Informed consent was obtained from all subjects and/or their legal guardian(s).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Eldeeb, A.I., Tamish, N.O. & Madian, A.M. Effect of Biomin F toothpaste and Diode laser on remineralization of white spot lesions (in vitro study). BMC Oral Health 24, 866 (2024). https://doi.org/10.1186/s12903-024-04589-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04589-9