Abstract
Background
Oral cancer (OC) is a common malignancy in clinical practice. Saliva testing is a convenient and noninvasive early diagnostic technique for OC. Several salivary cytokines have been identified as potential biomarkers for OC, including IL-8, IL-6, TNF-α, IL-1β, and IL-10. Nonetheless, the optimal cytokine for OC diagnosis remains inconclusive and highly contentious.
Methods
PubMed, Embase, Web of Science, and Cochrane Library databases were comprehensively retrieved to collect all case–control studies on OC. A meta-analysis was performed to compare the levels of salivary IL-8, IL-6, IL-10, TNF-α, and IL-1β in OC patients and healthy controls. Network meta-analysis (NMA) was carried out to probe into the accuracy of these salivary cytokines in diagnosing OC.
Results
This analysis included 40 studies, encompassing 1280 individuals with OC and 1254 healthy controls. Significantly higher levels of salivary IL-8, IL-6, TNF-α, IL-1β, and IL-10 were observed in patients with OC in comparison to healthy controls. The results of NMA showed that TNF-α had the highest diagnostic accuracy for OC, with a sensitivity of 79% and a specificity of 92%, followed by IL-6 (sensitivity: 75%, specificity: 86%) and IL-8 (sensitivity: 80%, specificity: 80%).
Conclusion
This study suggests that IL-8, IL-6, IL-10, TNF-α, and IL-1β may be potential diagnostic biomarkers for OC. Among them, TNF-α, IL-6, and IL-8 are highly accurate in the diagnosis of OC. Nevertheless, further studies that eliminate other confounding factors are warranted, and more standardized procedures and large-scale studies are needed to support the clinical use of saliva testing.
Similar content being viewed by others
Introduction
Oral cancer (OC) is one of the most frequent aggressive malignancies worldwide. It was estimated that there were 377,713 new OC cases (approximately 2% of all cancer cases) and 177,757 deaths (1.8% of all cancer-related deaths) in 2020 around the world [1]. This cancer may locally invade the tongue, lips, lower and upper gums, hard palate, retromolar trigone, and floor of the mouth, and even metastasize to distant sites at an advanced stage [2]. Squamous cell carcinoma (OSCC) represents approximately 90% of OC cases [3], and other OC types encompass salivary gland tumors, lymphomas, and sarcomas [4]. Due to asymptomatic characteristics, around 50% of individuals with OC are diagnosed at a late stage. Accordingly, the treatment of such patients is often aggressive and mutilating, adversely affecting their quality of life [5]. The 5-year relative survival rate is about 85.1% for localized OC, and is as low as 69.1% and 39.3% for lymphatic metastasis and distant metastasis, respectively [6]. Therefore, early diagnosis of OC is essential to reduce the mortality rate and ameliorate the quality of life of OC patients.
Currently, commonly-used diagnostic approaches for OC include traditional oral visual examination (VOE), classical biopsy followed by histopathological assessment, vital staining (such as toluidine blue), and radiographic imaging [7]. Among them, biopsy and histopathological examinations are still the standard procedures for the diagnosis of OC [8]. Besides, the analysis of body fluids, especially saliva, is a promising and potential alternative to biopsy for early OC detection since it is adjacent to cancer cells, readily available, non-invasive, and inexpensive [9]. Human saliva consists of cytokines, circulating cells, DNA and RNA molecules, and derivatives of tissue and extracellular vesicles (EVs) [10]. Cytokines, as key mediators of cell communication, can control complex and dynamic cell–cell interactions and regulate various cancer-related pathways in the tumor microenvironment [11]. In histiocytology, cytokines such as IL-6 and IL-8 that are important in pro-inflammatory and pro-angiogenic responses can be detected in cell lines, tissue specimens, and serum of patients with Head and Neck squamous cell carcinoma (HNSCC includes OSCC, pharyngeal squamous cell carcinoma, laryngeal squamous cell carcinoma, nasal squamous cell carcinoma, paranasal sinuses squamous cell carcinoma etc. [12, 13]. Moreover, a large scale gene expression profiling assisted by laser capture microdissection and microarray analysis was carried out, identifying the expression of 2 cellular genes: interleukin (IL)6 and IL-8 which are uniquely associated with OSCC [14]. Besides, a direct link between oral inflammation and cancer invasion was established by showing that neutrophils increase OSCC invasion through a tumor necrosis factor (TNFα)-dependent mechanism [15]. According to many case–control studies and previous systematic reviews and meta-analyses, the average levels of salivary cytokines such as IL-6, IL-8, TNF-α, IL-1β, and IL-10 are significantly different between OSCC, oral potentially malignant disorders (OPMD), oral leukoplakia (OL) and control saliva. Previous studies also showed that IL-6 and IL-8 concentrations in saliva were associated with different stages of OSCC and the presence of cervical metastasis [6, 16, 17]. Taken together, these findings suggest that these salivary cytokines may be potential diagnostic biomarkers for OC.
Nevertheless, there is no consensus on which biomarkers have the best diagnostic value for OC. Network meta-analysis for diagnostic tests (NMA-DT) is a new analysis approach that allows simultaneously comparing multiple diagnostic tests, at multiple test thresholds [18]. This novel technique can lessen bias and enhance statistical accuracy in the comparison of the diagnostic performance of multiple tests by borrowing strength from indirect evidence [19]. Therefore, this network meta-analysis was implemented to evaluate and compare the accuracy of five common salivary cytokines (IL-8, IL-6, TNF-α, IL-1β, and IL-10) in diagnosing OC and to rank these diagnostic tests based on a superiority index.
Methods
Protocol and registration
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Diagnostic Test Accuracy (PRISMA-DTA) Checklist (The prisma-DTA checklist is in the Supplementary Table S5) [20]. The study protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO; registration ID: CRD42023430533).
Inclusion and exclusion criteria
The following studies were included: (1) case–control studies on human subjects; (2) patients diagnosed with OC; (3) OC was confirmed pathologically; (4) the controls were healthy subjects without systemic diseases; (5) studies that reported at least one of the diagnostic sensitivity and specificity of IL-8, IL-6, IL-10, TNF-α, or IL-1β in OC patients compared to healthy controls with no systemic disease and the concentrations of these salivary biomarkers.
The exclusion criteria were as follows: (1) duplicate publications; (2) reviews, systematic reviews, meta-analyses; (3) conference summaries/abstracts, case reports, guidelines, letters to editors, editorials, study protocols, brief correspondences, animal experiments; (4) full texts unavailable; (5) outcome data unextractable; (6) non-English articles.
Literature search
As of 10 April 2023, two independent investigators (Lijun, Huang (L, H) and Mingsi, Deng (M, D)) extensively retrieved electronic databases, including PubMed, Embase, Web of Science, and Cochrane Library. No restrictions were imposed on study type, date/time, or publication status. Search terms encompassed “cytokine”, “saliva”, and “oral cancer” in combination with “interleukin” or “interferon”. The specific search strategy is delineated in Table S1. Besides, the reference lists of related studies and reviews were manually retrieved.
Study selection
All retrieved studies were imported into EndNote 20 to eliminate duplicate records. Two investigators (L, H and Fen, Luo (F, L)) independently checked the titles and abstracts to remove irrelevant articles. A full-text review was then conducted to select eligible studies. A third reviewer (M, D) was consulted to settle any disagreements that arose throughout the literature screening process.
Data extraction and quality assessment
Relevant data were independently extracted from included articles by two investigators, encompassing title, first author, publication year, country, study design, sample size, gender, patient age, the outcomes (levels of different cytokines in saliva, sensitivity and specificity, true positives (TP), false positives (FP), true negatives (TN) and false negatives (FN) in predicting OC). Dissents, if any, were settled by consulting a third investigator.
The Newcastle–Ottawa Scale (NOS) [21] was employed to evaluate the quality of the included articles. Two researchers (L, H and M, D) independently assessed 8 items in three domains: selection of case and control groups, comparability between groups, and exposure factors. Except for comparability, which has 2 points, each item in the remaining domains has 1 point. The total score ranges from 0 to 9 points. Studies rated 8 or higher were regarded as high quality, while those rated 4 or less were deemed to be of low quality. Meanwhile, studies with a score of 5–7 were considered to have a medium quality. The Quality Assessment on Diagnostic Accuracy Studies (QUADAS) 2 tool were used to assess the risk of bias [22], too. The risk of bias was assessed in four key domains including patient selection, index test(s), reference standard, and flow and timing. Concerns regarding applicability (patient selection, index test(s), and reference standard) were determined. The degree of bias and applicability were expressed as high, low, or unclear, in accordance with the guidance documents. The quality assessment was implemented independently by two researchers. Any dissents were resolved by a third researcher (F, L).
Statistical analyses
A traditional meta-analysis was carried out to pool data from studies comparing the levels of salivary IL-8, IL-6, IL-10, TNF-α, and IL-1β in OC patients and controls (non-OC). Standardized mean difference (SMD) with 95% confidence intervals (CIs) was used as the effect size. A p < 0.05 signals statistical significance. Forest plots were generated to visually present the results. The I2 statistic was utilized to examine heterogeneity among studies. I2 > 50% indicates statistically significant heterogeneity, and therefore, a random-effects model was applied for data analysis. Potential publication bias was determined by using a funnel plot and Egger’s test.
Furthermore, a network meta-analysis for diagnostic tests (NMA-DT) was conducted to delve into which saliva cytokine is the most accurate for predicting OC. NMA-DT enables us to concurrently compare several diagnostic tests of saliv a cytokines with the gold standard, at various thresholds [23]. The relative performance, sensitivity, and specificity of the index tests were assessed in relation to standard diagnostic method for OC, and these tests were ranked utilizing the diagnostic odds ratios (DORs) and superiority index (Table S2 in the Supplementary Materials explains all statistical terms). Higher DOR and superiority values indicate higher accuracy of tests in detecting diseases. This network meta-analysis was conducted using the R package “rstan” (version 4.1.3; R Foundation for Statistical Computing, Vienna, Austria). Analysis of Variance (ANOVA) model based on the Bayesian algorithm was applied to exhibit network meta-analysis among four systems by utilizing two independent binomial distributions to describe the true positive and true negative rates between OC and non-OC patients, meantime considering the correlation between sensitivity and specificity [24]. In order to improve accuracy and compare diagnostic assays one by one, calculations were repeated 7 times (model_code = model, chains = 2, iterations = 10,000, warmup = 5000, thin = 5), and then, league tables for relative comparations were drawn. Review manager version 5.4 was employed to calculate summary receiver operating characteristic curve (SROC) values.
Results
Study selection
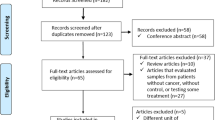
Initially, 3075 articles were obtained from the database search. After removing 1082 duplicates, 1993 articles remained. After screening the titles and abstracts, 58 articles were potentially eligible. Based on a full-text review, 18 studies were further excluded, including 4 studies without healthy-control group, 8 studies without required experimental group, 4 studies without cytokines of interest, 1 study with no outcome of interest, and 1 commentary. Finally, 40 articles were included in the traditional meta-analysis and 12 articles in the network meta-analysis. The study selection process is delineated in Fig. 1.
Study characteristics
The characteristics of the 40 included studies are presented in Table 1. These studies were published between 2004 and 2023. 19 studies were conducted in Asia [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43], 11 studies in Europe [16, 44,45,46,47,48,49,50,51,52,53], and 10 studies in North America [54,55,56,57,58,59,60,61,62,63]. A total of 1280 patients with OC (most of the patients had OSCC) and 1254 healthy controls were included. The sample size of the included studies ranged from 9 to 100, with a mean age of 46 to 73 years. All the case–control studies enrolled both adult males and females. The enzyme-linked immunosorbent assay (ELISA) was the most often utilized detection method for determining the levels of salivary cytokines, followed by the Luminex-based immunoassay, the bead-based multiplex immunoassay, and the chemiluminescent enzyme immunoassay. For example, in the study by Piyarathne et al. [44], protein levels of ILs were quantified using a commercially available sandwich enzyme-linked immune-sorbent assay (ELISA), with pre-coated plates: E-EL-H0149, E-EL-H0102 and E-EL-H0048 kits (Elabscience; Wuhan, Hubei, China). In the study by Laliberté et al. [54], cytokines were analyzed according to the immunoassay protocol for the Millipore Human Cytokine/Chemokine Magnetic Bead Assay Panel (HCYTMAG-60K-PX30 by EMD Millipore, USA) and Luminex detection. In the study by Sato, J. et al. [42], IL-6 concentrations were measured using a highly sensitive chemiluminescent enzyme immunoassay (Fujirebio Inc., Tokyo, Japan).
Ten studies reported salivary IL-1β concentrations [25, 35, 36, 44, 47, 53,54,55, 57, 60], 4 of which reported its sensitivity and specificity in the diagnosis of OC [25, 36, 44, 60]. 24 studies reported salivary IL-6 concentrations [16, 31, 33,34,35,36,37,38,39,40,41,42, 44,45,46, 49, 50, 52,53,54, 56, 58, 61, 63], 5 studies reported sensitivity and specificity [16, 36, 44, 46, 47]. 20 studies reported salivary IL-8 concentrations [16, 25, 29, 32, 34,35,36, 39, 44, 45, 47, 54,55,56,57,58,59,60,61, 63], 8 studies reported sensitivity and specificity [16, 25, 29, 36, 44, 55, 60, 62]. 15 studies reported salivary TNF-α concentrations [16, 26,27,28,29, 34, 36, 45, 48, 52,53,54, 58, 61, 63], 5 of which reported sensitivity and specificity [16, 27, 28, 36, 47]. 5 studies reported salivary IL-10 concentrations [36, 43, 48, 51, 54], but none of them investigated its sensitivity and specificity.
Quality assessment
The NOS scores are provided in Table 1. The quality assessment results revealed that 1 study [31] was of high quality and the other 39 studies were of medium quality. Besides, the mean score of all included studies was 6.5. Table S3 in the Supplementary Materials illustrates the thorough point-by-point evaluation. For the risk of bias and applicability of diagnostic accuracy studies, we considered the overall risk of bias to be relatively low, and all included studies generated only low concern in all aspects (details in the Supplementary Figure S1). To be specific, there was a high risk of bias in participant selection due to case–control designs and inappropriate exclusions, with unclear consecutive sample of patients enrolled in some studies. In the index test assessment, 2 [16, 44] out of 12 studies had a low risk of bias, 10 studies [25, 27,28,29, 36, 46, 47, 55, 60, 62] were judged to be unclear. Regarding reference standard tests, all studies had a low risk of bias. For the flow and timing aspects, all studies demonstrated a low risk of bias in statements regarding the interval time between the reference test and the index test.
Meta-analysis for comparing saliva cytokines between OC patients and healthy controls
IL-6, as the most extensively investigated cytokine, was reported in 24 studies [16, 31, 33,34,35,36,37,38,39,40, 42, 44,45,46, 49, 52,53,54, 56, 58, 61,62,63,64], including 708 OC cases and 652 controls. The meta-analysis suggested an obvious increase in salivary IL-6 levels in OC patients (SMD = 2.32, 95% CI (1.61, 3.03), p < 0.001). There was a high degree of heterogeneity (I2 = 95.8%, Fig. 2A). However, the sensitivity analysis did not find the source of heterogeneity (Supplementary Figure S2) Furthermore, subgroup analyses were conducted depending on patients’ age (> 60, ≤ 60, or not grouped), the assay kits used (ELISA, Luminex-based Multiplex immunoassay or chemiluminescent enzyme immunoassay), and the geographic locations among the included studies (Europe, north America, or Asia), and the results showed that patients’ age and the assay kits were the source of heterogeneity (> 60, I2 = 0%, ≤ 60, I2 = 90.7%; ELISA kit, I2 = 96.5%; the Luminex-based Multiplex kit and the chemiluminescent enzyme kit, I2 = 0%, details see Supplementary Table S4). Egger's test pointed to the evidence of publication bias across these 24 studies (p < 0.001).
The second most commonly studied cytokine was IL-8, which was investigated in 20 studies [16, 25, 29, 32, 34,35,36, 39, 44, 45, 54,55,56,57,58,59,60,61,62, 65] encompassing 691 OC patients and 750 controls. According to the pooled analysis (Fig. 2B), salivary IL-8 levels were found to be markedly increased in the OC population (SMD = 1.73, 95%CI [1.20, 2.26], p < 0.001). The heterogeneity between studies was significant (I2 = 93.8%). The exclusion of individual studies did not alter the analysis results (Supplementary Figure S2). The results of subgroup analyses indicated that the assay kits were the source of heterogeneity (ELISA kit, I2 = 94.9%; the Luminex-based Multiplex kit and the chemiluminescent enzyme kit, I2 = 0%, details see Supplementary Table S4). Egger's test revealed significant publication bias (p = 0.03).
The salivary TNF-α level (Fig. 3A) was discussed in 15 studies [16, 26, 27, 29, 34, 36, 45, 48, 52,53,54, 58, 61, 62, 65], covering 460 cases and 435 controls. The pooled analysis showed significantly elevated TNF-α levels in OSCC patients (SMD = 2.27, 95% CI (1.27, 3.26), p < 0.001). Heterogeneity was high (I2 = 96.3%). Sensitivity analysis suggested that the exclusion of each study did not alter the pooled effect size (Supplementary Figure S2). Subgroup analyses showed that participants’ age and the assay kits were the source of heterogeneity (> 60, I2 = 0%, ≤ 60, I2 = 91.2%; ELISA kit, I2 = 96.3%; the Luminex-based Multiplex kit, I2 = 0%, Supplementary Table S4). Egger's tests (p = 0.065) revealed no publication bias.
IL-1β was evaluated in 10 studies [25, 35, 36, 44, 53,54,55, 57, 59, 60], including 381 OC cases and 392 controls. The meta-analysis found that OC patients exhibited a considerably higher IL-1β level in comparison to the healthy controls (SMD = 0.79, 95% CI (0.58, 1.00), p < 0.001, Fig. 3B). Heterogeneity was low (I2 = 47.1%). The funnel plot (Supplementary Figure S3) and the Egger’s test suggested no publication bias (p = 0.393).
IL-10 levels were reported in 5 included studies [36, 43, 48, 51, 54], involving 99 OC cases and 139 controls. Higher levels of IL-10 were noted in OC patients in comparison to the control group (SMD = 0.80, 95% CI (0.12, 1.48), p = 0.022, Fig. 3C). High heterogeneity was observed (I2 = 88%). The sensitivity analysis demonstrated that leaving out any one study did not have an impact on the pooled results (Supplementary Figure S2), while the assay kits and the geographic locations were the source of heterogeneity (ELISA kit, I2 = 92.0%; the Luminex-based Multiplex kit, I2 = 0%; Asia, I2 = 0%; Europe, I2 = 92.0%, Supplementary Table S4). Egger's test and the funnel plot (Supplementary Figure S3) indicated no publication bias (p = 0.231).
Diagnostic accuracy estimate
Since no studies have mentioned the sensitivity and specificity of IL-10 in the diagnosis of oral cancer only the remaining 4 kinds of saliva cytokines (i.e., IL-6, IL-8, TNF-α, and IL-1β) were included in this network meta-analysis. 12 studies [16, 25, 27,28,29, 36, 44, 46, 47, 55, 60, 62] provided their sensitivity and specificity, involving 1058 participants, of whom 574 (54.3%) were OC patients. Among the included studies, 5 studies [16, 36, 44, 46, 47] assessed the diagnostic accuracy of IL-6 for OC, with the sensitivity varying from 0.75 to 1.00 and specificity from 0.49 to 0.80 (Fig. 4). The pooled sensitivity and specificity of IL-6 were 0.75 (95% CI: 0.71, 0.81) and 0.86 (95%CI: 0.82, 0.90), respectively (Table 2). 8 studies [16, 25, 29, 36, 44, 55, 60, 62] assessed the diagnostic accuracy of IL-8 for OSCC, with the sensitivity varying from 0.67 to 0.97, and specificity from 0.58 to 0.97 (Fig. 4). The pooled sensitivity and specificity of IL-8 were 0.80 (95%CI: 0.77, 0.83) and 0.80 (95%CI: 0.77, 0.84), respectively (Table 2). 5 studies [16, 27, 28, 36, 47] assessed the diagnostic accuracy of TNF-α for OC, with the sensitivity varying from 0.83 to 1.00, and specificity from 0.49 to 1.00 (Fig. 4). The pooled sensitivity and specificity of TNF-α were 0.79 (95%CI: 0.76, 0.84) and 0.92 (95%CI: 0.90, 0.95) (Table 2), respectively. 4 studies [25, 36, 60, 65] assessed the diagnostic accuracy of IL-1β for OC, with the sensitivity varying from 0.61 to 0.74, and specificity from 0.76 to 0.84 (Fig. 4). The pooled sensitivity and specificity of IL-1β were 0.66 (95%CI: 0.61, 0.72) and 0.75 (95%CI: 0.70, 0.81), respectively (Table 2).
The network plot for the diagnostic accuracy of salivary cytokines for OC is illustrated in Fig. 5A. The NMA suggested that TNF-α ranked first, with the highest DOR (72.42, 95%CI: 34.00, 89.45), the second highest sensitivity (0.79, 95%CI: 0.76, 0.84), highest specificity (0.97, 95%CI: 0.69, 1.00), and the highest superiority index (Table 2). IL-6 ranked second, and the pooled sensitivity, specificity and DOR of IL-6 were 0.75 (95% CI: 0.71, 0.81), 0.86 (95% CI: 0.82, 0.90) and 25.13 (95% CI:13.48, 31.77), respectively, followed by IL-8 (sensitivity: 0.80, 95% CI: 0.77,0.83; specificity: 0.80, 95% CI: 0.77, 0.84; DOR: 19.09, 95% CI: 12.71, 23.33) and IL-1β (sensitivity: 0.66, 95% CI: 0.61, 0.72; specificity: 0.75, 95% CI: 0.70, 0.81; DOR: 7.57, 95% CI: 4.22, 9.42). Summary ROC results are presented in Fig. 5B.
Discussion
A traditional meta-analysis was carried out based on all available evidence from 40 case–control studies to compare the levels of salivary IL-8, IL-6, IL-10, TNF-α, and IL-1β in OC patients versus controls. Our research indicated that OC patients exhibited considerably higher levels of salivary IL-8, IL-6, TNF-α, IL-1β and IL-10 than healthy controls. To our knowledge, this is the first network meta-analysis to delve into the diagnostic accuracy of these cytokines for OC. Our NMA found that IL-8 had the highest sensitivity (0.80), followed by TNF-α (0.79) and IL6 (0.75). TNF-α had the highest specificity (0.92), followed by IL6 (0.86) and IL8 (0.80). Overall, the DOR results indicated that TNF-α had the highest accuracy for the diagnosis of OC.
In the tumor microenvironment, there are a large number of cytokines, which inhibit tumor-specific immune response and promote the proliferation of tumor cells [66, 67], thus contributing to the tumorigenesis and progression of tumors. Our results suggested that OC patients exhibited considerably higher levels of salivary IL-8, IL-6, TNF-α, IL-1β and IL-10 in comparison to healthy controls, which were consistent with previous studies [17, 68]. Specifically, Rezaei's meta-analysis implied that levels of IL-6 and IL-8 in saliva were markedly elevated in OC patients [68]. The meta-analysis by Chiamulera demonstrated significantly higher levels of salivary IL-8, IL-6, TNF-α, IL-1β and IL-10 in OC patients [17]. It has been proposed that cytokines in oral chronic/acute inflammation recruit neutrophils to form a feedback loop with OC cells, resulting in a pro-tumor phenotype [15]. Our research further supports this finding, suggesting that these cytokines can be used as potential biomarkers for OC.
The main purpose of our study was to compare common saliva cytokines so as to identify the best cytokine for diagnosing OC. According to our results, TNF-α was the most accurate and ranked first with a specificity of 0.92 and a sensitivity of 0.79, and its DOR value was much higher than others. TNF-α, a member of the enormous TNF cytokine family, plays a key role in numerous physiological and pathological cellular processes, such as cell proliferation, differentiation, and death, regulation of immune response to various cells and molecules, local and vascular invasion of tumors, and destruction of the tumor vascular system [69]. In both in vitro and in vivo models, as well as in patients with OC, the upregulation of TNF-α has been shown to enhance cell proliferation, whereas its downregulation inhibits the proliferation and migration of tumors [70, 71]. Moreover, elevated TNF-α in the OC tumor microenvironment has been reported to facilitate invasion through two mechanisms: (i) it fosters the pro-inflammatory and pro-invasive phenotype of OC cells; (ii) it acts as a paracrine mediator to promote the recruitment and activation of inflammatory cells [15, 72]. Simultaneously, TNF-α gene polymorphisms are strongly associated with an elevated risk of oral pre-cancer [73]. Based on our results, TNF-α could be a preferred biomarker for the diagnosis of OSCC.
Furthermore, our NMA implied that IL-6 and IL-8 ranked second (sensitivity: 75%; specificity: 86%) and third (sensitivity: 80%; specificity: 77%), respectively, while IL-1β ranked last (sensitivity: 66%; specificity: 75%). The diagnostic performance of IL-10 was not analyzed because the included studies did not provide the specificity and sensitivity of IL-10 for the diagnosis of OC. Previous studies and the results of the present study have shown that the IL-6 level in saliva is significantly elevated in OC patients [17], and there is a statistical difference in the concentration of IL-6 between pre-cancer state and the normal population [31, 63]. The concentration of IL-6 in saliva may be used as a biological marker for the early diagnosis of OC. For instance, an elevated IL-6 concentration indicates a higher probability of local OC recurrence [31]. Moreover, IL-6, as a member of the IL-6 cytokine family, is involved in the recruitment of neutrophils and macrophages, which is related to the pathogenesis of chronic inflammatory diseases. It not only contributes to the tumorigenesis and rapid progression of tumors, but also promotes the metastasis and spread of aggressive cancer cells [74]. Hence, IL-6 may serve as a major contributor to the occurrence and development of OC. IL-8, as a member of the CXC chemokine family, is a pro-inflammatory chemokine produced by immune cells under inflammatory conditions [75]. In the tumor microenvironment, IL-8 can not only enhance tumor cell proliferation or transformation into a migratory or stromal phenotype but also foster tumor angiogenesis or recruit additional immunosuppressive cells to the tumor, thereby promoting tumor progression [76]. In addition, cancer cells secrete IL-8, thus up-regulating the expression of matrix metalloproteinase-7 (MMP-7), which also contributes to OSCC invasion [77].
IL-1β stimulates the tyrosine phosphorylation of epidermal growth factor receptor (EGFR) through the chemokine ligand 1-receptor 2(CXCL1-CXCR2) axis, and regulates EGFR signal to promote the proliferation of dysplasia oral mucosa keratinocyte (DOK) and OSCC cells. However, a significant decrease in tyrosine phosphorylation of EGFR and a sharp decrease in DOK cell proliferation were observed by transfecting CXCL1-targeted short hairpin RNA (shRNA) with lentivirus or by using CXCR2 antagonists [78]. Lee et al. (2015) also find that IL-1β can promote the proliferation of DOK and OSCC cells, enhance the angiogenesis ability and the expression of epithelial-mesenchymal transition (EMT)-related genes Snail and Slug, and down-regulate expression of cadherin E, thereby resulting in OCC invasion and metastasis [79]. It is worth noting that IL-1β can activate the nuclear factor-κB (NF-κB) pathway and foster the expression and secretion of IL-6 and IL-8. As a powerful pro-inflammatory cytokine, IL-1β has been widely demonstrated to be upregulated in ovarian, lung, and gastrointestinal cancers, which are often associated with poor prognosis [80]. IL-10 is a representative anti-inflammatory and immunosuppressive factor that promotes immune escape of tumor cells [81]. A previous study has shown that in most OC samples, the expression of IL-10 is higher in tumor cells and stromal cells than in controls [82]. Based on our research results, IL6, IL8 and IL1β could be used as biomarkers of OC and serve as auxiliary diagnostic methods. Nonetheless, further studies are needed to explore the specific role of these cytokines in OSCC and investigate their diagnostic accuracy.
Strength, limitations, and Inspiration for future research
First of all, saliva detection provides a low-cost method for early diagnosis of oral cancer due to its advantages of non-invasive, convenient collection, processing and storage. And because saliva is in constant contact with oral lesions, it may be superior to blood or other body fluids. In our study, we found that several salivary cytokines (salivary cytokines) have strong diagnostic ability in oral cancer diagnosis. This suggests that in future studies, we can perform multiple cytokine tests on these three cytokines.
However, the study still has several limitations. The heterogeneity of the included studies was significant, similar to a previous meta-analysis [17]. As a result, the operating procedures for saliva collection, storage, and cytokine quantification should be standardized in the future. Additionally, a multicenter study with a larger sample size is warranted to rule out possible bias. Future studies should also consider some lifestyle factors, such as smoking and drinking, which may also influence IL levels (between cases and controls), although their influence may be wakened upon the occurrence of cancer [44, 65]. Few studies provided data on the sensitivity and specificity of IL-10 in diagnosing OC, so no relevant NMA was conducted. Further studies are desired to provide a more accurate evaluation of IL-10 in the diagnosis of OC. Some included studies conducted subgroup analysis by the stages of OC [16, 36, 45], but the correlation between cancer stages and cytokines was not investigated in our analysis. Since the early diagnosis of OSCC is closely related to the clinical treatment and prognosis of patients, further research in this area is necessary.
Previous studies [6] observed that IL-8 and il-6 concentrations in OC patients were significantly higher than those in OPMD patients, and also significantly increased compared with healthy subjects. This suggests that the amount of the increase may distinguish between oral cancer and precancerous states, lichen planus, periodontitis, or other inflammatory and infectious diseases. We look forward to conducting more control studies in the future to compare oral cancer with potential oral malignancies, oral inflammatory diseases, and even systemic inflammatory states to further improve its specificity in the diagnosis of oral cancer.
In addition, many studies have demonstrated the effects of cytokines in the treatment of cancers [83,84,85,86], so these cytokines provide an insight into future clinical treatment of OC.
Conclusion
This study suggests that salivary cytokines can be used as potential biomarkers for early diagnosis of OC. Given its high diagnostic specificity and sensitivity, TNF-α is recommended, followed by IL-6 and IL-8. Notably, salivary cytokine levels may be affected by other factors, such as potential malignant states, chronic local inflammation, and autoimmune diseases. Therefore, it is necessary to further distinguish the diagnostic accuracy of these cytokines in different disease states and compare them with OC and different OC stages. At the same time, more standard operating procedures and large-scale multi-center studies are needed to reduce bias and heterogeneity. It is believed that the TNF-α, IL-6, and IL-8 saliva test is an affordable technique for early clinical diagnosis of OC, which can provide novel insights into the targeted therapy of OC.
Availability of data and materials
All data supporting the findings of this study are available within the paper and its Supplementary Materials.
References
Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Sand L, Jalouli J. Viruses and oral cancer. Is there a link? Microbes Infect. 2014;16(5):371–8.
Chamoli A, et al. Overview of oral cavity squamous cell carcinoma: Risk factors, mechanisms, and diagnostics. Oral Oncol. 2021;121: 105451.
Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508.
Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009;45(4–5):309–16.
Ferrari E, et al. Salivary Cytokines as Biomarkers for Oral Squamous Cell Carcinoma: A Systematic Review. Int J Mol Sci. 2021;22(13):6795.
Su YF, et al. Current Insights into Oral Cancer Diagnostics. Diagnostics (Basel). 2021;11(7):1287.
Chen XJ, et al. Nanotechnology: a promising method for oral cancer detection and diagnosis. J Nanobiotechnology. 2018;16(1):52.
Meleti M. Salivary biomarkers for diagnosis of systemic diseases and malignant tumors. A systematic review. Med Oral Patol Oral Cir Bucal. 2020;25(2):e299–e31.
Cristaldi M, et al. Salivary Biomarkers for Oral Squamous Cell Carcinoma Diagnosis and Follow-Up: Current Status and Perspectives. Front Physiol. 2019;10:1476.
Nisar S. Chemokine-Cytokine Networks in the Head and Neck Tumor Microenvironment. Int J Mol Sci. 2021;22(9):4584.
Chen Z, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999;5(6):1369–79.
Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382(1):60–72.
Alevizos I, et al. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene. 2001;20(43):6196–204.
Goertzen C, et al. Oral inflammation promotes oral squamous cell carcinoma invasion. Oncotarget. 2018;9(49):29047–63.
Dikova V, Jantus-Lewintre E, Bagan J. Potential Non-Invasive Biomarkers for Early Diagnosis of Oral Squamous Cell Carcinoma. J Clin Med. 2021;10(8):1658.
Chiamulera MMA, et al. Salivary cytokines as biomarkers of oral cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21(1):205.
O’Sullivan JW. Network meta-analysis for diagnostic tests. BMJ Evid Based Med. 2019;24(5):192–3.
Veroniki AA, et al. Diagnostic test accuracy network meta-analysis methods: A scoping review and empirical assessment. J Clin Epidemiol. 2022;146:86–96.
Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36.
Ma X, et al. A Bayesian hierarchical model for network meta-analysis of multiple diagnostic tests. Biostatistics. 2018;19(1):87–102.
Nyaga VN, Aerts M, Arbyn M. ANOVA model for network meta-analysis of diagnostic test accuracy data. Stat Methods Med Res. 2018;27(6):1766–84.
Singh P, Verma JK, Singh JK. Validation of Salivary Markers, IL-1β, IL-8 and Lgals3bp for Detection of Oral Squamous Cell Carcinoma in an Indian Population. Sci Rep. 2020;10(1):7365.
Sabarathinam J, Selvaraj J, Devi S. Estimation of Levels of Glutathione Peroxidase (Gpx), Malondialdehyde (Mda), Tumor Necrosis Factor Alpha (Tnf Alpha) and Alpha Feto Protein (Afp) In Saliva of Potentially Malignant Disorders and Oral Squamous Cell Carcinoma. Biomed Pharmacol J. 2019;12(4):1881–6.
Deepthi G, Nandan SRK, Kulkarni PG. Salivary Tumour Necrosis Factor-α as a Biomarker in Oral Leukoplakia and Oral Squamous Cell Carcinoma. Asian Pac J Cancer Prev. 2019;720(7):2087–93.
Ameena M, Rathy R. Evaluation of tumor necrosis factor: Alpha in the saliva of oral cancer, leukoplakia, and healthy controls - A comparative study. Journal of International Oral Health. 2019;11(2):92–9.
Rajkumar K, et al. Validation of the diagnostic utility of salivary interleukin 8 in the differentiation of potentially malignant oral lesions and oral squamous cell carcinoma in a region with high endemicity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118(3):309–19.
Krishnan R, et al. Association of serum and salivary tumor necrosis factor-α with histological grading in oral cancer and its role in differentiating premalignant and malignant oral disease. Asian Pac J Cancer Prev. 2014;15(17):7141–8.
Sato J, et al. Correlation between salivary interleukin-6 levels and early locoregional recurrence in patients with oral squamous cell carcinoma: preliminary study. Head Neck. 2013;35(6):889–94.
Punyani SR, Sathawane RS. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin Oral Investig. 2013;17(2):517–24.
Sato J, et al. Changes in saliva interleukin-6 levels in patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110(3):330–6.
SahebJamee M, et al. Salivary concentration of TNFalpha, IL1 alpha, IL6, and IL8 in oral squamous cell carcinoma. Med Oral Patol Oral Cir Bucal. 2008;13(5):E292–5.
Katakura A, et al. Comparison of salivary cytokine levels in oral cancer patients and healthy subjects. Bull Tokyo Dent Coll. 2007;48(4):199–203.
Lee LT, et al. Evaluation of saliva and plasma cytokine biomarkers in patients with oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2018;47(6):699–707.
Zhang S, et al. Variation and significance of secretory immunoglobulin A, interleukin 6 and dendritic cells in oral cancer. Oncol Lett. 2017;13(4):2297–303.
Shahidi, M, et al. Predictive value of salivary microRNA-320a, vascular endothelial growth factor receptor 2, CRP and IL-6 in Oral lichen planus progression. Inflammopharmacology. 2017.
Khyani IAM, et al. Detection of interleukins-6 and 8 in saliva as potential biomarkers of oral pre-malignant lesion and oral carcinoma: A breakthrough in salivary diagnostics in Pakistan. Pak J Pharm Sci. 2017;30(3):817–23.
Dineshkumar T, et al. Salivary and Serum Interleukin-6 Levels in Oral Premalignant Disorders and Squamous Cell Carcinoma: Diagnostic Value and Clinicopathologic Correlations. Asian Pac J Cancer Prev. 2016;17(11):4899–906.
Panneer Selvam N, Sadaksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11(3):236–41.
Sato J, et al. Differences in sequential posttreatment salivary IL-6 levels between patients with and patients without locoregional recurrences of oral squamous cell carcinoma: Part III of a cohort study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(6):751–60.e2.
Aziz S, et al. Salivary Immunosuppressive Cytokines IL-10 and IL-13 Are Significantly Elevated in Oral Squamous Cell Carcinoma Patients. Cancer Invest. 2015;33(7):318–28.
Piyarathne NS, et al. Salivary Interleukin Levels in Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia: Findings from a Sri Lankan Study. Cancers (Basel). 2023;15(5):1510.
Babiuch K, et al. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J Clin Med. 2020;9(3):867.
Márton, IJ. et al. Salivary IL-6 mRNA is a Robust Biomarker in Oral Squamous Cell Carcinoma. J Clin Med. 2019;8(11):1958.
Csősz É, et al. Proteomics investigation of OSCC-specific salivary biomarkers in a Hungarian population highlights the importance of identification of population-tailored biomarkers. PLoS ONE. 2017;12(5): e0177282.
Polz-Dacewicz M, et al. Salivary and serum IL-10, TNF-α, TGF-β, VEGF levels in oropharyngeal squamous cell carcinoma and correlation with HPV and EBV infections. Infect Agent Cancer. 2016;11:45.
Bagan L, et al. Salivary and serum interleukin-6 levels in proliferative verrucous leukoplakia. Clin Oral Investig. 2016;20(4):737–43.
Radulescu R, et al. Biomarkers of Oxidative Stress, Proliferation, Inflammation and Invasivity in Saliva from Oral Cancer Patients. Journal of Analytical Oncology. 2015;4:52–7.
Gonçalves AS, et al. Immunosuppressive mediators of oral squamous cell carcinoma in tumour samples and saliva. Hum Immunol. 2015;76(1):52–8.
Juretić M, et al. Salivary levels of TNF-α and IL-6 in patients with oral premalignant and malignant lesions. Folia Biol (Praha). 2013;59(2):99–102.
Brailo V, et al. Salivary and serum interleukin 1 beta, interleukin 6 and tumor necrosis factor alpha in patients with leukoplakia and oral cancer. Med Oral Patol Oral Cir Bucal. 2012;17(1):e10–5.
Laliberté C, et al. Characterization of Oral Squamous Cell Carcinoma Associated Inflammation: A Pilot Study. Front Oral Health. 2021;2: 740469.
Gleber-Netto FO, et al. Salivary Biomarkers for Detection of Oral Squamous Cell Carcinoma in a Taiwanese Population. Clin Cancer Res. 2016;22(13):3340–7.
Lisa Cheng YS, et al. Salivary interleukin-6 and -8 in patients with oral cancer and patients with chronic oral inflammatory diseases. J Periodontol. 2014;85(7):956–65.
Elashoff D, et al. Prevalidation of salivary biomarkers for oral cancer detection. Cancer Epidemiol Biomarkers Prev. 2012;21(4):664–72.
Korostoff A, et al. The role of salivary cytokine biomarkers in tongue cancer invasion and mortality. Oral Oncol. 2011;47(4):282–7.
Brinkmann O, et al. Oral squamous cell carcinoma detection by salivary biomarkers in a Serbian population. Oral Oncol. 2011;47(1):51–5.
Arellano-Garcia ME, et al. Multiplexed immunobead-based assay for detection of oral cancer protein biomarkers in saliva. Oral Dis. 2008;14(8):705–12.
Rhodus NL, et al. The feasibility of monitoring NF-kappaB associated cytokines: TNF-alpha, IL-1alpha, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. 2005;44(2):77–82.
St John MA, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004;130(8):929–35.
Rhodus NL, et al. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005;29(1):42–5.
Selvam NP, Sadaksharam J. Salivary interleukin-6 in the detection of oral cancer and precancer. Asia Pac J Clin Oncol. 2015;11(3):236–41.
Piyarathne NS, et al. Diagnostic salivary biomarkers in oral cancer and oral potentially malignant disorders and their relationships to risk factors - A systematic review. Expert Rev Mol Diagn. 2021;21(8):789–807.
Galdiero MR., Marone G, Mantovani A. Cancer Inflammation and Cytokines. Cold Spring Harb Perspect Biol,. 2018;10(8):a028662.
Li L, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol. 2020;88: 106939.
Rezaei F, et al. Evaluation of Serum and Salivary Interleukin-6 and Interleukin-8 Levels in Oral Squamous Cell Carcinoma Patients: Systematic Review and Meta-Analysis. J Interferon Cytokine Res. 2019;39(12):727–39.
Mahdavi Sharif P, et al. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine. 2020;130: 155066.
Sun Z, et al. Effect of interleukin-1β and tumor necrosis factor α gene silencing on mouse gastric cancer cell proliferation and migration. Oncol Lett. 2016;11(4):2559–65.
Ho MY, et al. TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol Cancer Res. 2012;10(8):1109–19.
Glogauer JE, et al. Neutrophils Increase Oral Squamous Cell Carcinoma Invasion through an Invadopodia-Dependent Pathway. Cancer Immunol Res. 2015;3(11):1218–26.
Serefoglou Z, et al. Genetic association of cytokine DNA polymorphisms with head and neck cancer. Oral Oncol. 2008;44(12):1093–9.
Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. 2018;18(12):773–89.
Matsushima K, Yang D, Oppenheim JJ. Interleukin-8: An evolving chemokine. Cytokine. 2022;153: 155828.
Fousek K, Horn LA, Palena C. Interleukin-8: A chemokine at the intersection of cancer plasticity, angiogenesis, and immune suppression. Pharmacol Ther. 2021;219: 107692.
Watanabe H, et al. Role of interleukin-8 secreted from human oral squamous cell carcinoma cell lines. Oral Oncol. 2002;38(7):670–9.
Lee CH, et al. Interleukin-1 beta transactivates epidermal growth factor receptor via the CXCL1-CXCR2 axis in oral cancer. Oncotarget. 2015;6(36):38866–80.
Lee CH, et al. IL-1β promotes malignant transformation and tumor aggressiveness in oral cancer. J Cell Physiol. 2015;230(4):875–84.
Zhang D, et al. Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer. 2007;7:45.
Kondoh N, et al. Immunomodulatory aspects in the progression and treatment of oral malignancy. Jpn Dent Sci Rev. 2019;55(1):113–20.
Arantes DA, et al. Overexpression of immunosuppressive cytokines is associated with poorer clinical stage of oral squamous cell carcinoma. Arch Oral Biol. 2016;61:28–35.
Nguyen KG, et al. Localized Interleukin-12 for Cancer Immunotherapy. Front Immunol. 2020;11: 575597.
Jaén M, et al. Interleukin 13 receptor alpha 2 (IL13Rα2): Expression, signaling pathways and therapeutic applications in cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(5): 188802.
Raeber ME, Sahin D, Boyman O. Interleukin-2-based therapies in cancer. Sci Transl Med. 2022;14(670):eabo5409.
Han Y, et al. IL-1β-associated NNT acetylation orchestrates iron-sulfur cluster maintenance and cancer immunotherapy resistance. Mol Cell. 2023;83(11):1887–1902.e8.
Acknowledgements
Not applicable.
Funding
1. Changsha Natural Science Foundation. (No: kq2208484).
2. Research Program Project of Hunan Provincial Health and Wellness Commission. (No: 202108030155).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Lijun Huang: Conceptualization, Methodology, Software, Writing- Original draft, Data curation, Visualization were performed; Fen Luo: Investigation, Writing - Original Draft, Writing – Reviewing and Editing, Funding acquisition were performed; Mingsi Deng: Methodology, Software, Writing- Original draft were performed; Jie Zhang: Conceptualization, Supervision, Project administration, Funding acquisition were performed. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Huang, L., Luo, F., Deng, M. et al. The relationship between salivary cytokines and oral cancer and their diagnostic capability for oral cancer: a systematic review and network meta-analysis. BMC Oral Health 24, 1044 (2024). https://doi.org/10.1186/s12903-024-04840-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12903-024-04840-3