Abstract
Background
Perivascular epithelioid cell tumours (PEComas) are soft tissue tumours. These neoplasms belong to the family of mesenchymal tumours, which include angiomyolipomas, clear-cell sugar tumours of the lung, and PEComas not otherwise specified (NOS). The probability of a perivascular epithelioid cell tumour (PEComa) occurring in the uterus is low, and the incidence, diagnosis, treatment, and outcomes of such tumours are still unclear.
Case presentation
A 51-year-old woman presented a 4-year history of natural menopause. An intrauterine mass was detected via ultrasound examination; the mass showed a tendency to increase but caused no symptoms. The levels of tumour markers were within the normal range. Pathological analysis of the curettage revealed perivascular epithelioid differentiation of the endometrial tumour. Consequently, a laparoscopic total hysterectomy with bilateral adnexectomy was performed. No distant metastasis was detected via whole-body positron emission computed tomography (PETCT) after the operation. Fluorescence in situ hybridization (FISH) revealed no TFE3 gene rearrangement. Next-generation sequencing of bone and soft tissue revealed negative TSC1/2 and TP53 expression. No recurrence or metastasis was observed during the 18-month follow-up period.
Conclusion
PEComa of the gynecologic tract is a rare and challenging entity. Diffuse HMB-45 expression, TSC alterations and TFE3 rearrangement are characteristic of uterine PEComas. Surgical resection is the first choice. Genetic testing is helpful for determining the nature of the mass and for choosing targeted therapy. Further research is needed to establish treatment protocols.
Similar content being viewed by others
Background
Perivascular epithelioid cell tumours (PEComas) were first proposed by Zamboni et al. in 1996 [1]. In 2003, the World Health Organization defined a PEComa as a mesenchymal tumour with perivascular epithelioid cell characteristics in terms of the histology and immunophenotype. PEComas of the female gynecological tract are rare, accounting for 25% of all PEComas, and they cause variable symptoms and yield different prognoses for each individual patient. The uterus is one of the most commonly involved sites (72%). Fewer cases of gynecological PEComas have also been reported in the cervix (11%), and even rarer cases have been reported in the vagina, broad ligament and ovary [2, 3]. Surgery is the main treatment. The rates of metastatic disease at diagnosis, recurrences, and/or death vary among larger studies, with percentages ranging from 35 to 64% of patients [4,5,6]. The etiology of PEComas remain unclear and may be related to estrogen levels [7]. Some studies have suggested that gene mutations in the tuberous sclerosis complex (TSC) [8] and the rearrangement of transcription Factor E3 (TFE3) [9] are associated with pathogenesis. PEComas with TFE3 gene rearrangements are a group of subtypes with a unique morphology. Compared with PEComas without rearrangement, these subtypes are more aggressive and have malignant morphological characteristics. Here, a case of a malignant uterine PEComa without TFE3 gene rearrangement is reviewed.
Case presentation
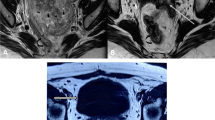
A 51-year-old woman with a uterine mass found 6 months prior was admitted to the gynecology department of Fujian Maternity and Child Health Hospital. The patient had undergone natural menopause 4 years prior. Ultrasonography revealed a 2 cm mass in the patient’s uterus 6 months prior, and a regular review was performed. The presurgical ultrasound examination showed an intrauterine mass that was 4.0 cm × 3.2 cm × 5.1 cm in size (uterine size, 4.9 cm × 4.5 cm × 5.3 cm; endometrial thickness, 0.3 cm). Colour Doppler revealed that the blood flow signal in the tumour had a pulsatility index of 0.52 and a resistive index of 0.40. The preoperative diagnostic hypothesis was uterine submucosal fibroids. There was no family history or clinical evidence of tuberous sclerosis. Pathological analysis of the curettage revealed perivascular epithelioid differentiation of the endometrial tumour. The results of laboratory tests, including carcinoembryonic antigen (CEA), cancer antigen (Ca) 125, Ca153, Ca199, alpha fetoprotein (AFP) and squamous cell carcinoma antigen (SCC) levels, were all within the normal range. As showed in Fig. 1, magnetic resonance imaging (MRI) revealed an abnormal signal indicating uterine mass (3.7 cm × 3.2 cm × 4.3 cm); and the internal strengthening was uneven after enhancement. No enlarged pelvic lymph nodes were observed.
A laparoscopic total hysterectomy with bilateral adnexectomy was performed. During the intraoperative exploration, no abnormalities were found in the abdominal pelvic cavity, and no obviously enlarged pelvic lymph nodes were observed. The uterus was enlarged, measuring 8.0 cm × 6.0 cm × 5.0 cm, whereas both the ovaries and fallopian tubes were unremarkable. Upon bivalving the uterus, a greyish tumour measuring 4 cm×4 cm× 2.5 cm was observed in the endometrial cavity. Microscopically, the tumour had infiltrated into the superficial 1/2 of the muscle layer, resulting in necrosis and lymphovascular invasion. The mitotic count was > 1/50 HPF. As shown by the immunohistochemistry results in Fig. 2A-D, the tumour cells were negative for ER, PR, ALK, and SOX-10. However, they showed diffuse strong positivity for HMB-45 and Melan-A and focal positive (1+) staining for smooth muscle actin (SMA), TFE-3, CD34, and myogenin. The Ki-67 index was 8%. The peritoneal lavage fluid tested negative for tumour cells. A final diagnosis of a malignant uterine PEComa was made.
(A) The tumor was composed of sheets of epithelioid cells and bundles of spindle cells, hematoxylin and eosin (H&E, × 100). (B) Spindle cells were positive for smooth muscle actin (SMA) (× 100). (C) Epithelioid cells were positive for HMB-45 (× 100). (D) Epithelioid cells showed nuclear positivity of TFE3 (× 100). (E) No TFE3 gene rearrangement detected by FISH
The whole-body PET-CT scan was performed and revealed no abnormalities after the operation. Moreover, FISH showed no TFE3 gene rearrangement (Fig. 2E). Next-generation sequencing for bone and soft tissue revealed negative TSC1/2 and TP53 expression.
A postoperative follow-up assessment was performed every 3 months for 2 years, every 6 months during years 3–5 and yearly thereafter. Routine follow-up appointments included a physical examination, vaginal examination, laboratory testing (including Ca125), chest radiography, and pelvic ultrasonography. Lung CT or pelvic MRI was performed when necessary. No recurrence or metastasis was observed during the 18-month follow-up period. Moreover, the gynecological ultrasound and tumour marker data remained normal.
Discussion and conclusions
Herein we report a case of uterine malignant PEComa without TFE3 gene rearrangement detected by FISH. A combination of immunohistochemical and genetic testing is helpful for diagnosing PEComas. Genetically targeted therapy is more effective at improving overall survival.
PEComas in the uterus are rare. The clinical presentation is nonspecific and includes abnormal uterine bleeding, abdominopelvic pain, diagnosis of “fibroids,” or the identification of a mass on imaging. PEComas are easily misdiagnosed preoperatively as uterine fibroids [8]. Most cases are diagnosed accidentally or via quick-frozen pathology during surgery.
PEComas can be comprised of both epithelioid and spindle cells. PEComas uniquely show immunohistochemical positivity for both melanocytic (HMB-45, Melan-A) and myoid markers (SMA, desmin, caldesmon, and calponin), whereas cytokeratin and S-100 are generally negative [3]. PEComas can generally be considered present when HMB-45 is positively expressed because of its specificity. Currently, there is no unified standard for differentiating between benign and malignant uterine PEComas. According to Folpe’s criteria, PEComas are categorized as malignant if they demonstrate ≥ 2 of the following poor prognostic indicators: size ≥ 5 cm, significant nuclear atypia, invasive growth, mitosis ≥ 1/50 HPF, necrosis, or evidence of lymphovascular invasion [7]. The pathologic features indicating a malignant PEComa in the index patient included invasive growth of the superficial 1/2 muscle layer, mitosis ≥ 1/50 HPF, necrosis, and lymphovascular invasion.
Molecular and genomic profiling of endometrial cancer has increased in popularity in recent years. L1 cell adhesion molecule (L1CAM) is frequently mutated in endometrial cancer and is associated with a greater risk of distant recurrence, which provides a potentially useful tool for tailoring the need for adjuvant therapy [10, 11]. A few PEComas also show abnormal gene expression. Some PEComa patients have mutations in the TSC1 and TSC2 genes. A subset of PEComas has shown TFE3 rearrangement [12]. TFE3 is ubiquitously present at low levels in normal cells. When TFE3 gene rearrangements occurs, TFE3 protein overexpression is promoted, which interferes with cell transcriptional regulation and leads to tumour formation [13]. Argani et al. [14] performed FISH analyses on TFE3-positive PEComas and confirmed that the TFE3 gene rearrangement was accompanied by T (X; 1) (P11.2; P34) chromosome translocation, resulting in PSF-TFE3 gene fusion, thereby promoting TFE3 overexpression in tumour cells. TFE3 protein is often strongly positive in immunohistochemistry, but this result does not indicate TFE3 gene abnormalities in the FISH test. However, recent studies have shown that TFE3 immunohistochemistry plays only a minor role in the diagnosis of TFE3-rearranged tumours. Thus, TFE3 protein detected by immunohistochemistry alone is not sufficient to be used as a surrogate indicator of TFE3 gene rearrangement; FISH analysis is recommended [15]. In the present case, the expression of TFE3 was positive by immunohistochemistry but negative by FISH analysis, demonstrating the superior accuracy of FISH analysis.
Since PEComas with TFE3 rearrangement are very rare, their exact biological behavior remains to be determined. Liu et al. [16] reported a more invasive case of malignant cervical PEComa accompanied by TFE3 gene rearrangement. PEComas with TFE3 gene rearrangements are considered more aggressive and should be considered independent subtypes of PEComas [7]. However, recent studies also revealed that PEComas with TFE3 gene expression are benign [17]. In this case, the patient’s tumour exhibited malignant biological behavior without TFE3 rearrangement. Therefore, the relationship between TFE3 gene rearrangement and the disease prognosis, as well as whether this factor should be considered a criterion for benign or malignant evaluation, needs to be proven with further research.
Standard treatment protocols are not yet available for these tumours owing to their rarity. Currently, surgical resection remains the preferred treatment. The choice of surgery depends on the patient’s age and fertility requirements. Tumour resection alone may be considered only for patients who have fertility requirements and whose tumours are thought to be “benign”. Shan et al. reported the case of a woman who had a natural pregnancy after tumour resection alone; she delivered a child and had a disease-free survival of 6 years [18]. According to the literature, total hysterectomy is the preferred treatment for patients without a fertility requirement [18]. The necessity for pelvic lymph node dissection is controversial because of the hematogenous metastasis of mesenchymal tumours ingeneral, which needs to be verified by further studies. Whether postoperative adjuvant therapy is neceaasry for malignant PEComas has also been explored. The efficacy of chemoradiotherapy is uncertain [19]. Mutations in the TSC1 and TSC2 genes are driving factors in the development of some PEComas, resulting in activation of the mammalian target of rapamycin (mTOR) pathway [8]. These alterations constitute the basis of mTOR inhibitor therapy. However, these findings need to be confirmed in additional clinical trials. The present patient’s genetic test showed a negative TSC gene mutation, and she may have obtained limited benefit from mTOR inhibitors.
Currently, the diagnosis and treatment of uterine PEComa are predominantly based on case reports. Because of the lack of unified standards, surgery is the main treatment. However, genetically targeted therapy may be more effective. Further studies on the genomics, transcriptomics, proteomics, and epigenetics of PEComas are needed to identify criteria for accurately predicting outcomes and guiding disease-management decisions.
In conclusion, this case highlights the importance of a comprehensive approach for diagnosing PEComas, including genetic testing and immunochemical markers. Surgical resection is the first choice for treatment. Genetically targeted therapy is effective in improving the prognosis for patients with malignant PEComas. Long-term monitoring and follow-up are also needed.
Data availability
The data supporting the conclusions of this article is available from corresponding author on reasonable request.
Abbreviations
- PEComa:
-
Perivascular epithelioid cell tumour
- TFE3:
-
Transcription factor E3
- TSC:
-
Tuberous sclerosis complex
- CEA:
-
Carcinoembryonic antigen
- AFP:
-
Alpha fetoprotein
- SCC:
-
Squamous cell carcinoma antigen
- PET-CT:
-
Positron emission computed tomography
- FISH:
-
Fluorescence in situ hybridization
- mTOR:
-
The mammalian target of rapamycin
References
Zamboni G, Pea M, Martignoni G, Zancanaro C, Faccioli G, Gilioli E, et al. Clear cell sugar tumor of the pancreas. A novel member of the family of lesions characterized by the presence of perivascular epithelioid cells. Am J Surg Pathol. 1996;20(6):722–30.
Liu CH, Chao WT, Lin SC, Lau HY, Wu HH, Wang PH. Malignant perivascular epithelioid cell tumor in the female genital tract: Preferred reporting items for systematic reviews and meta-analyses. Med (Baltim). 2019;98(2):e14072.
Bennett JA, Oliva E. Perivascular epithelioid cell tumors (PEComa) of the gynecologic tract. Genes Chromosomes Cancer. 2021;60(3):168–79.
Bennett JA, Braga AC, Pinto A, Van de Vijver K, Cornejo K, Pesci A, et al. Uterine PEComas: a morphologic, immunohistochemical, and molecular analysis of 32 tumors. Am J Surg Pathol. 2018;42:1370–83.
Schoolmeester JK, Howitt BE, Hirsch MS, Dal Cin P, Quade BJ, Nucci MR. Perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: clinicopathologic and immunohistochemical characterization of 16 cases. Am J Surg Pathol. 2014;38:176–88.
Conlon N, Soslow RA, Murali R. Perivascular epithelioid tumours (PEComas) of the gynaecological tract. J Clin Pathol. 2015;68:418–26.
Cao B, Huang Y. Malignant perivascular epithelioid cell tumor (PEComa) of the uterus. BMC Womens Health. 2022;22(1):523.
Dhanesar GK, Rengarajan H, Chakraborty B. Malignant perivascular epithelioid cell tumor of the Uterus. Cureus. 2023;15(7):e41685.
Bennett JA, Ordulu Z, Pinto A, Wanjari P, Antonescu CR, Ritterhouse LL, et al. Uterine PEComas: correlation between melanocytic marker expression and TSC alterations/TFE3 fusions. Mod Pathol. 2022;35(4):515–23.
Vizza E, Bruno V, Cutillo G, Mancini E, Sperduti I, Patrizi L, et al. Prognostic role of the removed vaginal cuff and its correlation with L1CAM in low-risk endometrial adenocarcinoma. Cancers (Basel). 2021;14(1):34.
Giannini A, D’Oria O, Corrado G, Bruno V, Sperduti I, Bogani G, et al. The role of L1CAM as predictor of poor prognosis in stage I endometrial cancer: a systematic review and meta-analysis. Arch Gynecol Obstet. 2024;309(3):789–99.
Vannucchi M, Minervini A, Salvi M, Montironi R, Raspollini MR. TFE3 gene rearrangement in Perivascular Epithelioid Cell Neoplasm (PEComa) of the Genitourinary Tract. Clin Genitourin Cancer. 2020;18(6):e692–7.
Argani P, Ladanyi M. The evolving story of renal translocation carcinomas. Am J Clin Pathol. 2006;126(3):332–4.
Argani P, Aulmann S, Illei PB, Netto GJ, Ro J, Cho HY, et al. A distinctive subset of PEComas harbors TFE3 gene fusions. Am J Surg Pathol. 2010;34(10):1395–406.
Sharain RF, Gown AM, Greipp PT, Folpe AL. Immunohistochemistry for TFE3 lacks specificity and sensitivity in the diagnosis of TFE3-rearranged neoplasms: a comparative, 2-laboratory study. Hum Pathol. 2019;87:65–74.
Liu F, Zhang R, Wang ZY, Xia Q, Shen Q, Shi S, et al. Malignant perivascular epithelioid cell tumor (PEComa) of cervix with TFE3 gene rearrangement: a case report. Int J Clin Exp Pathol. 2014;7(9):6409–14.
Chen XF, Yeong J, Chang KTE, Lim AST, Kuick CH, Lim TH, et al. TFE3-Expressing Epithelioid Rich Perivascular Epithelioid Cell Neoplasm (PEComa) of the bladder with unusual Benign Course. Ann Clin Lab Sci. 2018;48(1):110–5.
Shan W, Shi Y, Zhu Q, Yang B, Xie L, Li B, et al. Five cases of uterine perivascular epithelioid cell tumors (PEComas) and review of literature. Arch Gynecol Obstet. 2019;299(1):185–90.
Gentile M, Zinna M, Zanella C, Costanza A, Dalfior D, Sina S, et al. Uterine PEComa with aggressive behavior: a review with an additional case of spontaneous vaginal expulsion. Pathol Res Pract. 2020;216(6):152991.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MX participated in the acquisition of clinical data and drafted the manuscript. JHF carried out the pathological examination and interpretation. LZC revised the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Fujian Provincial Maternity and Children’s Hospital (No. 2024KY040).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xu, M., Fu, J. & Cai, L. Malignant Perivascular epithelioid cell tumour of the uterus without TFE3 gene rearrangement: a case report. BMC Women's Health 24, 527 (2024). https://doi.org/10.1186/s12905-024-03364-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12905-024-03364-w