Abstract
Background
Women with high-risk breast lesions, such as atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS), have a 4- to tenfold increased risk of breast cancer compared to women with non-proliferative breast disease. Despite high-quality data supporting chemoprevention, uptake remains low. Interventions are needed to break down barriers.
Methods
The parent trial, MiCHOICE, is a cluster randomized controlled trial evaluating the effectiveness and implementation of patient and provider decision support tools to improve informed choice about chemoprevention among women with AH or LCIS. For this pre-implementation analysis, 25 providers participated in semi-structured interviews prior to accessing decision support tools. Interviews sought to understand attitudes/beliefs and barriers/facilitators to chemoprevention.
Results
Interviews with 25 providers (18 physicians and 7 advanced practice providers) were included. Providers were predominantly female (84%), white (72%), and non-Hispanic (88%). Nearly all providers (96%) had prescribed chemoprevention for eligible patients. Three themes emerged in qualitative analysis. The first theme describes providers’ confidence in chemoprevention and the utility of decision support tools. The second theme elucidates barriers to chemoprevention, including time constraints, risk communication and perceptions of patients’ fear of side effects and anxiety. The third theme is the need for early implementation of decision support tools.
Conclusions
This qualitative study suggests that providers were interested in the early inclusion of decision aids (DA) in their chemoprevention discussion workflow. The DAs may help overcome certain barriers which were elucidated in these interviews, including patient level concerns about side effects, clinic time constraints and difficulty communicating risk. A multi-faceted intervention with a DA as one active component may be needed.
Trial registration
This trial was registered with the NIH clinical trial registry, clinicaltrials.gov, NCT04496739.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Breast cancer is the most common malignancy among women in the United States [1]. Women with high-risk benign breast lesions, such as atypical hyperplasia (AH) and lobular carcinoma in situ (LCIS), have up to a 4- to tenfold increased risk of breast cancer compared to women with non-proliferative breast disease [2]. Chemoprevention with selective estrogen receptor modulators (SERMs) (i.e., tamoxifen and raloxifene) and aromatase inhibitors (AIs) (i.e.,anastrozole and exemestane) is effective. Large-scale randomized controlled trials (RCTs) have shown breast cancer incidence is reduced by up to 50–65% among high-risk women, with relative risk reduction of 70–80% among women with AH or LCIS [3,4,5,6,7]. Low-dose tamoxifen has been shown to similarly reduce risk while improving tolerance [8]. Yet, uptake of breast cancer chemoprevention remains low, with rates ranging from less than 1 to 24% [9,10,11,12,13].
Significant barriers to uptake of chemoprevention include inadequate time for counseling, insufficient clinician and patient knowledge about breast cancer chemoprevention, and side effects [11, 14, 15]. In our prior qualitative work, patients described a desire for tailored and holistic approaches to reducing breast cancer risk [16]. Padamsee et al.found that amongst a population of high risk women, only 45% were aware of chemoprevention options, with those who were aware expressing significant reluctance related to potential side effects, perceived extremeness and hesitancy of taking medications in general [17]. Additionally, in a questionnaire-based survey of women diagnosed with AH, LCIS or ductal carcinoma in situ (DCIS), Trivedi et al.found that there was a strong interest in learning more about chemoprevention with 78.6% of participants indicating that they would like to learn more about chemoprevention drugs in the future [18]. Importantly, while barriers exist, several studies have shown that physician recommendation and effective communication influence uptake of chemoprevention [12, 19, 20], therefore, emphasizing the need to consider provider-level barriers in addition to patient-level barriers to address low uptake of chemoprevention.
We developed web-based decision support tools, RealRisks for high-risk women and BNAV (Breast cancer risk NAVigation tool) for providers (Supplementary Fig. 1) [21,22,23,24]. The patient-facing web-based RealRisks decision aid (DA) provides general education in graphic novel format and slide presentations and is organized into modules: 1) Breast cancer risk; 2) Family History and Genetic Testing; 3) Chemoprevention; 4) Lifestyle Behaviors. Within RealRisks,information is collected on breast cancer risk factors, including family history, in order to calculate 5-year and 10-year absolute invasive breast cancer risk according to the Breast Cancer Surveillance Consortium (BCSC) risk calculator, version 2 [25]. Upon completion of RealRisks, an action plan is generated summarizing the patient’s personalized breast cancer risk profile and preference elicitation for chemoprevention. On the provider end, the BNAV tool includes educational modules on breast cancer risk, genetic testing, screening, chemoprevention, and patient-centered care, and a patient dashboard with a summary of their patient's breast cancer risk profile based upon her interactions with RealRisks [24, 26]. We implemented these tools among high-risk women identified during screening mammography and in primary care [27]. After using these tools, we demonstrated an improvement in accurate breast cancer risk perceptions, chemoprevention knowledge, and informed choice; however, exposure to the tools did not significantly increase chemoprevention uptake [27, 28]. Given that targeting high-risk women or primary care providers (PCPs) alone may not increase chemoprevention delivery, our objective in this trial is to expand the use of this multilevel intervention to high risk women with AH or LCIS, who may benefit most from intervention [29].
In this ongoing trial entitled MiCHOICE (Making Informed Choices Incorporating Chemoprevention into carE), we are leveraging the clinical trials infrastructure of the National Cancer Institute (NCI) Community Oncology Research Program (NCORP). The pre-implementation phase has the potential to refine implementation strategies and contextualize implementation outcomes using a basic convergent design within an intervention mixed-methods framework [30]. Within this pre-implementation study, mixed methods are used to assess characteristics related to the organizational environment, perceived barriers/facilitators, potential adaptions, pros/cons of each intervention aspect, organizational support for and comfort with aspects of patient engagement, and the “primed patient” (exposure to RealRisks). The overarching goal of MiCHOICE is to address important barriers to chemoprevention uptake. The current study aimed to assess provider-perceived barriers to chemoprevention uptake and, specifically, how decision support tools may address these barriers.
Methods
Study Procedures
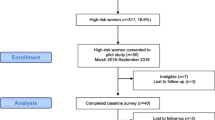
The MiCHOICE trial is a cluster randomized trial targeting women with high-risk benign breast lesions and clinicians, including PCPs and specialists (breast surgeons, medical oncologists, gynecologists). The trial was designed to enroll 415 patients and 200 healthcare providers and was activated in September 2020. As of June 14, 2024, 210 healthcare providers have been enrolled, and 412 patients out of the total target accrual of 415 have been enrolled [24]. The Site Principal Investigator (PI) obtained the permission of providers interested in enrolling to the study to share their contact information. The NCI Central Institutional Review Board (CIRB) approved the trial. Following the identification of eligible providers, research staff at Columbia University Irving Medical Center (CUIMC) obtained informed consent and administered an online questionnaire that collected information on demographics, professional and practice characteristics, use of breast cancer risk assessment tools, and chemoprevention prescribing patterns. The questionnaire also assessed provider confidence in medical statistics and risk communication [31].
For this qualitative study, a subset of providers randomized to the intervention group were invited via email to participate in pre-implementation semi-structured interviews. Twenty-five providers were interviewed based on the literature which suggests data saturation to occur after 12–20 interviews [32,33,34]. Interviews assessed implementation processes, including identifying barriers and facilitators in the discussion and implementation of chemoprevention. The role-based interview guide was informed by the Consolidated Framework for Implementation Research (CFIR) [35], a meta-framework that provides a menu of constructs associated with effective implementation, and developed for this study. The interview questions probe: 1) the intervention, 2) inner setting, 3) outer settings, 4) individuals involved and 5) the process for sustaining the chemoprevention intervention following study completion.
The CFIR-guided interviews (Supplementary Table 1) were conducted using Zoom videoconferencing. Audio recording of the interviews enabled subsequent coding of primary themes. Clinicians completed one-on-one 45-to 60-min CFIR guided video interviews (Supplementary Table 1) with a team member (JA). Interviews were audio-recorded with permission and transcribed. Consolidated Criteria for Reporting Qualitative Research (COREQ) were used to guide the methodologic approach [36].
Data Analysis
Descriptive statistics were generated to compare baseline characteristics of the total study population of healthcare providers enrolled in the parent MiCHOICE trial and the subset of providers enrolled in the pre-implementation interview study. Two-sample t-tests and chi-squared tests were used to compare continuous and categorical variables, respectively.
Transcribed and deidentified data were analyzed using ATLAS.ti software. Data analyses were performed using inductive thematic analysis, whereby the researchers immersed themselves in the interview transcripts to identify themes that emerged from the participant responses [37]. Two coders (HY and JA) met weekly to review the transcripts and to develop the codebook, with researchers generating the codes inductively [37,38,39,40]. Notably, HY (MPA) was a doctoral candidate in health education and a certified ATLAS.ti consultant with several years of experience in qualitative research, and JA had a Masters of Public Health degree with training in qualitative data analysis and also served as project manager. Initially, the coders (HY and JA) coded the 2 transcripts independently using line-by-line coding [37] and discussed potential codes with the rest of the team (HY, JA, RK) to develop consensus. As part of the coding process, the analysis team met regularly to discuss memos and compare constructs as they emerged from interview transcripts as part of the constant comparative method, an analytical process used in grounded theory for coding [38]. Modifications to the codebook were made as needed, going back to previously coded interviews to re-code as needed. Discrepancies regarding codes were discussed between the two coders. The coding of each transcript was compared consecutively. After defining the final codes, the two coders independently coded the remaining transcripts. Inter-rater reliability was calculated with a Scott’s Pi measure of 0.69 (scale 0–1), or 69% agreement between the coders, indicating substantial agreement. As a final step, themes were reviewed and further defined as a group (HY, JA, AV, RK, AM). We involved an additional study team member (AM) in the analysis and write-up phases of this qualitative analysis which provided alternative perspectives and enhanced the interrogation of assumptions. All co-authors approved the final exemplar quotations included in the results section. To ensure trustworthiness, direct quotations were provided to connect the results to the raw data.
Results
Table 1 summarizes the characteristics of the providers who participated in the semi-structured interviews compared to the total study population enrolled in the trial. Providers (N = 25) were predominantly female (84%), white (72%), and non-Hispanic (88%) (Supplementary Table 2). Eighteen (72%) were physicians, 6 (24%) nurse practitioners (NPs) and 1 (4%) physician assistant (PA). Specialties included Family Medicine (8%), Internal Medicine (8%), Oncology (40%), Surgery (32%), and other (12%). Nearly all providers (96%) reported they had previously prescribed anti-estrogens for chemoprevention. Providers reported high confidence levels (range 1–6, with 1 being not at all confident and 6 being extremely confident) in their knowledge of medical statistics (mean 4.64, SD 0.76), communication of medical statistics (mean 4.88, SD 0.73), and risk communication (mean 5.08, SD 0.57). The interview population was representative of the overall provider population taking part in the study.
Qualitative results
From the qualitative analysis of semi-structured interviews with participating providers, ten codes were generated with 873 coded quotations (Fig. 1). Three themes (each with subthemes) relating to providers’ experiences with chemoprevention use in high-risk women emerged from qualitative analysis: (1) beliefs about chemoprevention and suitability of decision support to facilitate decision-making and support patient autonomy; (2) barriers affecting chemoprevention uptake; and (3) suggestions for successful implementation and roll-out of the decision support tools. Exemplar quotations for each theme are included in Table 2.
Representation of the coding tree, with from left to right, sub-codes, codes, themes and sub-themes. In parenthesis, the number of quotations included in each category. Notably, three codes (role in facility, structural characteristics and engaging) were excluded from this coding tree given data was captured quantitatively and included in Table 1
Theme 1: Beliefs about chemoprevention and suitability of decision support tools to be helpful to their practices and support the autonomy of patient decision-making
Subtheme: Confidence in the Quality of the Data
Providers (N = 14/25, 56%) explicitly expressed confidence in the quality of data surrounding supporting recommendations for using chemoprevention. Specifically, 20% (N = 5/25) cited the existence of robust data from large RCTs as a key factor in support of chemoprevention. As one oncologist (ID #2, with 11–15 years of experience) stated, “I think the quality of evidence for chemoprevention definitely exists. We have several large randomized trials showing that it works.”
Subtheme: Suitability of Decision Support Tools
Providers (N = 22/25, 88%) generally felt the patient decision aid (DA), RealRisks, would be adaptable and well-suited to their workflow. Several providers (N = 6/25, 24%) believed it could save time in busy clinical practices, given the time required to explain chemoprevention. One oncologist (ID #6, < 5 years of experience) emphasized, “It didn’t seem that an hour was enough to kind of go over all of those things and sometimes… it could take more time [compared] with someone who has invasive cancer.”
Subthemes: Patient Autonomy in Decision-Making and Use of Risk Models
While expressing their support for using chemoprevention for risk reduction, providers (N = 12/25, 48%) also emphasized their commitment to respecting patient autonomy in the decision-making process. Providers emphasized the importance of conducting risk versus benefit analyses with patients; but ultimately one stated, “I always let them know that it’s their choice in the end” (ID #5, Oncology NP, 16–20 years of experience). To allow for patient autonomy, providers shared the general discussions of the risks and benefits that occur in the clinic regarding chemoprevention. Sixteen (64%) mentioned using risk assessment tools, such as the Gail [25] and Tyrer-Cuzick [41] models, as well as visual aids such as drawings and journal articles with charts to communicate risk effectively. Given the complexity of the issues, providers recognized the need for improved patient understanding and emphasized the decision does not have to be made at the first visit.
Theme 2: Barriers to chemoprevention uptake
Despite the availability of high-quality data and the belief that decision support tools could be beneficial to discussing chemoprevention use, various barriers were identified at different levels of healthcare delivery.
Subtheme: Time
The most common barrier cited by providers (N = 6/25, 24%) was limited time with patients due to clinic time constraints.
At the patient level, providers perceived numerous barriers to chemoprevention uptake.
Subtheme: Concerns about Side Effects
Fifteen providers (60%) highlighted patients’ medication beliefs and their preference for avoiding medications. One oncologist emphasized that despite evidence for chemoprevention, “Some patients are afraid of side effects and they don’t want to be on any medication, and they choose to be monitored by breast imaging” (ID #4, 11–15 years of experience). Concerning side effects, providers (N = 4/25,16%) felt patients often lack information about risk factors and prevention, which in turn leads them to turn to the internet for information on the side effects.
Subtheme: Stigma of cancer
Regarding patients’ reasoning for avoiding chemoprevention, three providers (12%) discussed the perception or “stigma” amongst high-risk women about chemoprevention medications, given their traditional association with cancer treatment. For example, the same oncologist (ID #4, 11–15 years of experience) who discussed patients’ concern for side effects, elaborated on patients’ fear of side effects and described grappling with patients’ perceptions that chemoprevention is like chemotherapy. She emphasized the confusion surrounding the practice of prescribing the same medications to both women diagnosed with breast cancer and those at high-risk for breast cancer saying, “Sometimes patients question if they don’t have any breast cancer, why do they need to take medications we actually prescribe to breast cancer patients?”.
Subtheme: Anxiety
Four providers (16%) described patient anxiety as a perceived barrier to chemoprevention discussions. They described the high-risk population as a highly anxious group given their previous lack of experience with the health care system and the various decisions they are faced with at the initial visit. One provider (ID #10, Surgery NP, 16–20 years of experience) posited, “The newer patients, are very anxious, you know, they’re talking to lots of different people. They’re not quite sure what to do.” Another provider (ID #12, Surgery NP, > 20 years of experience) felt that the anxiety may be “a little bit misguided” and suggested that the education tool may help with that.
Subtheme: Health Literacy
Additional provider-perceived barriers related to patient characteristic mentioned education level, health literacy, and cultural and socioeconomic issues. One oncologist (ID #25, 5–10 years of experience) identified low health literacy as a barrier in talking about chemoprevention and they discussed feeling “a little bit uncomfortable” given the implications of lower health literacy for shared-decision making.
Subtheme: Difficulty interpreting and communicating risk
Another challenge providers (N = 5/25, 20%) discussed included interpreting and communicating the risk of high-risk breast lesions to their patients. They suggested risk can be difficult for patients to grasp. One oncologist (ID #1, > 20 years of experience) explained, “The idea of risk and you’re going to try to reduce risk, is a hard concept. I’m going to take this pill that makes me sick with the hope maybe I won’t get cancer, but I might get cancer anyway.” Risk was further explained as difficult to grasp given chemoprevention’s delayed benefit over the course of a lifetime. Furthermore, providers acknowledged their own difficulty in communicating risk, saying, “Risk is really tough” (ID #2, Oncologist, 11–15 years of experience) and adding that their own difficulty with risk is complicated by the varying perceptions amongst patients of what constitutes an elevated risk.
Theme 3: Suggestions for successful implementation and roll-out of decision support tools
Regarding suggestions for successful trial implementation, seventeen providers (68%) suggested introducing the patient DA early, at the time of diagnosis of AH or LCIS within the first clinic visit to maximize impact. Providers supported multiple visits for reinforcing information regarding chemoprevention given their impression that patients often feel overwhelmed due to the volume of information provided at the initial visit. Additional suggestions to help with increasing chemoprevention uptake included the need for symptom management resources, given the likelihood of side effects. One surgeon suggested greater involvement of PCPs and gynecologists saying, “There’s not enough breast surgeons or high-risk clinics to handle all of the patients who probably would benefit from chemoprevention” (ID #21, > 20 years of experience). The DA was seen as a valuable tool to educate women, particularly given the time constraints in clinical settings and a lack of other resources for high-risk women. Providers also noted the absence of tools or risk calculators tailored to racial/ethnic minorities. There was some acknowledgment that the RealRisks tool addresses the needs of Hispanic patients, but not other groups such as Pacific Islanders.
Discussion
In this pre-implementation study of the MiCHOICE parent trial, we found providers generally felt their practices supported decision support tools focusing on chemoprevention. Providers acknowledged the existence of sufficient, high-quality data to support the use of chemoprevention in high-risk women. Providers identified barriers to the informing of patients, including time constraints, patients’ concerns about side effects, stigma of cancer, patient anxiety, and various patient-level factors such as education level, health literacy, cultural and socioeconomic issues.
Prior intervention trials of clinical decision support tools designed to increase uptake of breast cancer chemoprevention have been met with limited success. A web-based, personally tailored DA, targeting high-risk postmenopausal women, called Guide to Decide, demonstrated lower decisional conflict [42], but low chemoprevention uptake rates of 0.5% with raloxifene and no tamoxifen uptake [43]. Within the primary care setting, a tablet-based intervention called BreastCARE, consisting of an individualized risk report, led to more high-risk women being referred for specialized risk counseling within the intervention arm (18.8% vs 4.1%); yet discussions surrounding chemoprevention remained limited (1% vs 0%) [44]. In a program designed to increase risk screening, without targeted decision support, the Ready, Set, GO GAIL!program used the Gail model to screen more than 5700 women in a primary care setting [45]. While 15.2% of women were deemed high risk, only 14.7% were referred for risk counseling and only 2% started chemoprevention, with PCPs expressing concerns about the accuracy of the Gail model and the additional time needed to administer it [45]. Comparatively, our decision support tools are both personally tailored and contain individualized risk reports which are shared between patients and providers. In a prior RCT of decision support for 300 high-risk women and 50 providers, we found that our web-based DA, RealRisks,compared to standard educational materials did not significantly increase chemoprevention uptake but improved: 1) accurate breast cancer risk perceptions (56% vs 39%, p = 0.017); 2) adequate chemoprevention knowledge (49% vs. 27%, p < 0.001); 3) informed choice (41% vs. 23%, p = 0.003); and 4) decreased mean decision conflict (34.0 vs. 47.0, p < 0.001) [46]. In the MiCHOICE trial, we have targeted a population of high-risk women with AH or LCIS, who derive a greater benefit from chemoprevention, and their providers, predominantly surgeons and medical oncologists. Importantly, compared to prior research, our tools have been validated in ethnically diverse high-risk women with various educational backgrounds and health literacy [21, 46, 47] and our trial seeks to understand how the combined effect of targeting patients and their providers would impact chemoprevention informed choice.
In this qualitative study, we sought to understand how providers envisioned chemoprevention DAs would fit into their practices. Importantly, all providers reported having used breast cancer risk assessment tools. Nearly all providers (96%) surveyed had previously prescribed chemoprevention. Amongst these providers, they were generally willing and eager to incorporate our DA into their practices. Consistent with prior research, which has attributed inadequate time and competing demands to low chemoprevention uptake [48, 49], providers in our interviews cited time constraints as a challenge and discussed how offering resources such as our RealRisks DA could facilitate risk communication and chemoprevention decisions in a timely, in-depth manner. While Bychkovsky et al.found that the median time from first visit to chemoprevention was 54 days, with 31% of participants starting chemoprevention > 6 months after their initial visit [9], interviews suggested there may be a valuable role for our DA either prior to or after the initial visit to aid in decision making.
Prior qualitative and quantitative studies have identified patients’ medication beliefs and concerns about side effects as playing a major role in the low uptake of chemoprevention [17, 18]. Among women with AH, LCIS or ductal carcinoma in situ, 71.6% previously reported they were very or extremely worried about the side effects and 56.9% reported they thought the side effects were very or extremely serious [18]. Within our interviews, providers similarly asserted patients were concerned with potential side effects of chemoprevention, often turning to the internet to better understand the risks. While side effects were repeatedly discussed, several physicians also discussed the perception of patient stigma regarding chemoprevention’s traditional association with cancer treatment and “chemo” in general, suggesting that more clarification around terminology via DAs could help to clarify this further for patients.
Prior literature has implicated anxiety in the complexity of decision-making surrounding chemoprevention and even suggested anxiety may motivate interest in tamoxifen [50]; however, in our interviews, four (16%) providers specifically reported the perception of patient anxiety as a barrier to chemoprevention uptake. Several reasons for anxiety were suggested, including a lack of prior experience with the health care system, as well as the stigma associated with concerns about taking a drug used to treat cancer when they don’t have breast cancer and hesitancy to discuss side effects affecting woman’s bodies.
Based upon our survey results, providers felt confident in their ability to communicate statistics regarding risk to patients. However, in the interviews, five providers (20%) cited difficulty in explaining risk as a barrier to chemoprevention uptake and suggested additional supportive tools would be beneficial. This finding is consistent with our group’s prior finding amongst PCPs that a key barrier is limited risk communication about breast cancer risk [49].
In relation to the provider-related barriers we have identified in these pre-implementation interviews, our tools may be uniquely suited to address these concerns. To address providers’ difficulty communicating risk, BNAV includes educational modules on risk as well as direct information regarding a patient’s risk score and preferences regarding chemoprevention. On the patient end, RealRisksincludes information on risk factors and calculates a personalized breast cancer risk score according to the BCSC model. Together these tools may facilitate a more robust conversation regarding chemoprevention. Given that our prior work demonstrates that our tools improve the accuracy of breast cancer risk perceptions without increasing breast cancer worry [28], our DA may address anxiety and concerns about side effects, through the personalized absolute risk calculations that are provided for risk of common side effects (i.e. hot flashes) and rare side effects (i.e. thromboembolic events, endometrial cancer) with and without chemoprevention. The direct inputting of this risk information into the provider decision support will potentially facilitate more robust and targeted conversations amongst patients and their providers. Additionally, while several providers discussed health literacy, language barriers and diverse patient populations, prior usability studies of RealRiskshave demonstrated similar efficacy across racially and ethnically diverse populations [21, 27, 46].
Strengths of our study include the collection of quantitative data from surveys with additional qualitative data via semi-structured interviews. Limitations include the relative lack of racial/ethnic and gender diversity amongst the providers who participated in semi-structured interviews with predominantly female (84%), white (72%), and non-Hispanic (88%) providers. While our findings are about the target population of providers caring for women with high-risk breast lesions, which comprises the patient population that is most likely to benefit from chemoprevention, this specialized population of providers, including only 2 PCPs and 2 family medicine physicians, limits the generalizability of our results in this pre-implementation study. Another limitation is that we did not include the patient perspective in this analysis. We are currently conducting mid-implementation interviews of patients enrolled in the intervention arm of the parent trial to gain the patient perspective on the use of RealRisks, which will be reported in a subsequent publication. Other future directions will include the applicability of our decision support tools to additional populations, including lower-risk women, who may benefit from improved risk perception and understanding of universal preventative measures, including healthy lifestyles and alcohol reduction.
We acknowledge many barriers are posited to affect chemoprevention uptake. While our web-based DA has improved the accuracy of perceived breast cancer risk and decreased breast cancer worry amongst women meeting family history criteria for BRCA1/2 testing [27], some barriers (i.e., lack of time, medication beliefs, and fear of side effects) may be amenable to the role of the DA. While DAs may increase knowledge, self-efficacy and intent, Reuland et al.found that adding a patient navigator to a patient DA increased the screening rate for colorectal cancer compared to usual care alone amongst a diverse and vulnerable patient population [51]. Given the complexity of decision making surrounding chemoprevention, we similarly may need to address the topic as a multi-faceted intervention, such as by including trained patient navigators to assist patients as they interact with the DA [52].
Overall, our current study illustrates that providers recognize the potential benefits of decision support tools, which provide personalized information on breast cancer risk and the harms and benefits of chemoprevention, while also highlighting significant barriers such as time constraints and patient-related concerns that these tools may help mitigate, demonstrating their potential to enhance both provider-patient communication and informed decision-making in clinical practice. We plan to conduct additional mid-implementation interviews of providers and patients to further understand how the decision support tools were implemented into clinical care and their effectiveness. Our overarching goal is to compare the frequency of chemoprevention informed choice at 6 months after enrolling women with AH or LCIS while assessing patients’ perceived breast cancer risk and worry, chemoprevention knowledge, chemoprevention intention/decision, decision conflict and decision regret. Simultaneously, using implementation science and mixed methods research, we can contextualize quantitative findings to better understand barriers and facilitators to implement a chemoprevention DA intervention into clinical workflow.
Availability of data and materials
The data analyzed during the current study are available from the corresponding author upon reasonable request.
References
Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–51.
Cuzick J, Sestak I, Cawthorn S, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16(1):67–75.
Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014;383(9922):1041–8.
Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–62.
Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364(25):2381–91.
Vogel VG, Costantino JP, Wickerham DL, et al. Update of the National Surgical Adjuvant Breast and Bowel Project Study of Tamoxifen and Raloxifene (STAR) P-2 Trial: Preventing breast cancer. Cancer Prev Res. 2010;3(6):696–706.
Lazzeroni M, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Recurrence in Breast Noninvasive Neoplasia: A 10-Year Follow-Up of TAM-01 Study. J Clin Oncol. 2023;41(17):3116–21.
Bychkovsky B, Laws A, Katlin F, et al. Initiation and tolerance of chemoprevention among women with high-risk breast lesions: the potential of low-dose tamoxifen. Breast Cancer Res Treat. 2022;193(2):417–27.
Flanagan MR, Zabor EC, Stempel M, Mangino DA, Morrow M, Pilewskie ML. Chemoprevention Uptake for Breast Cancer Risk Reduction Varies by Risk Factor. Ann Surg Oncol. 2019;26(7):2127–35.
Port ER, Montgomery LL, Heerdt AS, Borgen PI. Patient reluctance toward tamoxifen use for breast cancer primary prevention. Ann Surg Oncol. 2001;8(7):580–5.
Smith SG, Sestak I, Forster A, et al. Factors affecting uptake and adherence to breast cancer chemoprevention: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):575–90.
Waters EA, Cronin KA, Graubard BI, Han PK, Freedman AN. Prevalence of tamoxifen use for breast cancer chemoprevention among US women. Cancer Epidemiol Biomarkers Prev. 2010;19(2):443–6.
Ravdin PM. The lack, need, and opportunities for decision-making and informational tools to educate primary-care physicians and women about breast cancer chemoprevention. Cancer Prev Res. 2010;3(6):686–8.
Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–5.
Jones T, Guzman A, Silverman T, Freeman K, Kukafka R, Crew K. Perceptions of Racially and Ethnically Diverse Women at High Risk of Breast Cancer Regarding the Use of a Web-Based Decision Aid for Chemoprevention: Qualitative Study Nested Within a Randomized Controlled Trial. J Med Internet Res. 2021;23(6): e23839.
Padamsee TJ, Hils M, Muraveva A. Understanding low chemoprevention uptake by women at high risk of breast cancer: findings from a qualitative inductive study of women’s risk-reduction experiences. BMC Womens Health. 2021;21(1):157.
Trivedi MS, Coe AM, Vanegas A, Kukafka R, Crew KD. Chemoprevention Uptake among Women with Atypical Hyperplasia and Lobular and Ductal Carcinoma In Situ. Cancer Prev Res. 2017;10(8):434–41.
Meiser B, Wong WKT, Peate M, Julian-Reynier C, Kirk J, Mitchell G. Motivators and barriers of tamoxifen use as risk-reducing medication amongst women at increased breast cancer risk: a systematic literature review. Hered Cancer Clin Pract. 2017;15:14.
Rondanina G, Puntoni M, Severi G, et al. Psychological and clinical factors implicated in decision making about a trial of low-dose tamoxifen in hormone replacement therapy users. J Clin Oncol. 2008;26(9):1537–43.
Coe AM, Ueng W, Vargas JM, et al. Usability Testing of a Web-Based Decision Aid for Breast Cancer Risk Assessment Among Multi-Ethnic Women. AMIA Annu Symp Proc. 2016;2016:411–20.
Kukafka R, Yi H, Xiao T, et al. Why Breast Cancer Risk by the Numbers Is Not Enough: Evaluation of a Decision Aid in Multi-Ethnic, Low-Numerate Women. J Med Internet Res. 2015;17(7): e165.
Yi H, Xiao T, Thomas PS, et al. Barriers and Facilitators to Patient-Provider Communication When Discussing Breast Cancer Risk to Aid in the Development of Decision Support Tools. AMIA Annu Symp Proc. 2015;2015:1352–60.
Crew KD, Anderson GL, Arnold KB, et al. Making Informed Choices On Incorporating Chemoprevention into carE (MiCHOICE, SWOG 1904): Design and methods of a cluster randomized controlled trial. Contemp Clin Trials. 2024;142: 107564.
Gail MH, Brinton LA, Byar DP, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81(24):1879–86.
Finkelstein J, Wood J, Crew KD, Kukafka R. Introducing a Comprehensive Informatics Framework to Promote Breast Cancer Risk Assessment and Chemoprevention in the Primary Care Setting. AMIA Jt Summits Transl Sci Proc. 2017;2017:58–67.
Kukafka R, Pan S, Silverman T, et al. Patient and Clinician Decision Support to Increase Genetic Counseling for Hereditary Breast and Ovarian Cancer Syndrome in Primary Care: A Cluster Randomized Clinical Trial. JAMA Netw Open. 2022;5(7):e2222092.
Kukafka R, Fang J, Vanegas A, Silverman T, Crew KD. Pilot study of decision support tools on breast cancer chemoprevention for high-risk women and healthcare providers in the primary care setting. BMC Med Inform Decis Mak. 2018;18(1):134.
Crew K, Anderson G, Arnold K. SWOG 1904: Cluster-randomized controlled trial of patient and provider decision support to increase chemoprevention informed choice among women with atypical hyperplasia or lobular carcinoma in situ (MiCHOICE). 2022.
Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs-principles and practices. Health Serv Res. 2013;48(6 Pt 2):2134–56.
Han PK, Joekes K, Elwyn G, et al. Development and evaluation of a risk communication curriculum for medical students. Patient Educ Couns. 2014;94(1):43–9.
Creswell J. Qualitative Inquiry and Research Design: Choosing Among Five Traditions. Thousand Oaks, CA: SAGE Publications; 1998.
Green J, Thorogood N. Qualitative methods for health research. 2nd ed. Los Angeles: SAGE; 2009.
Guest G, Bunce A, Johnson L. How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18:59. https://doi.org/10.1177/1525822X05279903.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–57.
Clarke V, Braun V, Hayfield N. Thematic Analysis. In: Handbook of Research Methods in Health Social Sciences. 2019.
Chun Tie Y, Birks M, Francis K. Grounded theory research: A design framework for novice researchers. SAGE Open Med. 2019;7:2050312118822927.
Charmaz K. Constructing grounded theory. London ; Thousand Oaks, Calif: Sage Publications; 2006.
Charmaz K. Teaching Theory Construction With Initial Grounded Theory Tools: A Reflection on Lessons and Learning. Qual Health Res. 2015;25(12):1610–22.
Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23(7):1111–30.
Banegas MP, McClure JB, Barlow WE, et al. Results from a randomized trial of a web-based, tailored decision aid for women at high risk for breast cancer. Patient Educ Couns. 2013;91(3):364–71.
Fagerlin A, Dillard AJ, Smith DM, et al. Women’s interest in taking tamoxifen and raloxifene for breast cancer prevention: response to a tailored decision aid. Breast Cancer Res Treat. 2011;127(3):681–8.
Kaplan CP, Livaudais-Toman J, Tice JA, et al. A randomized, controlled trial to increase discussion of breast cancer in primary care. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1245–53.
Owens WL, Gallagher TJ, Kincheloe MJ, Ruetten VL. Implementation in a large health system of a program to identify women at high risk for breast cancer. J Oncol Pract. 2011;7(2):85–8.
Crew KD, Bhatkhande G, Silverman T, et al. Patient and Provider Web-Based Decision Support for Breast Cancer Chemoprevention: A Randomized Controlled Trial. Cancer Prev Res. 2022;15(10):689–700.
Trivedi MS, Manley H, Yi H, et al. Pilot study of a decision aid on BRCA1/2 genetic testing among Orthodox Jewish women. Fam Cancer. 2024. https://doi.org/10.1007/s10689-024-00371-6.
Crew KD. Addressing barriers to uptake of breast cancer chemoprevention for patients and providers. Am Soc Clin Oncol Educ Book. 2015:e50–58. https://doi.org/10.14694/EdBook_AM.2015.35.e50.
Jones T, Silverman T, Guzman A, et al. Qualitative analysis of shared decision-making for chemoprevention in the primary care setting: provider-related barriers. BMC Med Inform Decis Mak. 2022;22(1):208.
Dillard AJ, Scherer L, Ubel PA, et al. Breast cancer anxiety’s associations with responses to a chemoprevention decision aid. Soc Sci Med. 2013;77:13–9.
Reuland DS, Brenner AT, Hoffman R, et al. Effect of Combined Patient Decision Aid and Patient Navigation vs Usual Care for Colorectal Cancer Screening in a Vulnerable Patient Population: A Randomized Clinical Trial. JAMA Intern Med. 2017;177(7):967–74.
Molina Y, Kim SJ, Berrios N, et al. Patient Navigation Improves Subsequent Breast Cancer Screening After a Noncancerous Result: Evidence from the Patient Navigation in Medically Underserved Areas Study. J Womens Health (Larchmt). 2018;27(3):317–23.
Acknowledgements
This work was supported by the National Institutes of Health, National Cancer Institute UG1CA189974, R01CA226060; The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding
This work was supported by the National Institutes of Health, National Cancer Institute UG1CA189974, R01CA226060; The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
AM: Formal analysis, Writing– original draft, review & editing. HY: Formal analysis, Writing- original draft, review & editing. JA: Project administration, investigation, review & editing. NC: Project administration; review & editing, investigation. AV: Writing- original draft. SU: Formal analysis, Writing- original draft, review & editing. GA: Writing – review & editing, Methodology, Formal analysis. KA: Writing – review & editing, Project administration, Methodology, Investigation, Formal analysis, Data curation. CL: Writing – review & editing, Project administration, Investigation. SP: Writing – review & editing, Project administration. ASL: Writing – review & editing, Project administration. RS: Writing – review & editing, Project administration. MGP: Writing – review & editing, Project administration. SC: Writing – review & editing, Project administration. SK: Writing – review & editing, Project administration. TK: Writing – review & editing, Project administration. LY: Writing – review & editing, Project administration. TB: Writing – review & editing, Project administration. CBI: Writing – review & editing, Project administration. DM: Writing – review & editing, Project administration. KW: Writing – review & editing, Project administration. CD: Writing – review & editing, Project administration. MR: Writing – review & editing, Project administration. JF: Writing – review & editing, Project administration. AK: Writing – review & editing, Project administration. LV: Writing – review & editing, Project administration. TS: Writing – review & editing, Project administration. CZ: Writing – review & editing, Project administration. SL: Writing – review & editing, Project administration. CG: Writing – review & editing, Project administration. AC: Writing – review & editing, Project administration. KY: Writing – review & editing, Project administration. DA: Writing – review & editing, Project administration. CJ: Writing – review & editing, Methodology. DH: Writing – review & editing, Project administration, Methodology, Investigation, Funding acquisition. MN: Writing – review & editing, Methodology, Investigation. BA: Writing – review & editing, Supervision, Methodology, Investigation. KC: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. RK: Writing – review & editing, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The trial was approved by the National Cancer Institute Central Institutional Review Board (CIRB). Informed consent to participate was obtained from all of the participants in the study.
Consent for Publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Michel, A., Yi, H., Amenta, J. et al. Use of web-based decision support to improve informed choice for chemoprevention: a qualitative analysis of pre-implementation interviews (SWOG S1904). BMC Med Inform Decis Mak 24, 272 (2024). https://doi.org/10.1186/s12911-024-02691-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-024-02691-0