Abstract
Background
The benefits of comprehensive geriatric assessment (CGA) are well established for hospital care but less so for primary care. Our primary objective was to assess the effect of two multifaceted interventions based on a CGA adapted for primary care on a composite criterion combining all-cause mortality, emergency department visits, unplanned hospital admissions, and institutionalisation.
Methods
This open-label, pragmatic, three-arm, cluster-randomised controlled trial involved 39 general practices in France. It included 634 patients aged 70 years or over with chronic health conditions and/or an unplanned hospital admission in the past 3 months, between 05/2016 and 08/2018. Interventions were in arm 1: a systematic nurse-led CGA; arm 2: a GP-led CGA, at the GP’s discretion; arm 3: standard care. The primary composite endpoint was assessed at 12 months. The secondary endpoints included: components of the composite endpoint, health-related quality of life (Duke Health Profile), functional status (Katz Activities of Daily Living Index) and medications (number) at 12 months. Pairwise comparisons between the experimental groups and the control were tested. The main analysis was performed on the intention-to-treat (ITT) population, after imputing missing information and adjusting for baseline imbalances by mixed effects regressions.
Results
For the primary composite outcome, no statistically significant difference was found between arm 1 and the control (adjusted odds ratio [aOR] = 0.81 [95%CI 0.54–1.21], P = 0.31), whereas arm 2 and the control differed significantly (aOR = 0.60 [0.39–0.93], P = 0.022). A statistically lower risk of unplanned hospital admission in arm 2 vs control (aOR = 0.57 [0.36–0.92], P = 0.020)) was observed, while no statistically significant differences were found for the other components and between arm 1 and the control. None of the other secondary endpoints differed between arms.
Conclusions
Our study led in community-dwelling older patients with chronic conditions found no significant effect of a CGA adapted for primary care on mortality, functional independence and quality of life, but suggests that a GP-led CGA may reduce the risk of unplanned hospital admission. Our study demonstrates the feasibility of incorporating CGA into clinical practice and highlights its potential benefits when applied on a case-by-case basis, guided by the GPs who develop the resulting PCP.
Trial registration
NCT02664454.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Worldwide, the population is ageing: according to the United Nations, the number of people aged 65 and over will rise from 700 million in 2019 to 1.5 billion in 2050 [1]. In the European Union, the proportion of over-65 s is expected to increase from 18.5% in 2014 to 30% in 2080 [2]. Population ageing will raise crucial challenges for healthcare systems and primary care settings for the coming decades [3,4,5,6]. The assessment and management of ageing has to change [7, 8]. The World Health Organization (WHO) defined the concept of “healthy ageing” as helping older people to develop and maintain the functional ability that enables well-being [9]. The prevalences of multimorbidity, frailty and functional decline increase with age and thus lead to a greater risk of falls, hospital admission, disability, long-term care and death [10]. Thus, strategies for preventing, slowing or reverse the declines in older people’s abilities are essential for healthy ageing [11].
Multidimensional comprehensive geriatric assessments (CGAs) were developed to better define the sometimes complex care required by older adults and to improve patient-centred care [12]. This is a holistic diagnostic process for determining overall health status, with a focus on the person’s physical, functional, psychological and social capabilities [13]. A CGA is not limited to assessment because it prompts the formulation of a coordinated, integrated, multicomponent, personalised care plan (PCP) for treatment and follow-up [12, 14]. The PCP takes account of the patients’ preferences and priorities with regard to managing the identified health problems [12, 15, 16].
The benefits of performing a CGA have been well established in hospital care [17] but less so in primary care [18, 19]. A recent Cochrane Collaboration review (2022) found that a CGA in a primary care setting might not have an impact on death or institutionalisation among community-dwelling older patients but might (according to low-certainty evidence) reduce the risk of unplanned hospital admission [18]. The researchers concluded that further studies (using validate scales) were required to examine the effects of primary care CGAs on emergency department visits, functional independence and quality of life [18].
To contribute to provide primary care data, we conducted the Clinical Epidemiology and Ageing (CEpiA) three-arm, cluster-randomised trial. We hypothesised that relative to standard care (arm 3), a complex intervention including a specific training seminar, a dedicated helpline for GPs, and a CGA modified for use in primary care and led by a nurse (arm 1) or a GP (arm 2) would be associated with lower morbidity and mortality rates in over-70 patients with chronic health conditions. We chose not to focus on vulnerable older patients with acute care needs because the literature suggests that frail older adults living in the community would benefit the most from CGA [18]. With increasing global longevity and imminent demographic shifts, it is crucial to find ways to reduce the anticipated surge in hospitalisations. Furthermore, interventions targeting community-dwelling older patients could positively influence the course of their chronic diseases by reducing functional decline at an earlier stage. Therefore, we chose to focus on regular older patients with chronic conditions. This choice was intended to reflect the type of older patients GPs usually encounter in their daily practice, making the results of this study more relevant to their clinical practice.
We also hypothesised that the effect of the intervention would be greatest in arm 1 (i.e., with a nurse-led CGA). We assumed that the design for arm 1, with a systematic CGA provided by a trained nurses (unlike in arm 2, where it is left to the GP’s discretion), along with the delegation of tasks to nurses by GPs, would enable the full deployment of the intervention and increase its likelihood of effectiveness. The implementation of CGA in primary care is challenging, because GPs are already facing a workload and this new task may be seen as burdensome [4], thus leading to the design of delegation of this time-consuming task to nurses in arm 1, and the hypothesis of arm 2 (with GPs only) could potentially be less effective. Multidomain assessments are core nursing competencies [20], and nurse-led care reportedly gives similar biomedical outcomes, and even slightly better results for the patient’s quality of life, relative to GP-led care [21,22,23]. Few large randomised controlled trials (RCTs) found benefits of a nurse-led CGA (geriatric nurses or intensively trained practice nurses) among the community-dwelling older patients with multiple comorbidities on their daily functioning (24) and mental well-being [24]. Also, the WHO has recommended that older patients receive integrated interventions from a multidisciplinary primary care team to improve healthy ageing [7] and the British Geriatrics Society has advised to delegate a part of CGA in primary care to nurses [14].
The primary objective of the present study was to assess the impact on 12-month morbidity and mortality rates of nurse-led or GP-led CGAs, compared with standard care with a composite endpoint. The secondary objectives were to assess these interventions’ effects on the individual components of the composite endpoint (all-cause mortality, unplanned hospital admissions, emergency department visits, and institutionalisation), together with quality of life, functional independence, and polypharmacy.
Methods
Trial design
The CEpiA multicentre, open-label, three-arm, parallel-group, pragmatic, cluster-randomised controlled trial involved 39 general practices in France and was conducted between May 2016 and August 2018. The detailed protocol has been published elsewhere [10] and preliminary (partial) results of this study were presented in scientific meetings [25,26,27]. The study was approved by an independent ethics committee (CPP Ile-de-France IV, Paris, France; reference: 2015/48SC). The study was conducted in accordance with the Declaration of Helsinki. All followed procedures were in accordance with relevant guidelines and French regulations. The study database was registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés, Paris, France). All the study participants provided their verbal, informed consent. This study was registered at ClinicalTrials.gov (NCT02664454) before the first recruitment. The results were reported in accordance with the CONSORT guidelines.
Participants
The inclusion criteria were age 70 or over, a long-term health condition (see reference 10) or an unplanned hospital admission in the previous 3 months, and consultation with their usual GP or another GP in the same investigating centre. The main exclusion criteria were an estimated life expectancy below 12 months, inability to speak or understand French, residence in an institutional, and the absence of health insurance coverage. Once GP practices were randomised to one of the three arms of the trial, patients seen consecutively during consultations or home visits by GPs and in compliance with eligibility criteria were invited to participate to the study. Patients then received oral and written information about the trial by the GP investigator. Eligible patients were included after giving verbal informed consent (the date of given verbal consent was written in the Case Report Form).
Interventions
The two interventions combined three components. In arm 1: firstly, a 1-day multidisciplinary seminar on CGA in primary care was organised for the GPs and nurses. Secondly, a CGA was systematically performed by a nurse. Lastly, a dedicated hotline (staffed by geriatricians) was made available for the GPs all along the follow-up. In arm 2: the same 1-day multidisciplinary seminar on CGA in primary care was organised for the GPs. Secondly, a CGA was performed by the GP if he deemed it necessary. Lastly, the dedicated hotline was made available for the GPs all along the follow-up. In Arm 3: there were no specific interventions (usual care). A figure describing the two interventions is available in Additional file 1: Fig. S1. The detailed description of each component of the intervention was published elsewhere [10]. The CGAs in arms 1 and 2 had to be performed within a month of the patient’s inclusion and could be performed at the patient’s home or in the GP’s office. CGA tools used in arms 1 and 2 were identical (Additional file 2: Table S1).
The CGA led to the formulation of a PCP including shared objectives and care actions planned in the short term (within 3 months) and medium term (within 6 months) [10] (Additional file 3: Table S1). The combination of tailored care actions initiated with the PCP and an optimised and regular follow-up were expected to improve geriatric outcomes, notably by allowing medicines optimisation and earlier detection and management of nutritional, functional or cognitive deficits. This hypothesis was in line with a recent review article [28] on complex community-based interventions to sustain independence in older patients, whose findings indicate that multifactorial action from individualised care planning and regular follow-up reviews, tailored to the needs of older patients, may contribute to their health and wellbeing and maximise their independence.
Collaboration between nurses and GPs in Arm 1
Nurse-led GCAs were performed by registered nurses (community or practices nurses) with no specific specialisation in geriatrics, apart from the training provided in the 1-day seminar. The collaboration modalities between GPs and nurses were not imposed by the study and were left to the discretion of the study actors. GPs were responsible for proposing the inclusion in the study to patients meeting the eligibility criteria. Then, nurses performed the CGA within a month after inclusion. Finally, GPs were expected to establish the PCP based on the results of the nurse-led CGA.
Outcomes and follow-up
Patients were evaluated three times by the GPs: at baseline and after 6 and 12 months. Printed Case Report Forms (CRFs) were to be completed by GPs based on consultation findings and medical records. CRFs were collected and monitored for consistency and missing data by clinical research technicians at M6 and M12 based on available hospital stay reports.
The primary outcome (a composite of all-cause mortality, unplanned hospital admissions, emergency department visits and institutionalisation) was assessed at 12 months. The secondary endpoints included an individual measurement of each component of the composite outcome at 12 months, as well as the change from baseline to 12 months in the number of medications, health-related quality of life (assessed with the validated French-language version of the Duke Health Profile) and functional independence (on the Katz Index of Independence in Activities of Daily Living scale). The Duke Health Profile comprises 17 items that can be combined into six health measures (physical, mental, social, general and perceived health, and self-esteem) and four dysfunction measurements (anxiety, depression, pain, and disability) on 0-to-100 scales (a higher score = better health).
Process indicators were used to measure intervention coverage in the two interventional arms, up to 12 months post-inclusion. They included the number of CGAs performed and PCPs drafted, the number of calls to the helpline, and number of care actions delivered. The feasibility and the GPs’ and nurses’ levels of satisfaction were assessed qualitatively and will be published elsewhere.
Randomisation
Cluster-randomisation was applied at the practice level because of potential organisational changes in participating practices and in order to avoid contamination bias between control and experimental groups. The computerised randomisation used an allocation list prepared by an independent statistician who was not involved in patient enrolment or the final analyses. To limit potential differences between arms, we applied a “best balance” allocation procedure to the GP centres [29,30,31] based on the following prespecified characteristics: rural/urban setting, proportion of over-70 s in the past year, the number of GPs, and the presence/absence of a nurse in the practice. All units were enrolled before randomisation, allowing for collecting this information beforehand. In a nutshell, the procedure is based on the calculation of all possible allocations with estimation of a balanced statistic for each one. A subset of all allocations with the highest level of balance (i.e., 1% lowest measures of imbalance) is then identified, from which the final allocation is randomly selected. Because of the extremely large total number of possible allocations—more than 1*10^17— too computationally intensive to allow direct calculations, randomisation was performed in three blocks, with block sizes of 14, 13 and 13 units, respectively. Allocation involved 5 v 4 v 4 units for the two arms with 13-unit blocks and 5 v 5 v 4 units for the arm with the 14-unit block. Because of the nature of the intervention, the trial is an open-label study, but primary and secondary outcomes were analysed with blinding of the trial statistician masked to arm allocation.
Sample size
Based on French national health insurance databases, the primary endpoint was expected to be 35% in the control group (standard care). The greatest intervention effect was expected in arm 1 (a systematic nurse-led CGA). With a two-sided type 1 error of 5%, a maximal intraclass correlation coefficient of 0.01, and 5% loss-to-follow-up rate, we calculated that a total of 750 patients (250 per group) in 40 clusters was required to achieve a power of 80% for detecting an absolute difference of − 15% (i.e. 35% [control] vs 20% [intervention]) between the interventional arms and the control group.
Statistical analysis
Standard descriptive statistics were used to evaluate baseline characteristics and process indicators. Quantitative data were expressed as the mean (standard deviation) or the median (interquartile range (IQR)), and categorical data were expressed as the frequency (percentage). The groups’ baseline variables and care actions at 12 months were compared in a Kruskal–Wallis test (for continuous variables) and Pearson’s chi-squared test or Fisher exact test (for categorical variables).
We evaluated the primary and secondary outcomes in the intention-to-treat (ITT) population. The analyses featured mixed-effects logistic regression models for categorical variables and mixed-effects linear regression models for quantitative variables, using the GP’s practice as a random effect. Pairwise comparisons between the interventional groups and the control group (i.e. arm 1 vs arm 3, arm 2 vs arm 3) were tested. Data that were missing at 12 months in the ITT population were imputed using the missForest nonparametric machine learning imputation method [32], assuming data to be missing at random conditional on other predictors and on the outcome. A comparison of patients with complete information on the primary endpoint to those with incomplete information is shown in Additionalfile 4: Table S1, finding no significant differences in patients’ main characteristics.
To account for potential inter-arm imbalances in important prognostic factors after randomisation, we performed multivariable analyses and adjusted for potential confounders (sex, age, depression and loss of functional independence). To verify the robustness of the results, additional supporting analyses without missing data imputation were conducted on the complete-case population.
All tests were two-sided and the threshold for statistical significance was set to P < 0.05. Odds ratios were calculated and their 95% confidence intervals (CIs) were obtained with the Wald estimation. All analyses were performed with STATA software v14.2 (StataCorp, TX, USA) and R software v4.03 (R Foundation, Austria).
Results
Study population
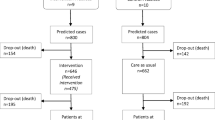
Forty general practices (comprising 90 GPs) in three French regions were initially enrolled and 39 (comprising 89 physicians) were randomised (Table 1). Most practices were in urban areas (74.4%). A total of 634 patients were recruited. Complete data on the primary outcome at 12 months were available for 586 (92%) patients (Fig. 1).
The median [IQR] age of the included patients was 82 [77.1–86.5] (Table 2). At baseline, the three arms did not differ significantly with regard to marital status, living arrangements, comorbidity, polypharmacy, emergency department visits and hospital admissions in the previous 3 months, and quality of life (Table 2). However, there were significant inter-arm differences at baseline in age, sex ratio, depression and functional independence. The patients in arm 3 (standard care) were notably younger and less likely to lose functional independence. The patients in arm 1 (systematic nurse-led CGA) were more likely to be depressed, with a higher proportion of women.
Primary outcome
For the primary composite endpoint main analysis led in the ITT population after missing data imputation and adjusting for baseline imbalances (Table 3, right lower section), no statistically significant difference was found between arm 1 and the control (adjusted odds ratio [aOR] 0.81 [0.54–1.21], P = 0.31), whereas arm 2 and the control differed significantly (aOR 0.60 [95%CI 0.39–0.93); P = 0.022). Analyses of the components of the primary endpoint revealed a statistically lower risk of unplanned hospital admission in arm 2 vs control (aOR 0.57 (0.36–0.92); P = 0.020)), while no statistically significant differences were found for the other components (all-cause mortality, emergency department visits, institutionalisation) and between arm 1 and the control. Unadjusted analyses (Table 3, left sections) and/or led on complete cases without missing information (Table 3, upper section) found no statistically significant differences between the two interventional arms and the control arm with regard to the primary composite endpoint and its components.
Secondary outcomes and process indicators
There were no statistical differences between the two interventional arms and the control arm regarding changes from baseline in the number of medications, functional independence and health-related quality of life (Additional file 5: Table S1). Sensitivity analyses on the final 12-month follow-up value adjusting for baseline information were performed and yielded largely similar results (Additional file6: Table S1).
At 12 months, 211 CGAs (93.4%) had been performed by nurses (arm 1) and 158 (85.0%) had been performed by GPs (arm 2) (Additional file 7: Table S1). There were no CGAs in the control arm 3. The median (IQR) duration of a CGA was significantly (P < 0.001) longer in arm 1 (50 min (45–60) than in arm 2 (40 min (30–46)). The GPs in arm 2 were more likely to divide the CGA into two sessions (P < 0.001).
There were 192 (85.3%) PCPs in arm 1 and 141 (78.8%) in arm 2 (Additional file 7: Table S1). The median time spent drawing up a PCP was longer in arm 1 (15 min (10–20)) than in arm 2 (10 min (10–20); P = 0.007). In arm 1, the PCPs were provided mainly by nurses (52.7%) but also by GPs (23.4%), a GP-nurse collaboration (11.2%) and by teams of other healthcare professionals (12.2%).
None of the GPs in arms 1 and 2 called the helpline during the study period.
Fewer care actions at 12 months were delivered in arm 2 (P < 0.001) (Table 4). Laboratory tests (P < 0.001) and medical imaging (P < 0.001) were prescribed more frequently in the control group. Nutritional care was prescribed more frequently (P < 0.001) in arm 2.
Discussion
Our results suggest that GP-led CGAs of community-dwelling older patients with chronic conditions treated in primary care may reduce unplanned hospital admissions. We did not observe significant associations between GP-led CGAs and the other secondary endpoints: death, emergency department visits, institutionalisation, quality of life, functional independence and polypharmacy. There were no significant differences in any of the endpoints between arm 1 (nurse-led CGAs) and arm 3 (control).
Most of our results are consistent with the literature data. A recent Cochrane review [18] suggested that a CGA of community-dwelling older patients in primary care might not have an impact on death or institutionalisation, as we also found in our analysis of secondary outcomes. There is low-certainty evidence of an association between CGAs and fewer unplanned hospital admissions [18, 33]; we also observed this association but only for GP-led CGAs. Our study suggests that a CGA adapted for use in primary care does not reduce the risk of emergency department visits and does not influence the patient’s functional. These findings are in line with those of the Cochrane review. Of the nine studies reviewed, only one [34] used the same scale as we did (the Katz Index) to evaluate the change in functional status, and it also found no significant differences. Our results suggest that a primary care CGA may have no impact on health-related quality of life; this finding differs from that of the Cochrane review, where CGA was associated with small changes in quality of life in the few included studies. However, the level of evidence was estimated to be very low and the impact was ultimately considered to be uncertain by the investigators. Furthermore, none of the six reviewed studies used the scale that we did; this might well explain the disparity. It is noteworthy that we used a generic, health-related quality of life instrument (i.e. the Duke Health Profile) in our CGA. Since some domains specific for older patients are not addressed by this instrument, some subtle changes in quality of life might have been overlooked. To the best of our knowledge, our study is the first to have looked at the impact of CGA on polypharmacy and care actions delivered in primary care settings, and so a comparison with the literature data is not possible.
The results in arm 2 suggest that GP-led CGA was associated with fewer unplanned hospital admissions at 12 months. Care actions were less frequent in arm 2, although nutritional support was more frequent. However, patients in arm 2 were similar to those in arm 1 in terms of malnutrition (data not shown). We suspect that the care actions delivered in arm 2 were probably better targeted, with fewer prescriptions overall but with more appropriate prescriptions in some domains (e.g. nutrition). We also suspect that nutritional assessments might often be neglected during a consultation with a GP, compared with higher-priority concerns in other domains; hence, the CGA might have made GPs more aware of this topic. Improving the prevention or management of malnutrition by GPs via a CGA might explain the reduction in unplanned hospital admissions in this arm. It has been reported in the literature that unidentified or untreated malnutrition is associated with a higher risk of hospital admission [35, 36]. It is also well established that a strengthened follow-up in general practice is associated with fewer hospital admissions for ambulatory patients with chronic health issues [37, 38].
We did not find any significant benefits in Arm 1 where CGA was systematically performed by a trained nurse. Yet, when the CEpiA study was designed, arm 1 was expected to have the greatest intervention effect because all the assigned patients would have a CGA. The lack of an effect in arm 1 might be due to the delegation of tasks to nurses by GPs, rather than a formalised and effective collaboration, notably involving common objectives and shared decisions-making. Approximately 53% of the PCPs in arm 1 were provided solely by nurses, revealing issues in the collaboration process with GPs. This raises questions about the GPs’ involvement in the establishment of PCPs and their participation in implementing the interventions in arm 1, when CGA was not guided by GPs but systematically performed by nurses. This is highlighted by the increased number of interventions conducted in arm 2, as shown in Table 4. The first potential explanation for the collaboration issues is that the 1-day training seminar focused on the CGA tool but not on teamwork; it did not explore how to work together. Secondly, the nurses and GPs in the two-person teams did not necessarily know each other before the study. Thirdly, some nurses did not work in the same practice as their partner GP; organisational issues might have occurred in some teams. Fourthly, 53% of the PCPs in arm 1 were developed by a nurse without supervision by a GP; some of these prescriptions might not have been relevant. Lastly, the CGAs and PCPs in arm 1 were often conducted or drafted a long time after study inclusion (median time interval: 21 days and 46 days, respectively). A complementary analysis using qualitative methodology is currently underway, and its results will be presented in a separate dedicated article specifically studying the collaboration modalities between GPs and nurses, and their issues. Even though the associations in arm 1 were not statistically significant, there were nevertheless trends towards fewer emergency department visits (P = 0.105) and institutionalisations (P = 0.109). Our results are mostly consistent with literature data. Few large RCTs found benefits of a nurse-led CGA (geriatric nurses or intensively trained practice nurses) among the community-dwelling older patients with multiple comorbidities on their daily functioning [39] and mental well-being [24]. However, and similarly to our study, some other RCTs found no benefits of a CGA performed by an interdisciplinary collaborative team (involving advanced practice nurses or trained practice nurses) among primary care older adults on health-related quality of life and physical function outcomes [40, 41].
Strengths and limitations
One of the study’s main strengths was the large number of patients in primary care (over 600) and the provision of high-quality data. Few published studies have examined the effect of a CGA on emergency department visits or have used using standardised scales to gauge a change in functional independence or quality of life [18]. Our data on secondary outcomes were collected with validated instruments, such as the Katz Index for functional independence and the Duke Health Profile for health-related quality of life. The trial was designed rigorously to minimise bias [10] and incorporated a number of sensitivity analyses. Lastly, the trial’s pragmatic design and relatively broad inclusion criteria provided results of relevance to routine clinical practice.
Our study had several limitations. Firstly, given that the GPs knew to which arm they (or their centre) had been assigned, they might have tended to choose the most fragile or seriously ill patients for inclusion in the interventional arms. To limit this risk and facilitate enrollment, investigators were invited to establish a pre-screening list of patients potentially eligible prior to the study inception, and all analyses were further adjusted for baseline imbalances between randomised groups. Secondly, the arms differed significantly with regard to some covariates (despite the cluster randomisation), and some data of the primary and secondary endpoints were missing. However, the proportion of missing data was low (7.6% on the primary endpoint), and all analyses were performed after adjusting for covariate imbalances and imputing missing data in the ITT population. It should also be noticed that difficulties in interpretation may arise from the use of the proposed composite criteria, as its individual components do not have equal clinical weight (dying being obviously worse than an emergency department visit). The choice of this criterion was based on the clinical relevance of its components to reflect frailty outcomes and to assess the efficacy of the CGA-based intervention on pejorative geriatric events, but recently developed alternative methodological approaches such as win ratios based on hierarchical endpoints could have been valuable to deal with this issue. Lastly, our calculation of the sample size was based on the hypothesis that the highest intervention effect would be observed in arm 1, with a prespecified fixed sequence of pairwise comparisons between groups starting with 1 v 3, followed by 1 v 2 and 2 v 3, where each comparison is made if the previous one in the sequence was statistically significant to keep an overall global alpha risk at the 5% level. The highest effect was actually observed in arm 2. As a result, this fixed sequence was not applied and our results should not be interpreted as confirmatory and should be confirmed in further research.
Implications for research and practice
Our results indicate that GPs can integrate the use of CGA adapted to primary care into their daily routines, as evidenced by 83.2% of the patients in arm 2 receiving a GP-led CGA, albeit on a case-by-case basis. Furthermore, the absence of calls to the geriatric helpline in both arms suggests a good appropriation of the CGA tool for their daily practice, with no perceived need for help or supervision from the study’s dedicated geriatricians. Our findings suggest that GP-led CGA may reduce the number of unplanned hospitalisations among this population. We hypothesise that the underlying mechanisms explaining this are likely GPs being less involved in the establishment of PCPs in arm 1 with nurse-led CGA, which results in lower implementation of the interventions. Our results also suggest that GP-led CGA may improve nutritional care among this population, with probably better targeted prescriptions. The GPs likely had a more holistic view of the patient’s situation and were able to prioritise their actions more pragmatically (implementing more targeted interventions). About clinical implications, these results suggest that the person conducting the CGA and the one establishing the PCP should be the same. They also represent a significant contribution to primary care research in context of imminent demographic shifts and an anticipated surge in hospitalisations. The study conditions were very similar to current GPs’ practices, suggesting that these results could be generalised to the broader population of community-dwelling older patients with chronic conditions, although further evaluation is warranted.
Further studies are needed to examine the perceived utility of CGA-based interventions in primary care. It will notably be important to (i) identify barriers to and facilitators of CGAs in routine practice in primary care and (ii) understand the lack of effectiveness of nurse-led CGAs.
Conclusions
Our study led in community-dwelling older patients with chronic conditions found no significant effect of a CGA adapted for use in primary care on mortality, functional independence and quality of life, but suggests that a GP-led CGA may reduce the risk of unplanned hospital admission. It also suggests that a GP-led CGA may improve nutritional care with better-targeted actions. GPs could integrate the use of CGA adapted to primary care into their daily routines for their registered older patients with chronic conditions. Our study demonstrates the feasibility of incorporating CGA into clinical practice and highlights its potential benefits when applied on a case-by-case basis, guided by the GPs who develop the resulting PCP. Further research using qualitative methods is needed to better understand the CGA’s perceived utility in routine practice and the lack of effectiveness observed for nurse-led CGAs.
Availability of data and materials
The data that support the findings of this study are not openly available but are available from the corresponding author upon reasonable request.
Abbreviations
- aOR:
-
Adjusted odds ratio
- CEpiA:
-
Clinical Epidemiology and Ageing
- CGA:
-
Comprehensive geriatric assessment
- CI:
-
Confidence interval
- CRF:
-
Case Report Forms
- GP:
-
General practitioner
- HRQoL:
-
Health-related quality of life
- IQR:
-
Interquartile range
- ITT:
-
Intention-to-treat
- PCP:
-
Personalised care plan
- RCT:
-
Randomised controlled trial
- SD:
-
Standard deviation
- WHO:
-
World Health Organization
References
United Nations, Department of Economic and Social Affairs, Population Division (2019) World population ageing 2019: highlights (ST/ESA/SER.A/430). https://www.un-ilibrary.org/content/books/9789210045537/read.
Eurostat. 1 out of every 8 persons in the EU could be 80 or above by 2080. https://ec.europa.eu/eurostat/web/products-euro-indicators/-/3-29092015-ap.
Christensen K, Doblhammer G, Rau R, et al. Ageing populations: the challenges ahead. Lancet. 2009;374(9696):1196–208.
Reeves D, Pye S, Ashcroft DM, et al. The challenge of ageing populations and patient frailty: can primary care adapt? BMJ. 2018;362: k3349.
Sander M, Oxlund B, Jespersen A, et al. The challenges of human population ageing. Age Ageing. 2015;44(2):185–7.
Whitty CJM, MacEwen C, Goddard A, et al. Rising to the challenge of multimorbidity. BMJ. 2020;368: l6964.
World Health Organization. WHO Clinical Consortium on healthy ageing. Topic focus: frailty and intrinsic capacity. Report of Consortium Meeting 1–2 December 2016 in Geneva, Switzerland. 2017. https://www.who.int/publications/i/item/WHO-FWC-ALC-17.2.
Cesari M, Sumi Y, Han ZA, et al. Implementing care for healthy ageing. BMJ Glob Health. 2022;7: e007778.
World Health Organization. World report on ageing and health. Geneva; 2015. https://www.who.int/publications/i/item/9789241565042.
Ferrat E, Bastuji-Garin S, Paillaud E, et al. Efficacy of nurse-led and general practitioner-led comprehensive geriatric assessment in primary care: protocol of a pragmatic three-arm cluster randomised controlled trial (CEpiA study). BMJ Open. 2018;8: e020597.
World Health Organization. Decade of healthy ageing baseline report. Geneva; 2020. https://www.who.int/publications/i/item/9789240017900.
Pilotto A, Martin FC. Comprehensive geriatric assessment practical. Issues in geriatrics. Cham: Springer International Publishing AG; 2018.
Rubenstein LZ, Stuck AE, Siu AL, et al. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39(9 Pt 2):8S-16S; discussion 17S-18S.
British Geriatric Society. Comprehensive geriatric assessment toolkit for primary care practitioners. 2019. https://www.bgs.org.uk/resources/resource-series/comprehensive-geriatric-assessment-toolkit-for-primary-care-practitioners.
Coulter A, Entwistle VA, Eccles A, et al. Personalised care planning for adults with chronic or long-term health conditions. Cochrane Database Syst Rev. 2015;2015(3):CD010523.
Mangin D, Stephen G, Bismah V, et al. Making patient values visible in healthcare: a systematic review of tools to assess patient treatment priorities and preferences in the context of multimorbidity. BMJ Open. 2016;6: e010903.
Ellis G, Gardner M, Tsiachristas A, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9(9):CD006211.
Briggs R, McDonough A, Ellis G, et al. Comprehensive geriatric assessment for community-dwelling, high-risk, frail, older people. Cochrane Database Syst Rev. 2022;5(5):CD012705.
Sidorkiewicz S, De Chanaud N. Compared efficacy of GP-led geriatric and nurse-led geriatric assessment in primary care a pragmatic 3-arm controlled cluster randomized trial (CEPIA study). Exercer. 2017;132:160–1.
American Association of Colleges of Nursing. The essentials: core competencies for professional nursing education. https://www.aacnnursing.org/Portals/42/AcademicNursing/pdf/Essentials-2021.pdf.
Laurant M, Van der Biezen M, Wijers N, et al. Nurses as substitutes for doctors in primary care. Cochrane Database Syst Rev. 2018;7(7):CD001271.
Horrocks S, Anderson E, Salisbury C. Systematic review of whether nurse practitioners working in primary care can provide equivalent care to doctors. BMJ. 2002;324(7341):819–23.
Clark CE, Smith LF, Taylor RS, et al. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341: c3995.
Melis RJ, van Eijken MI, Teerenstra S, et al. A randomized study of a multidisciplinary program to intervene on geriatric syndromes in vulnerable older people who live at home (Dutch EASYcare Study). J Gerontol A Biol Sci Med Sci. 2008;63(3):283–90.
Orcel V, Fabre F, Bastuji-Garin S, et al. A geriatric assessment intervention in primary care provided by a nurse or a GP (CEPIA): a cluster-randomised trial. Poster session presented at: 18th Congress of the European Geriatric Medicine Society. London; 2022.
Orcel V, Fabre F, Bastuji-Garin S, et al. A geriatric assessment intervention in primary care provided by a nurse or a GP (CEPIA): a cluster-randomised trial. In: EGPRN, Peremans L, editor-in-chief. Programme Book of the 95th Meeting of the European General Practice Research Network. Antwerp; 2022. https://www.egprn.org/page/conference-abstracts.
Orcel V, Fabre F, Renard V, et al. Comparaison de l'Efficacité d'une évaluation gériatrique en soins Primaires réalisée par un Infirmier ou un médecin générAliste: un essai pragmatique contrôlé randomisé en 3 bras (étude CEPIA). Presented at: 21ème congrès du Collège National des Generalistes Enseignants. Lille; 2021.
Crocker TF, et al. Community based complex interventions to sustain independence in older people: systematic review and network meta-analysis. BMJ. 2024;384: e077764.
De Hoop E, Teerenstra S, Van Gaal BG, et al. The “best balance” allocation led to optimal balance in cluster-controlled trials. J Clin Epidemiol. 2012;65(2):132–7.
Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol. 2008;8: 65.
Raab GM, Butcher I. Randomization inference for balanced cluster-randomized trials. Clin Trials. 2005;2(2):130–40.
Stekhoven DJ, Buhlmann P. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012;28(1):112–8.
Nord M, Lyth J, Alwin J, et al. Costs and effects of comprehensive geriatric assessment in primary care for older adults with high risk for hospitalisation. BMC Geriatr. 2021;21(1):263.
Spoorenberg SLW, Wynia K, Uittenbroek RJ, et al. Effects of a population-based, person-centred and integrated care service on health, wellbeing and self-management of community-living older adults: a randomised controlled trial on embrace. PLoS One. 2018;13(1): e0190751.
Stratton R, Smith T, Gabe S. Managing malnutrition to improve lives and save money. British Association for Parenteral and Enteral Nutrition (BAPEN). 2018. https://www.bapen.org.uk/reports/malnutrition/managing-malnutrition-to-improve-lives-and-save-money/.
Valmorbida E, Trevisan C, Imoscopi A, et al. Malnutrition is associated with increased risk of hospital admission and death in the first 18 months of institutionalization. Clin Nutr. 2020;39(12):3687–94.
Barker I, Steventon A, Deeny SR. Association between continuity of care in general practice and hospital admissions for ambulatory care sensitive conditions: cross sectional study of routinely collected, person level data. BMJ. 2017;356: j84.
Sawicki OA, Mueller A, Klaaßen-Mielke R, et al. Strong and sustainable primary healthcare is associated with a lower risk of hospitalization in high-risk patients. Sci Rep. 2021;11(1):4349.
Bleijenberg N, Imhof L, Mahrer-Imhof R, et al. Patient characteristics associated with a successful response to nurse-led care programs targeting the oldest-old: a comparison of two RCTs. Worldviews Evid Based Nurs. 2017;14(3):210–22.
Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–33.
Hoogendijk EO, van der Horst HE, van de Ven PM, et al. Effectiveness of a geriatric care model for frail older adults in primary care: results from a stepped wedge cluster randomized trial. Eur J Intern Med. 2016;28:43–51.
Acknowledgements
The authors want to thank all the professionals and patients who participated and made this study possible. The authors are indebted to David Fraser (Biotech Communication, France) for his language review of the manuscript. They warmly thank Julie Fabre for her useful discussions.
Funding
The CEpiA trial was supported by public funding from the French Ministry of Health (Programme de Recherche sur la Performance du Système des soins, PREPS14370_K140707).
Author information
Authors and Affiliations
Contributions
VO did the literature search, conducted statistical analyses, designed the figures, interpretated data, and wrote the first draft of the manuscript. LB aided in interpreting the results, critically revised the manuscript. SBG, VR, PC, and EP designed the study and critically revised the manuscript. EB conducted statistical analyses and critically revised the manuscript. AG helped collect the data. EA and EF supervised the project, designed the study and the analyses, contributed analysis tools, aided in performing the analyses, and critically revised the manuscript (directed). All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by an independent ethics committee (CPP Ile-de-France IV, Paris, France; reference: 2015/48SC) in October 2015. The study was conducted in accordance with the Declaration of Helsinki. All followed procedures were in accordance with relevant guidelines and French regulations. The study database was registered with the French National Data Protection Commission (Commission nationale de l'informatique et des libertés, Paris, France). The protocol was in conformity with the French legislation applied to usual care. All the study participants provided their verbal, informed consent. Patients giving their verbal consent had previously received oral and written information about the trial by the GP investigator. The date the patients gave their verbal consent was reported in the Case Report Form (CRF).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12916_2024_3613_MOESM4_ESM.docx
Additional file 4. Table S1. Comparison of patients with or without complete information on the primary composite endpoint (N=634).
12916_2024_3613_MOESM5_ESM.docx
Additional file 5. Table S1. Results from secondary outcome analyses in patients alive at 12 months: changes from baseline to 12-month follow-up (N=598).
12916_2024_3613_MOESM6_ESM.docx
Additional file 6. Table S1. Results from secondary outcome analyses in patients alive at 12 months: 12-month follow-up values (N=598).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Orcel, V., Banh, L., Bastuji-Garin, S. et al. Effectiveness of comprehensive geriatric assessment adapted to primary care when provided by a nurse or a general practitioner: the CEpiA cluster-randomised trial. BMC Med 22, 414 (2024). https://doi.org/10.1186/s12916-024-03613-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-024-03613-7