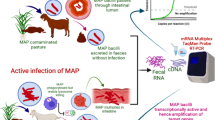
Abstract
Background
The appearance of the novel porcine haemotrophic mycoplasma (HM) species ‘Candidatus Mycoplasma haemosuis’ was reported in apparently healthy but also in clinically sick animals in China, Korea and in a case report from Germany. Outside of Asia, however, nothing further is known about the frequency of ‘Ca. M. haemosuis’ in pigs to date. To investigate the distribution of this novel HM species in Germany, fattening pigs, sows and pre-suckling piglets were examined using a herein developed quantitative real-time PCR assay (qPCR). Because the piglets were sampled before the first colostrum uptake, additional information on a possible vertical transmission from dams to their offspring was obtained.
Results
Our novel qPCR assay successfully detected ‘Ca. M. haemosuis’ in all blood samples from the ‘Ca. M. haemosuis’-infected pigs. No cross-reactivity was detected when DNA from non-target Mycoplasma spp. and other bacterial species representing 105 bacteria/reaction were used as a template. The lower limit of detection of the qPCR was thus 10 gap gene copies per reaction and 2.5 x 103 genome equivalents (GE) per mL blood.
‘Candidatus M. haemosuis’ was detected by this qPCR in blood samples from a total out of 6.25% sows (13/208), 4.50% pre-suckling piglets (28/622) and 17.50% fattening pigs (35/200). On farm level, 3 out of 21 piglet producing farms (14.28%) and 9 out of 20 fattening farms (45.00%) were positive for ‘Ca. M. haemosuis’. Co-infections with M. suis were evident in all age groups.
Conclusion
‘Candidatus M. haemosuis’ infection is present in German pig farms and the detection of the novel porcine HM species in piglets immediately after birth before colostrum intake indicates vertical transmission. The novel qPCR assay specific for ‘Ca. M. haemosuis’ described herein will be a prerequisite for future studies on the prevalence, epidemiology as well as the clinical and economic impact of ‘Ca. M. haemosuis’ infections.
Similar content being viewed by others
Background
Haemotrophic mycoplasmas (HMs) are a group of cell-wall less bacteria with a unique tropism for erythrocytes. HMs were found worldwide in a wide range of mammals, including livestock and companion animals as well as wild animals and humans [1,2,3,4,5]. In pigs, the two HM species Mycoplasma suis and Mycoplasma parvum were first described in the first half of the last century [6]. While M. parvum seems to be an apathogenic HM species, M. suis is the causative agent of infectious anaemia in pigs (IAP) [4, 7, 8]. Acute M. suis infections are characterised by haemolytic anaemia, high fever, icterus, hypoglycaemia, intravascular coagulopathy, and endothelial damages [4, 6, 9, 10]. Of most importance are chronic infections with subclinical or mild to moderate clinical signs including low-grade anaemia, poor reproduction performance, and reduced growth rates [1, 11]. Both, acute and chronic M. suis infections have been reported worldwide causing significant economic losses to the pig industry [12,13,14,15,16,17].
Recently, a third porcine HM species was discovered in Zhejiang, China by Fu and co-workers in 2017 and designated as ‘Candidatus Mycoplasma haemosuis’ [18]. Phylogenetic analyses of the 16S rDNA revealed that this novel HM species is most closely related to the feline HM ‘Candidatus Mycoplasma turicensis’, the murine species Mycoplasma muris and the human HM ‘Candidatus Mycoplasma haemohominis’ [18]. This novel porcine HM species was also described in obviously healthy animals in Korea [19] and, most recently, in clinically affected fattening pigs in Germany with skin alterations, fever, and anaemia [20]. This clinical case indicated the need to establish a specific and sensitive detection method to investigate the spread of the novel emerging pathogen in the pig population.
In the last two decades, molecular detection methods for the so far uncultivable HMs have proven to be the basic prerequisite to get insights into the epidemiology, species, and strain differentiation and the clinical impact of HM infections [14, 21,22,23,24,25,26].
Thus, the aims of the present study were (i) to develop a specific real-time PCR diagnostic assay (qPCR) for the detection and quantification of ‘Ca. M. haemosuis’ in pigs and (ii) to apply this novel qPCR and investigate the occurrence of ‘Ca. M. haemosuis’ in fattening pigs as well as in sows and piglets in Germany. Because the piglets were sampled before the first colostrum uptake, additional information on a possible vertical transmission from dams to their offspring was obtained.
Results
Development of a real-time PCR assay
We developed a real-time qPCR assay for the specific detection of ‘Ca. M. haemosuis’ from the blood of pigs. The real-time qPCR is targeting the gap gene encoding the NAD-dependent glyceraldehyde 3-phosphate dehydrogenase of ‘Ca. M. haemosuis’ (GAPDH). For the establishment of the qPCR ‘Ca. M. haemosuis’ positive samples were available from a previous study [20]. In this study, pigs (n = 7) suffering from anaemia, fever, and skin alterations, were tested ‘Ca. M. haemosuis’-positive by 16S rDNA PCR, sequencing, and subsequent sequence analysis [20]. Our novel qPCR assay successfully detected ‘Ca. M. haemosuis’ in all blood samples from the ‘Ca. M. haemosuis’-infected pigs. Melting curve analyses revealed a specific melting temperature of 74.676 °C (± 0.32 °C). Moreover, we tested the specificity of the assay using bacterial strains and isolates as listed in Table 3. Positive qPCR reactions were found for ‘Ca. M. haemosuis’ DNA. No cross-reactivity was detected when DNA from non-target Mycoplasma spp. and other bacterial species representing 105 bacteria/reaction were used as a template.
Sequence analyses of the qPCR amplicons from all available ‘Ca. M. haemosuis’ isolates (n = 7; 20) revealed 100% identity among each other and to the gap gene deposited in GenBank (Accession No. KU246051). The analytical sensitivity of the qPCR assay was determined using serial dilutions of the plasmid pC_CMhsuis standard corresponding to 107 to 100 GE. The highest dilution yielding consistently positive qPCR results (Ct < 35 cycles) contained 0.45 fg pC_CMhsuis DNA per reaction corresponding to 10 gene copies per reaction. The lower limit of detection of the qPCR was thus 10 gap gene copies per reaction and 2.5 x 103 genome equivalents per mL blood.
Quantitative PCR data analysis revealed a linear regression curve between 45 pg and 0.45 fg of the plasmid DNA. The PCR efficiency was calculated to be 97.3%. The intra-assay and inter-assay repeatability are shown in Table 1. All different concentrations in the standard dilutions from 107 to 101 GE were consistently detected by the qPCR assay.
Quantification of ‘Ca. M. haemosuis’ in the infected pigs [20] revealed blood loads from 3.08 × 102 to 3.96 × 107 bacteria/mL blood.
The ten randomly selected qPCR positive samples also revealed positive results in haemotrophic mycoplasma-specific16S rDNA amplification and sequencing. As all obtained sequences were identical, we uploaded one sequence to the GenBank (Accession No. MZ614253).
‘Candidatus M. haemosuis’ infections in fattening pigs
To investigate the occurrence of ‘Ca. M. haemosuis’ in fattening pigs, we tested a total of 200 animals from 20 fattening farms at the time of slaughter using the novel qPCR assay. ‘Candidatus. M. haemosuis’ was detected in blood samples of 35 out of 200 investigated animals (17.50%) originating from 9 out of the 20 farms (45.00%). Quantification of bacterial loads in qPCR-positive pigs revealed a mean value of 1.61 x 105 ‘Ca. M. haemosuis’/mL blood (range: 5.52 x 103 to 1.55 x 106 ‘Ca. M. haemosuis’/mL). A total of 12 ‘Ca. M. haemosuis’ positive fattening pigs were co-infected with M. suis (Table 2). Data of all animals are included in Supplementary File 1.
‘Candidatus M. haemosuis’ infections in farrowing sows and pre-suckling piglets
As shown in Table 2, a total of 13 out of 208 sows (6.25%) were positive for ‘Ca. M. haemosuis’ and the number of positive sows within a herd varied between one and ten. All 13 ‘Ca. M. haemosuis’ positive sows were co-infected with M. suis. On-farm level, ‘Ca. M. haemosuis’ was detected in three out of 21 investigated piglet-producing farms (14.29%) in at least one animal, whereas in the remaining 18 farms all animals were qPCR negative. In all farms, no clinical signs of IAP were obvious at the time of the investigation.
A total of 28 out of the 622 (4.50%) pre-suckling piglets reacted ‘Ca. M. haemosuis’ qPCR positive. All ‘Ca. M. haemosuis’ infected piglets originating from one farm and were born from 10 (76.92%) of the 13 qPCR positive sows. The number of positive piglets per sow varied between one and three. Four out of the 28 ‘Ca. M. haemosuis’ positive piglets were co-infected with M. suis.
Quantification of bacterial loads in qPCR-positive sows revealed a mean value of 3.83 x 104 ‘Ca. M. haemosuis’/mL blood (range: 3.21 x 104 to 6.44 x 104 ‘Ca. M. haemosuis’/mL) and in qPCR-positive piglets a mean value of 2.25 x 105 ‘Ca. M. haemosuis’/mL blood (range: 1.13 x 104 to 2.48 x 106 ‘Ca. M. haemosuis’/mL), respectively. Data of all animals are further included in Supplementary File 1.
Discussion
In this study, we describe the establishment of a novel quantitative real-time PCR for the specific detection of the emerging porcine HM species ‘Ca. M. haemosuis’ in blood samples as well as the quantitative detection of ‘Ca. M. haemosuis’ in sows, piglets and fattening pigs from Germany. To our knowledge, our SYBR® green qPCR assay is the first quantitative diagnostic tool specific for ‘Ca. M. haemosuis’. The novel qPCR assay is targeting the ‘Ca. M. haemosuis’ gene encoding the NAD-dependent glyceraldehyde-3-phosphate dehydrogenase. The gap was chosen over the 16S rRNA gene because of an identity of >97% of the 16S rRNA gene of Ca. M haemosuis to other hemotrophic mycoplasmas (e. g. ‘Ca. M. turicensis’, ‘Ca. M. haemobos’, ‘Ca. M. haemomuris’, M. haemocanis, and M. haemofelis), Glyceraldehyde-3-phosphate dehydrogenase encoding genes were used as targets in -specific qPCR assays for the detection of haemotrophic and non-haemotrophic Mycoplasma species including M. suis, M. wenyonii, ‘Ca. M. haemobos’, M. genitalium and M. hominis [28, 29]. All these PCR assays have proven to be reliable and robust for use in prevalence studies and routine diagnostics [13, 17, 26, 30].
So far, ‘Ca. M. haemosuis’ was detected using 16S rDNA targeting primers without quantification of bacterial loads in infected pigs [18, 19]. The assay described herein allows quantification with a sensitivity of 10 genome equivalent per PCR corresponding to 2.5 x 103 bacteria per mL blood. Comparable results have been obtained from previously published qPCR assays for the detection of other HMs, e.g. M. suis, the canine HM species M. haemocanis, ‘Ca. M. haematoparvum’ as well as the bovine HM species M. wenyonii and ‘Ca. M. haemobos’ [23, 26, 31]. This high analytical sensitivity enables the identification of asymptomatic chronically infected carrier animals that may serve as important epidemiological reservoirs [24, 32]. The analytical specificity of the novel ‘Ca. M. haemosuis’ qPCR assay as predicted in silico was confirmed by the negative PCR results obtained with all tested Mycoplasma spp. (HMs and non-haemotrophic mycoplasmas) and other porcine pathogens.
Reports about ‘Ca. M. haemosuis’ infections are scarce because the agent was recently discovered in 2017 [18]. In the study reported herein, we surveyed for the first time a European sample panel (n = 1080 pigs) for ‘Ca. M. haemosuis’ infections. We confirmed that ‘Ca. M. haemosuis’ is prevalent in Germany. Interestingly, we found considerable variation in the prevalence depending on the population studied. The highest detection rate with 17.5% was found in fattening pigs, followed by 6.25% in sows, and by 4.50% in piglets. In the two previous studies, the ‘Ca. M. haemosuis’ prevalence was higher in China with 36% positive sows and 24.1% positive growing pigs [18] and lower in Korea with only one ‘Ca. M. haemosuis’ positive animal (0.01%) out of 1867 tested pigs from 464 farms [19]. On-farm level, the higher detection rates for fattening pigs could be confirmed: only 3 out of the 21 piglet producing farms (14.29%) but 9 out of the 20 fattening farms (45.00%) were shown to be ‘Ca. M. haemosuis’ positive. Various factors could be responsible for the differences in the detection rates found between piglet producing and fattening farms including the purchase of pigs from different piglet-producing farms and potential higher biosecurity levels in piglet producing farms. In the current study, all positive sows were co-infected with ‘Ca. M. haemosuis’ and M. suis, but only 4 out of 28 piglets, and 12 out of 200 slaughter pigs were positive for both porcine HM species. The evidence of co-infections is in line with other HM studies. Co-infections with two or three HM species were also found in sows (26.7%) and growing pigs (13.9%) in China [18], in cattle [25, 26], in sheep [33], and cats [34].
So far, our knowledge regarding the pathogenicity of ‘Ca. M. haemosuis’ is rather limited. In the Chinese study, the novel porcine HM species was first detected in one diseased pig showing typical clinical signs of IAP [18]. In Europe, Stadler and co-workers (2020) identified ‘Ca. M. haemosuis’ first in diseased pigs showing also typical clinical signs of an M. suis induced IAP (i.e. anaemia, fever, skin alterations) [20]. In the present study ‘Ca. M. haemosuis’ was detected in obviously healthy pigs as it was also shown for M. suis in recent studies [13, 17, 35]. Typically, such chronic HM infections predominate in the pig population causing significant economic loss and welfare concern due to immune dysregulation, higher susceptibility to other infectious agents, extended feeding periods or increased stillbirth rates [4, 13, 17, 35, 36]. In addition, chronic HM infections can lead to increased and metaphylactic antibiotic usage contributing to the development of antibiotic resistance [36]. Further studies focusing on individual health parameters of ‘Ca. M. haemosuis’ positive pigs are certainly needed.
The ‘Ca. M. haemosuis’ loads found in the present study seem to be lower than those found for M. suis in sows (mean blood load of 3.15 × 107 M. suis/mL [17];), in pre-suckling piglets (mean loads of 5.09 × 107 M. suis/mL blood) or in fattening pigs (mean loads of 7.62 × 107 M. suis/mL blood [13];, respectively. Interestingly, 76.92% of the positive sows have born at least one ‘Ca. M. haemosuis’ positive piglet indicating that ‘Ca. M. haemosuis’ is transmitted vertically within the pig herds. The possibility of vertical transmission of HMs has also been described for M. suis [17] as well as for M. wenyonii and ‘Ca. M. haemobos’ [37, 38]. But it is worth noting when comparing the vertical transmission of M. suis [17] and ‘Ca. M. haemosuis’ (present study) that a considerably higher percentage of 76.92% of the ‘Ca. M. haemosuis’ infected sows delivered infected pre-suckling piglets whereas only 50% of the M. suis infected sows have born positive piglets.
Conclusion
We showed for the first time that ‘Candidatus Mycoplasma haemosuis’ infection is prevalent in Germany in piglet producing farms as well as in fattening farms and coinfections with M. suis are common. Our data on the detection of ‘Ca. M. haemosuis’ in pre-suckling piglets indicate that the pathogen is transmitted vertically. Further studies are needed to investigate the pathogenic potential, the clinical impact, prevalence, and epidemiology including transmission routes of ‘Ca. M. haemosuis’ to clarify the significance of this emerging pathogen. The herein-described novel qPCR assay can be used to accurately diagnose infections with the new HM species in pigs and to perform these studies.
Methods
Sample and data collection
Blood samples (n = 7) of ‘Ca. M. haemosuis’ positive fattening pigs were available from a previous study [20]. As samples were taken as diagnostic material during acute disease, no ethical approval was required according to the German Animal Welfare Law. The pigs suffering from skin alterations (urticaria, haemorrhagic diathesis), high fever and anaemia were shown to be ‘Ca. M. haemosuis’ positive by 16S rDNA amplification and subsequent sequence analysis [20]. The seven animals revealed a negative M. suis-qPCR result.
In addition, DNA samples extracted from the blood of farrowing sows (n = 208) and corresponding pre-suckling piglets (n = 622) from 21 piglet producing farms were available from a previous study [17]. From each farm, nine to ten farrowing sows and two or three pre-suckling piglets per sow have been sampled. DNA quality was checked using a NanoDrop™ 2000 to assure the quality of the samples. Animal sampling was performed in accordance with the German animal welfare law using a protocol officially approved by the Government (Az. 55.2–154–2532.2-16-13).
For the group of fattening pigs, EDTA-anticoagulated blood samples were taken from 200 animals (20 different farms in Germany, 10 animals per farm) at the time of slaughter. All 20 farms were located in the South of Germany (Federal States of Bavaria and Baden-Wuerttemberg). Blood collection was performed after slaughtering, thus, no ethical approval was needed for those samples according to the German Animal Welfare Law and the DIRECTIVE 2010/63/EU.
Bacterial DNA was extracted as described elsewhere [13, 17]. Briefly, blood samples were preconditioned by mixing 200 μl of EDTA-anticoagulated blood with an equal volume of lysis buffer (10 mM Tris pH 7.5, 5 mM MgCl2, 330 mM sucrose, 1% (v/v) Triton X-100). The mixure was centrifuged (8000 x g, 1 min, 20 °C) and the pellet was again washed twice with 400 μl lysis buffer. Subsequently, a total amount of 200 μl bacterial DNA was using the GenElute™ Bacterial Genomic DNA Kit (Sigma-Aldrich, Steinheim, Germany). One PBS control was included into each extraction run of ten blood samples. For all samples, the M. suis status was determined by a quantitative PCR [17, 23] either in a previous study (sows and piglets, [17]) or in the present study (fattening pigs).
Primer design and amplicon sequencing
To develop a specific ‘Ca. M. haemosuis’ qPCR two primers were designed based on the gene gap encoding the NADP-dependent glyceraldehyde-3-phosphate dehydrogenase of ‘Ca. M. haemosuis’ (Accession No. KU246051) using the Primer3 software [39, 40]: CMhsuisF 5´-TGCTTTGGCTCCTGTGGTTA-3´ and CMhsuisR 5´- GCAGCAGCACCTGTAGAAGTA-3`. The Blast algorithm, which is provided by NCBI, was used to test the specificity of the primers in silico. Specificity was further investigated by sequencing (Seqlab Sequence Laboratories, Göttingen, Germany) of the resulting 177-bp gap fragment of ‘Ca. M. haemosuis’. Obtained sequences were compared to GenBank entries using the Blast tool provided by NCBI.
Cloning and Preparation of Standard DNA
The 177-bp qPCR fragment of ‘Ca. M. haemosuis’ was cloned into the plasmid vector pCR2.1 (Invitrogen) according to the manufacturer’s instructions. Plasmid DNA was purified from the Escherichia coli transformant (pC_CMhsuis) using the GenElute™ Plasmid Miniprep Kit (Sigma-Aldrich). Plasmid DNA was quantified using a spectrophotometer (NanoDrop™ 2000, Thermo Fisher Scientific). DNA concentrations were adjusted to 45 pg/2 μL representing 1 x 107 GE according to the calculation described below.
Quantitative real-time PCR
‘Candidatus. M. haemosuis’ DNA was detected and quantified with the StepOne™ System (Applied Biosystems). The 20 μL reaction mixtures contained 10 μL of the 2x SYBR® Green PCR Master Mix (Thermo Fisher Scientific), 8 μL primer mixture (containing 0.5 μM primer each), and 2 μL template DNA. Cycling conditions consisted of 95 °C for 15 min and 40 cycles at 95 °C for 15 s and 60 °C for 1 min followed by a melting curve analysis. Plasmid pC_CMhsuis DNA standard dilutions (450 fg/2 μl, 45 fg/2 μl, and 4.5 fg/2 μl) representing 105, 104, and 103 GE per reaction were included in each qPCR run for quantification. Obtained Ct values were extrapolated into ‘Ca. M. haemosuis’ GE/reaction using the Standard Curve Method of the StepOne™ Software Version 2.2 (Applied Biosystems). The ‘Ca. M. haemosuis’ GE/mL blood were determined considering the factor 200 (2 μL template out of 200 μL DNA volume out of 200 μL EDTA blood (software Microsoft® Excel, 2016).
Since the diagnostics of hemotrophic mycoplasmas in Germany has to be covered by the farmers themselves, we decided to establish a SYBR Green assay, to use the range of 105–103 GE/reaction and to omit an internal control. Despite some disadvantages compared to the use of probe-based PCRs, the SYBR Green assay offers a clear economic advantage with nevertheless good diagnostic specificity [41]. This allows us to keep costs as low as possible and to offer farmers an incentive to send in diagnostic samples. Analytical specificity and lower limit of detection of the real-time qPCR.
The specificity of the qPCR assay was tested by using template DNA from the porcine HM species M. suis, M. parvum, other haemotrophic and non-haemotrophic Mycoplasma spp. and a panel of other porcine pathogens (Table 3). Bacteria were cultivated and/or DNA was isolated as described elsewhere [17, 23, 26].
For measuring the lower limit of detection (LOD) pC_CMhsuis plasmid DNA concentration was adjusted to 45 pg/2 μL representing 1 x 107 GE/2 μL and the LOD was measured by testing ten-fold dilutions (from 107 to 1 GE/reaction) in 15 runs.
The ‘Ca. M. haemosuis’ genome was calculated as 0.85 fg, and the pC_CMhsuis plasmid corresponds to 4.5 ag per copy (genome weight = genomic length (bp) x 105 x 665 Da/bp x 1.67 x 1024 g/Da) (http://cels.uri.edu/gsc/cndna.html). Since the actual genome size of ‘Ca. M. haemosuis is unknown so far, we used a mean genome size of all so far sequenced haemotrophic mycoplasmas of 750 kb. For the plasmid, a size of 4.106 kb was used to calculate the concentrations in plasmid copies per microliter corresponding to genome equivalents (GE) of ‘Ca. M. haemosuis’.
Four replicates of the plasmid dilutions (107 to 101 GE/reaction) were tested in the same run to assess the intra-assay repeatability. The inter-assay repeatability was determined by running duplicates of the same plasmid dilutions in five different runs on different days carried out by two persons.
Conventional PCR
For confirmation of the new developed qPCR, we tested a total out of ten randomly selected qPCR positive results by haemotrophic mycoplasma-specific16S rDNA PCR as described elsewhere [42]. The ten amplicons were sequenced (Seqlab Sequence Laboratories, Göttingen, Germany). Obtained sequences were compared to GenBank entries using the Blast tool provided by NCBI.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Ca.:
-
Candidatus
- IAP:
-
Infectious anaemia in pigs
- HM:
-
Haemotrophic mycoplasma
- M. :
-
Mycoplasma
- qPCR:
-
Quantitative polymerase chain reaction
References
Messick JB. Hemotrophic mycoplasmas (hemoplasmas): a review and new insights into pathogenic potential. Vet Clin Pathol. 2004;33(1):2–13.
Hoelzle K, Engels M, Kramer MM, Wittenbrink MM, Dieckmann SM, Hoelzle LE. Occurrence of Mycoplasma suis in wild boars (Sus scrofa L.). Vet Microbiol. 2010;143(2–4):405–9.
Guimaraes AM, Santos AP, do Nascimento NC, Timenetsky J, Messick JB. Comparative genomics and phylogenomics of hemotrophic mycoplasmas. PLoS One. 2014;9(3):e91445.
Stadler J, Jannasch C, Mack SL, Dietz S, Zöls S, Ritzmann M, et al. Clinical and haematological characterisation of Mycoplasma suis infections in splenectomised and non-splenectomised pigs. Vet Microbiol. 2014;172(1–2):294–300.
Hattori N, Kuroda M, Katano H, Takuma T, Ito T, Arai N, et al. Candidatus Mycoplasma haemohominis in Human, Japan. Emerg Infect Dis. 2020;26(1):11–9.
Hoelzle LE. Haemotrophic mycoplasmas: recent advances in Mycoplasma suis. Vet Microbiol. 2008;130(3–4):215–26.
Splitter EJ. Eperythrozoon parvum a Filterable Blood Parasite of Swine. Nature. 1953;172(4366):40.
do Nascimento NC, dos Santos AP, Chu Y, Guimaraes AM, Baird AN, Weil AB, et al. Microscopy and genomic analysis of Mycoplasma parvum strain Indiana. Vet Res. 2014;45(1):86.
Sokoli A, Groebel K, Hoelzle K, Amselgruber WM, Mateos JM, Schneider MK, et al. Mycoplasma suis infection results endothelial cell damage and activation: new insight into the cell tropism and pathogenicity of hemotrophic mycoplasma. Vet Res. 2013;44(1):6.
Stadler J, Ade J, Hermanns W, Ritzmann M, Wentzel S, Hoelzle K, et al. Clinical, haematological and pathomorphological findings in Mycoplasma suis infected pigs. BMC Vet Res. 2021;17(1):214.
Strait EL, Hawkins PA, Wilson WD. Dysgalactia associated with Mycoplasma suis infection in a sow herd. J Am Vet Med Assoc. 2012;241(12):1666–7.
Wu J, Yu J, Song C, Sun S, Wang Z. Porcine eperythrozoonosis in China. Ann N Y Acad Sci. 2006;1081:280–5.
Ritzmann M, Grimm J, Heinritzi K, Hoelzle K, Hoelzle LE. Prevalence of Mycoplasma suis in slaughter pigs, with correlation of PCR results to hematological findings. Vet Microbiol. 2009;133(1–2):84–91.
Guimaraes AM, Vieira RF, Poletto R, Vemulapalli R, Santos AP, de Moraes W, et al. A quantitative TaqMan PCR assay for the detection of Mycoplasma suis. J Appl Microbiol. 2011;111(2):417–25.
Watanabe Y, Fujihara M, Suzuki J, Sasaoka F, Nagai K, Harasawa R. Prevalence of swine hemoplasmas revealed by real-time PCR using 16S rRNA gene primers. J Vet Med Sci. 2012;74(10):1315–8.
Gatto IRH, Sonálio K, Amaral RBD, Morés N, Dalla Costa OA, André MR, et al. High frequency and molecular characterization of porcine hemotrophic mycoplasmas in Brazil. Vet Microbiol. 2019;231:33–9.
Stadler J, Willi S, Ritzmann M, Eddicks M, Ade J, Hoelzle K, et al. Detection of Mycoplasma suis in pre-suckling piglets indicates a vertical transmission. BMC Vet Res. 2019;15(1):252.
Fu Y, Shi T, Xu L, Wei W, Lu F, Zhang X, et al. Identification of a novel Hemoplasma species from pigs in Zhejiang province, China. J Vet Med Sci. 2017;79(5):864–70.
Seo MG, Kwon OD, Kwak D. Prevalence and phylogenetic analysis of hemoplasma species in domestic pigs in Korea. Parasit Vectors. 2019;12(1):378.
Stadler J, Ade J, Ritzmann M, Hoelzle K, Hoelzle LE. Detection of a novel haemoplasma species in fattening pigs with skin alterations, fever and anaemia. Vet Rec. 2020;187(2):66.
Tasker S, Helps CR, Day MJ, Gruffydd-Jones TJ, Harbour DA. Use of real-time PCR to detect and quantify Mycoplasma haemofelis and "Candidatus Mycoplasma haemominutum" DNA. J Clin Microbiol. 2003;41(1):439–41.
Willi B, Boretti FS, Cattori V, Tasker S, Meli ML, Reusch C, et al. Identification, molecular characterization, and experimental transmission of a new hemoplasma isolate from a cat with hemolytic anemia in Switzerland. J Clin Microbiol. 2005;43(6):2581–5.
Hoelzle LE, Helbling M, Hoelzle K, Ritzmann M, Heinritzi K, Wittenbrink MM. First LightCycler real-time PCR assay for the quantitative detection of Mycoplasma suis in clinical samples. J Microbiol Methods. 2007;70(2):346–54.
Meli ML, Kaufmann C, Zanolari P, Robert N, Willi B, Lutz H, et al. Development and application of a real-time TaqMan(®) qPCR assay for detection and quantification of 'Candidatus Mycoplasma haemolamae' in South American camelids. Vet Microbiol. 2010;146(3–4):290–4.
Meli ML, Willi B, Dreher UM, Cattori V, Knubben-Schweizer G, Nuss K, et al. Identification, molecular characterization, and occurrence of two bovine hemoplasma species in Swiss cattle and development of real-time TaqMan quantitative PCR assays for diagnosis of bovine hemoplasma infections. J Clin Microbiol. 2010;48(10):3563–8.
Ade J, Niethammer F, Schade B, Schilling T, Hoelzle K, Hoelzle LE. Quantitative analysis of Mycoplasma wenyonii and “Candidatus Mycoplasma haemobos” infections in cattle using novel gapN-based realtime PCR assays. Vet Microbiol. 2018;220:1–6.
Groebel K, Hoelzle K, Wittenbrink MM, Ziegler U, Hoelzle LE. Mycoplasma suis invades porcine erythrocytes. Infect Immun. 2009;77(2):576–84.
Svenstrup HF, Jensen JS, Björnelius E, Lidbrink P, Birkelund S, Christiansen G. Development of a quantitative real-time PCR assay for detection of Mycoplasma genitalium. J Clin Microbiol. 2005;43(7):3121–8.
Baczynska A, Svenstrup HF, Fedder J, Birkelund S, Christiansen G. Development of real-time PCR for detection of Mycoplasma hominis. BMC Microbiol. 2004;4:35.
Niethammer FM, Ade J, Hoelzle LE, Schade B. Hemotrophic mycoplasma in Simmental cattle in Bavaria: prevalence, blood parameters, and transplacental transmission of 'Candidatus Mycoplasma haemobos' and Mycoplasma wenyonii. Acta Vet Scand. 2018;60(1):74.
Barker EN, Tasker S, Day MJ, Warman SM, Woolley K, Birtles R, et al. Development and use of real-time PCR to detect and quantify Mycoplasma haemocanis and "Candidatus Mycoplasma haematoparvum" in dogs. Vet Microbiol. 2010;140(1–2):167–70.
Hoelzle LE, Felder KM, Hoelzle K. Porcine eperythrozoonosis: from Eperythrozoon suis to Mycoplasma suis. Tierarztliche Praxis Ausgabe G, Grosstiere/Nutztiere. 2011;39(4):215–20.
Tagawa M, Takeuchi T, Fujisawa T, Konno Y, Yamamoto S, Matsumoto K, et al. A clinical case of severe anemia in a sheep coinfected with Mycoplasma ovis and 'Candidatus Mycoplasma haemovis' in Hokkaido, Japan. J Vet Med Sci. 2012;74(1):99–102.
Willi B, Boretti FS, Baumgartner C, Tasker S, Wenger B, Cattori V, et al. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J Clin Microbiol. 2006;44(3):961–9.
Brissonnier M, Normand V, Lebret A, Moalic PY, Guyomard AS, Bachy V, et al. Frequency of infection with Mycoplasma suis in gestating sows using qPCR on ten commercial French herds, and impact of the infection on clinical, haematological and biochemical parameters. Porcine Health Manage. 2020;6:13.
Hoelzle K, Ade J, Hoelzle LE. Persistence in Livestock Mycoplasmas—a Key Role in Infection and Pathogenesis. Curr Clin Microbiol Rep. 2020;7(3):81–9.
Sasaoka F, Suzuki J, Hirata T, Ichijo T, Furuhama K, Harasawa R, et al. Vertical transmission of Mycoplasma wenyonii in cattle, supported by analysis of the ribonuclease P RNA gene - Short communication. Acta Vet Hung. 2015;63(3):271–4.
Girotto-Soares A, Soares JF, Bogado ALG, de Macedo CAB, Sandeski LM, Garcia JL, et al. 'Candidatus Mycoplasma haemobos': Transplacental transmission in dairy cows (Bos taurus). Vet Microbiol. 2016;195:22–4.
Koressaar T, Remm M. Enhancements and modifications of primer design program Primer3. Bioinformatics (Oxford, England). 2007;23(10):1289–91.
Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M, et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 2012;40(15):e115.
Thornton B, Basu C. Rapid and simple method of qPCR primer design. Methods Mol Biol. 2015;1275:173–9.
Volokhov DV, Gulland FM, Gao Y, Chizhikov VE. Ureaplasma miroungigenitalium sp. nov. isolated from northern elephant seals (Mirounga angustirostris) and Ureaplasma zalophigenitalium sp. nov. isolated from California sea lions (Zalophus californianus). Int J Syst Evol Microbiol. 2020;70(1):153–64.
Acknowledgements
The authors wish to thank the colleagues from the Clinic for Swine who assisted at sample collection. The authors are grateful to the farmers who contributed to this study.
The authors want to thank Dr. Anna-Caroline Wöhr, Animal Welfare Officer of the Faculty of Veterinary Medicine, for professional support concerning ethical issues.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
JA participated in design of the study, performed the study, analyzed the data and drafted the manuscript. JS performed animal sampling, contributed to data analysis, reviewed the study protocol and assisted with the interpretation of the data. CZ supported with primer design and in silico analyses. MR, KH and LEH designed the study protocol, reviewed the manuscript and assisted with interpretation of the data. All the authors read, revised, and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal sampling of sows and piglets was performed in accordance with the German animal welfare law using a protocol officially approved by the government (Regierung von Oberbayern) (Az. 55.2–154–2532.2-16-13). Sampling of fattening pigs was performed after the slaughtering process which does not require any ethical approval according to the German Animal Welfare Law. `Candidatus M. haemosuis` positive blood samples (availiable from a previous study [20] were taken during diagnostic procedures and therefore are not subject to approval requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ade, J., Stadler, J., Ritzmann, M. et al. Occurrence of ‘Candidatus Mycoplasma haemosuis’ in fattening pigs, sows and piglets in Germany using a novel gap-based quantitative real-time PCR assay. BMC Vet Res 18, 40 (2022). https://doi.org/10.1186/s12917-022-03147-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-022-03147-1