Abstract
Background
The zoonotic hookworms Ancylostoma caninum and Uncinaria stenocephala are widespread soil-transmitted helminths in dogs in Europe. Given the veterinary and public health importance of hookworms in dogs and the recent changes in the molecular epidemiology of some species, there is a need to continuously monitor the epidemiological and molecular prevalence of these parasites also at the “local” level. The present study aimed to update the epidemiological scenario of hookworm infections in both owned and stray dogs in southern Italy and to discriminate between different hookworm species (A. caninum and U. stenocephala) through molecular analyses. For this purpose, a retrospective analysis was performed over 10 years (2011–2021), including a total of 7008 owned dogs and 5642 stray dogs referred to our laboratory for copromicroscopic examinations. Moreover, 72 faecal samples, from dogs naturally infected by hookworms, were used to discriminate between A. caninum and U. stenocephala using two PCR protocols. Prior to molecular analyses, a subsample of 40/72 positive faecal samples was used for morphometric investigations on hookworm eggs.
Results
The results of the ten-year retrospective analysis (2011–2021) showed an overall prevalence of hookworm infection of 9.16%, specifically 5.1% in owned dogs and 14.2% in stray dogs. Logistic regression showed a significant association between positivity to hookworms and the variable “puppies” both in stray (13.84%; OR = 2.4) and owned (7.07%; OR = 2.2) dogs. The results of molecular analyses showed that positivity was confirmed only in 21/72 samples, specifically, 6 samples using protocol A and 19 with protocol B. Sequencing revealed 15 samples positive to U. stenocephala and 6 to A. caninum.
Conclusions
The findings of this study showed a high prevalence of hookworm infections in dogs in southern Italy, updating the epidemiological scenario of the last decade. Moreover, the results of the study revealed the first identification of hookworm species in dogs in Italy by molecular studies, highlighting that U. stenocephala is more prevalent than A. caninum.
Similar content being viewed by others
Background
Among the intestinal parasites that infect dogs, the hookworms Ancylostoma caninum and Uncinaria stenocephala play an important role in the health and welfare of canine populations worldwide, as well as in public health, due to their zoonotic potential [1,2,3]. Both pathogens might cause larva migrans syndrome or “ground itch” in humans [4, 5]. Moreover, the possibility of causing eosinophilic enteritis in human hosts which determines diarrhea, abdominal pain and weight loss has also been described [6].
The main source of infection in dogs is the soil contaminated with eggs excreted in dog faeces, where larvae hatch and develop to the infective stage L3 at suitable temperatures and humidity rates. Infection occurs mainly by percutaneous penetration of L3 or their ingestion per os [7]. In addition, hookworms are known to cause anaemia and hypoproteinemia in dogs, especially in puppies [8, 9].
Hookworms are common parasites in dogs and wild carnivores throughout the world, with prevalence values varying by climatic regions and dog population. In Europe, prevalence rates of hookworm infections in dogs range from 1.2% to 34% [9,10,11,12,13]. In particular, in Italy, hookworm infections have been reported in many studies, with high prevalence rates in stray (67.7%) and owned dogs (18.9%) in the southern area [14, 15], followed by prevalence rates between 0–9.3% in stray dogs and 0.4–3.6% in owned dogs in the northern area [16,17,18,19]. However, there are few studies to support the identification of hookworm species in dogs around the world. For example, in Central Europe, according to a recent study, U. stenocephala infection seems to be more prevalent than A. caninum infection in dogs [20]. On the other hand, in Africa [21,22,23], Asia [24,25,26] and Brasil [27], the occurrence of A. caninum was reported with higher frequency than U. stenocephala species. Moreover, the data also revealed mixed infections with other hookworm species such as Ancylostoma ceylanicum or Ancylostoma braziliense. Hence, considering the above information and the impact of hookworm infections on veterinary and public health, it should be imperative to continuously monitor the prevalence of hookworms in dogs in Europe and Italy. In addition, the fact that there are few studies [20, 28] reporting the differentiation between hookworm species in dogs in Europe, but no study conducted so far in Italy, demands future research to estimate and evaluate the zoonotic aspects of hookworm infections. Therefore, the present study aims to update the epidemiological scenario of hookworm infections in owned and stray dogs in southern Italy by performing a retrospective analysis of prevalence over ten years (2011–2021) and a molecular study to identify the occurence of A. caninum and U. stenocephala.
Results
The results of the ten-year retrospective analysis (2011–2021) in southern Italy showed an overall prevalence of hookworm infections of 9.16% (1159/12650; 95% CI = 8.67–9.68) in owned and stray dogs. More specifically, a prevalence of 5.1% (355/7008; 95% CI = 4.57–5.61) was found in owned dogs with a mean egg shedding of 222.9 eggs per gram (EPG) of feaces (2–6,440 EPG; SD = 601.07) and 14.2% (804/5642; 95% CI = 13.35–15.20) in stray dogs with a mean EPG of 20.5 (2–556 EPG; SD = 39.92). Prevalence values per year (2011–2021) of hookworm infection, both in owned and stray dogs are reported in Table 1. Co-infections with other helminths and protozoa (Trichuris, Toxocara, Capillaria, Isospora, Giardia) were also found (data not showed). Almost all dogs were apparently healthy (80%), while in a few cases abnormalities in faecal consistency such as diarrhea or the presence of blood (20%) were observed.
The results of the Chi-square test for the different variables considered (gender, age and dog breed size) are reported in Tables 2 and 3. Logistic regression revealed a significant association between positivity to hookworms and the variable “puppies” in both stray (13.84%; OR = 2.4; 95%CI = 12.50–15.21; P = 0.004) and owned (7.07%; OR = 2.2; 95%CI = 6.12–8.14 P = 0.000) dogs. Regarding the excretion of hookworm eggs in owned dogs, 193/355 (54.4%) were allocated in group A (2–50 EPG), 53/355 (14.9%) in group B (52–100 EPG), 69/355 (19.4%) in group C (102–500 EPG), 21/355 (5.9%) in group D (502–1000 EPG) and 19/355 (5.4%) in group E (≥ 1002 EPG); while, in stray dogs 759/804 (94.4%) were allocated in group A (P < 0.005), 32/804 (3.9%) in group B, 11/804 (1.4%) in group C, 2/804 (0.3%) in group D and 0/804 in group E.
The results of molecular analyses showed that 21/72 samples were confirmed with both protocols (A, B). Specifically, 6 samples were confirmed with protocol A and 19 with protocol B. In addition, only four samples were resulted positive at both PCR protocols (A, B) Sequencing revealed that 15 samples were identified as U. stenocephala (100% identity; MT345056) and 6 samples as A. caninum (100% identity; MT1309331). Co-infections with the two hookworm species were not detected in any sample.
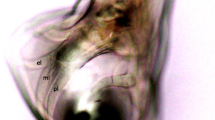
The results of morphometric analyses showed that 28/40 hookworm positive samples were similar to U. stenocephala (Fig. 1) (major axis of egg = 80.532 ± 3.120 μm; minor axis = 46.591 ± 3.691 μm; perimeter = 214.477 ± 3.703 μm) and 12/40 samples were similar to A. caninum (Fig. 2) (major axis of eggs = 66.305 ± 5.675 μm; minor axis = 41.348 ± 4.033 μm; perimeter = 175.375 ± 6.029 μm) [29].
Of the total 40 faecal samples subjected to the morphometric analyses, the results of molecular analysis could be assigned to only 10 samples as follows: 2 samples with protocol A and 8 with protocol B. Finally, the molecular results of the samples confirmed by PCR and sequencing (N = 10) agreed with the results of the morphometric analyses, i.e., U. stenocephala (n = 9) and A. caninum (n = 1)
Discussion
Hookworms in dogs cause clinically relevant parasitic infections that are common worldwide, with prevalence rates varying by geographic area and dog population [9,10,11]. In Asia, Africa, North America and Australia hookworm infection are widespread with different prevalence rates, e.g. 23–79% [30,31,32], 30–32% [21, 22, 33], 10–91% [34] and 6–10% [35, 36], respectively. The presence of hosts other than dogs, such as foxes and wolves, and climatic conditions favorable for larval development are important factors that could influence the distribution of hookworms in dog populations worldwide [37]. According to some studies conducted in Europe, the prevalence of hookworms ranges from 10 to 12% in foxes [11, 38] and from 30 to 90% in wolves [39, 40], whereas a recent study on intestinal parasites in dogs in cities across Western Europe revealed a hookworm prevalence of 3.2% [13].
In Italy, data on the prevalence of hookworm infections in dogs vary widely from north to south and depend on the diagnostic method used, the study area (rural, urban, and suburban), the dogs’ lifestyle, and the chemoprophylaxis regimes [14,15,16,17,18,19, 41]. The data obtained in the present study on the overall prevalence of hookworms (9.2%) in dogs in southern Italy is in line with the prevalence (11.6%) obtained in an harmonized survey recently conducted in Italy [15]. As expected, the prevalence was higher in stray dogs (14.2%) than in owned dogs (5.1%), confirming the data of previous studies from the same area [14, 15]. These findings showed that hookworms are still prevalent in dog populations in southern Italy, despite the increased awareness of veterinarians and owners promoted by the national and European guidelines of the European Scientific Counsel Companion Animal Parasites (ESCCAP) [42].
On the other hand, the present study showed greater egg shedding in owned dogs than in stray dogs. According to the authors' knowledge there is no explanation for this result but the only hypothesis that could justify this would be the immunological response induced by the infected stray dogs due to their constant exposure to hookworm eggs, resulting in elimination of low EPG levels than in owned dogs which their exposure to hookworm eggs could be less frequent but with intense elimination of parasitic eggs. Thus, further studies are needed in order to investigate the immunological response both in experimental and naturally infected dogs with hookworms. However one of the limit of this study was the impossibility to highlight the seasonality regarding the shedding of the eggs, as the number of analyzed samples varied greatly in different months and years.
Statistical results of the present study showed that positivity for hookworms was significantly related to the age of infected dogs with higher prevalence in puppies. According to Gates et al. (2009) [43], puppies are more likely to be infected through transmammary route during the lactation period in case of A. caninum. However, the possibility of transplacental transmission has not yet been described. In addition, the higher pathogenicity of hookworm species in dogs depending on the age of the dog must be considered. [8]. In fact, puppies affected by hookworms infection usually suffer from diarrhea and anemia and sometimes die in massive infections [8].
The results of the present study showed variable values of hookworm prevalence per year (2011–2021), ranging from 1 to 13% in owned dogs and from 11 to 20% in stray dogs, but no temporal trend was observed. In contrast, retrospective studies conducted in the USA (in 2012–2018 and 2013–2017) [44, 45] and in central Italy (in 2015–2020) [41] showed an increasing trend in the prevalence of hookworm infections (2.02–2.96%, 1.17–2.77%, and 6.8–16.5%) over their study periods. This could be due to multiple factors such as: climatic conditions influencing parasite development in the environment, possible resistance to commercially available anthelmintics [46], the use of copromicroscopic techniques with different detection limits, and the different size of dog population used, all of which must be taken into account.
Although several studies on the prevalence of hookworms in dogs have been carried out in Italy, there are no data on the identification and discrimination of the different hookworm species based on molecular studies. In fact, PCR and sequencing are the only tools available so far for hookworm species identification [20, 26]. This is the first molecular identification of hookworm species in dogs in Italy showing that U. stenocephala is more prevalent than A. caninum in dogs in southern Italy. It is interesting to note that U. stenocephala occurs in regions where the climate is not optimal for its development [20], such as the Mediterranean region. It is likely that our findings are due to both climatic changes and increasing animal movements as a result of globalization. It should also be noted that in the present study, not all the faecal samples which were positive to the hookworm eggs with the FLOTAC technique (N = 72), were also positive to the PCR protocols (A and B) (N = 21/72) used [47]. Moreover, the DNA used for the PCR analyses was extracted from the faecal samples naturally infected with Ancylostomidae eggs and the positive control was extracted from the Uncinaria stenocephala adult. However, there are other molecular studies that showed a high prevalence rates of hookworm infection [21, 22, 24, 27], using the same PCR protocol as described in the present study [21, 22, 24, 27]. This may suggest that either the PCR protocols used in the present study are less sensitive, or that a different substrate for DNA extraction should be considered, e.g. L3 larvae instead of eggs in the faecal samples, as shown in another study [22, 25]. In addition, the low prevalence rates of hookworm infections obtained with PCR analyses, reported herein, are similar with what reported in another study in which was performed a different PCR protocol, but using faecal samples naturally infected with high EPG of hookworms [26, 48]. The reduced detection limit of both PCR protocols (A, B) perfomed in the present study could be explained by two hypotheses: the type of matrix used for the DNA extraction, but perhaps, also the load of eggs excreted by the dogs naturally infected by hookworms. Therefore, further studies are needed to improve the sensitivity of the PCR protocol for hookworm detection, by investigating the detection limit and the best type of sample to use (i.e. faeces with eggs, floated suspension with eggs, L3 larvae). However, both hypotheses above mentioned (using spiked and naturally infected samples with hookworms) will be tested in another upcoming study by the authors of the present study.
The morphometric results obtained in this study agree with previous studies [29] and were also confirmed by the molecular analyses [20]. However, using only morphometric analyses of hookworm eggs, it is not possible to discriminate between different hookworm species. One of the limits of this study is that only a few number of samples were used for the morphometric analysis; in addition, all measurements were performed on samples naturally infected by hookworms without using a positive control from experimental infection. However, differentiation of different hookworm species such as A. caninum and U. stenocephala could also be possible by identification of L3 [49, 50], which was not performed in the present study.
Conclusions
In conclusion, the findings of this study revealed a high prevalence of hookworm infections in dogs in southern Italy, and updated the epidemiological scenario of the last decade. This study was the first to identify hookworm species (A. caninum and U. stenocephala) in dogs in Italy through molecular studies. Further studies are needed, especially to differentiate hookworm species and to develop increasingly sensitive, accurate and point-of-care diagnostics to provide more effective surveillance tools for the protection of human and animal health.
Methods
Study design
The study design is summarized in Fig. 3. To update the epidemiological scenario of hookworm infections in dogs in southern Italy, two objectives were pursued. The first objective was to determine the prevalence and analyze the risk factors for hookworm infections in dogs in a Mediterranean area. For this purpose, a retrospective study was conducted analysing ten-years (January 2011-December 2021) of parasitological data from routine diagnostics in dogs from the Campania region (southern Italy). A total of 7,008 owned dogs (males = 4,030; females = 2,978) and 5,642 stray dogs (males = 3,059; females = 2,583) were referred to our laboratories (Parasitology Service of the Veterinary Teaching Hospital, University of Naples Federico II, Italy) for copromicroscopic examination. All faecal samples were analyzed using the FLOTAC technique [51, 52]. Moreover, data on dog’s age, gender, lifestyle (stray/owned dogs) and breed size were collected.
The second objective was to identify hookworm species in dogs in the study area, by morphometric analysis of eggs and confirmation by molecular tests. To this end, all faecal samples collected in 2022 (total number = 1548) that tested positive for hookworms using the FLOTAC technique (N = 72) were tested by molecular analyses, using two different protocols: i) protocol A as described by Traub et al., (2004) [47], and; ii) protocol B with some modifications of the previous protocol. In addition, a subsample of 40 of the 72 positive faecal samples was used for morphometric studies of hookworm eggs prior to molecular analyses.
Laboratory analysis
Coprological analyses
Each canine faecal sample (pools of three consecutive days for each faecal sample/per animal) was tested for intestinal parasites (helminths and protozoa) using the FLOTAC dual technique [51, 52] with sodium chloride (specific gravity, s.g. = 1.20) and zinc sulphate (s.g. = 1.20) as flotation solutions. The detection limit (analytic sensitivity) was 2 eggs/oocysts/cysts/larvae per gram (EPG/OPG/CPG/LPG) of faeces.
Morphometric analysis of hookworm eggs, DNA extraction and molecular analysis
All samples used for molecular analyses (N = 72) were previously stored at -20 °C. Therefore, morphometric analyses (major axis, minor axis and perimeter) of 20 hookworm eggs were performed for each of the 40 faecal samples using LAS X Leica software (version 5.0.2, 2021). In addition, the faecal samples that were analysed morphometrically, as well as the samples (N = 32) for which the morphometric tests could not be performed (low quantity of faecal samples) were subjected to molecular analyses. Therefore, 72 faecal samples were subjected to DNA extraction using the Fast DNA Stool Kit (Qiagen, Germany) according to the manufacturer's instructions. Specific primers were used for amplification of the ITS1, 5.8S and ITS-2 regions according to Traub et al. (2004) [47] as follows: forward primer RTGHF1 (5′-CGTGCTAGTCTTCAGGACTTTG-3′) and reverse primer RTGHR1 (5′-CGTTGTCATACTAGCCACTGC-3″) for the detection of Ancylostoma spp (680 bp region of A. caninum). To also confirm the amplification of Uncinaria stenocepahala, an adult specimen of U. stenocepahala was used in the same PCR protocol. The results showed that the purified PCR product of the adult specimen was confirmed as U. stenocephala after sequencing the 680 bp region using the same primers as described above.
Finally, the first PCR protocol (A) was performed according to Traub et al. (2004) [47] and the second protocol (B) with some modifications of protocol A [47] as follows: in 25 µl volumes with the final mix containing 12.5 pmol of each primer, 1X buffer Mix (EmeralAmp® GT PCR Master Mix; Takara Bio Inc., Shiga, Japan), H2O and 2 µl of DNA. Samples were heated at 96 °C for 10 min, 95 °C for 45 s, 59 °C for 40 s, 72 °C for 1 min for 10 cycles, then followed by 30 cycles of 95 °C for 45 s, 58 °C for 40 s, 72 °C for 60 s and 1 cycle of 72 °C for 7 min. In addition, the U. stenocephala adult DNA was used as positive control in all the PCR runs performed in the study.
The purified PCR products were sequenced in both forward and reverse directions and analyzed using Chromas 2.6.6 software. They were then compared with the NCBI/GenBank database using the Basic Local Alignment Search Tool (BLAST) and ClustalW software, for the discrimination between the two different hookworm species (A. caninum and U. stenocephala).
Statistical analyses
Statistical analyses were considered only for the dogs that resulted positive to the hookworm infections; other co-infections were excluded. Hence, dogs were classified into five age groups: puppies (up to 12 months); young dogs (13–36 months); adult dogs (37–72 months); old dogs (73–120 months); and very old dogs (> 120 months). In addition, dogs were classified into three groups (small, medium and large) based on the breed size and into five groups (A, B, C, D, E) based hookworms egg excretion (A = 2–50 EPG; B = 52–100 EPG; C = 102–500 EPG; D = 502–1000 EPG; E ≥ 1002 EPG). Positivity for hookworms was analyzed in association with the above-mentioned variables (gender, age dog breed size and egg excretion) using univariate and logistic regression analysis. Data regarding the year of analysis were excluded from statistical analyses because the dog population of each year was variable. Moreover, data regarding previous antiparasitic treatments were very sparse and incomplete, therefore were also excluded from the statistical analyses.
Any association was considered significant at P < 0.005. The prevalence and the 95% confidence intervals (95%CI) were calculated using the free online software «Sample Size Calculator» (Creative Research Systems, CA, USA). All statistical analyses were performed using the SPSS® software (version 22,0, IBM Corporation, Armonk, USA).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ESCCAP:
-
European Scientific Counsel Companion Animal Parasites
- EPG:
-
Eggs per gram of feaces
- OPG:
-
Oocysts per gram of feaces
- LPG:
-
Larvae per gram of feaces
- CPG:
-
Cysts per gram of feaces
References
Raza A, Rand J, Qamar AG, Jabbar A, Kopp S. Gastrointestinal Parasites in Shelter Dogs: Occurrence, Pathology, Treatment and Risk to Shelter Workers. Animals (Basel). 2018;8(7):108.
Mircean V, Dumitrache MO, Mircean M, Colosi HA, Györke A. Prevalence and risk factors associated with endoparasitic infection in dogs from Transylvania (Romania): A retrospective study. Vet Parasitol. 2017;243:157–61.
Kostopoulou D, Claerebout E, Arvanitis D, Ligda P, Voutzourakis N, Casaert S, Sotiraki S. Abundance, zoonotic potential and risk factors of intestinal parasitism amongst dog and cat populations: The scenario of Crete, Greece. Parasit Vectors. 2017;10(1):43.
Del Giudice P, Hakimi S, Vandenbos F, Magana C, Hubiche T. Autochthonous Cutaneous Larva Migrans in France and Europe. Acta Derm Venereol. 2019;99(9):805–8.
Bowman DD, Montgomery SP, Zajac AM, Eberhard ML, Kazacos KR. Hookworms of dogs and cats as agents of cutaneous larva migrans. Trends Parasitol. 2010;26(4):162–7.
Traub RJ, Zendejas-Heredia PA, Massetti L, Colella V. Zoonotic hookworms of dogs and cats - lessons from the past to inform current knowledge and future directions of research. Int J Parasitol. 2021;51(13–14):1233–41.
Epe C. Intestinal nematodes: biology and control. Vet Clin North Am Small Anim Pract. 2009;39(6):1091–107 vi-vii.
Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:91.
Bajer A, Bednarska M, Rodo A. Risk factors and control of intestinal parasite infections in sled dogs in Poland. Vet Parasitol. 2011;175:343–50.
Wright I, Stafford K, Coles G. The prevalence of intestinal nematodes in cats and dogs from Lancashire, north-west England. J Small Anim Pract. 2016;57(8):393–5.
Lledó L, Giménez-Pardo C, Saz JV, Serrano JL. Wild Red Foxes (Vulpes vulpes) as Sentinels of Parasitic Diseases in the Province of Soria. Northern Spain Vector Borne Zoonotic Dis. 2015;15(12):743–9.
Ilić T, Nišavić U, Gajić B, Nenadović K, Ristić M, Stanojević D, Dimitrijević S. Prevalence of intestinal parasites in dogs from public shelters in Serbia. Comp Immunol Microbiol Infect Dis. 2021;76: 101653.
Drake J, Sweet S, Baxendale K, Hegarty E, Horr S, Friis H, Goddu T, Ryan WG, von Samson-Himmelstjerna G. Detection of Giardia and helminths in Western Europe at local K9 (canine) sites (DOGWALKS Study). Parasit Vectors. 2022;15(1):311.
Rinaldi L, Pennacchio S, Musella V, Maurelli MP, Guariglia I, Cappelli G, et al. Kennel dogs and helminth infections in the Campania region. In: Rolando, editor. Proceedings of the Italian Conference of the Italian Society of Parasitology (SOIPA): 26–29 June 2012. Italy: Alghero (SS). p. 45.
Brianti E, Arfuso F, Cringoli G, Di Cesare A, Ferroglio L, Falsone E, Frangipane di Regalbono A, Gaglio G, Galuppi R, Genchi M, Iorio R, Kramer L, Lia RP, Manfredi MT, Morganti G, Perrucci S, Pessarin C, Poglayen G, Otranto D, Rinaldi L, Scala A, Solari Basano F, Varcasia A, Venco L, Veneziano V, Veronesi F, Zanet S, Zanzani SA. Italian nationwide survey on endoparasites of dogs. Proceedings of the Italian Conference of the Italian Society of Parasitology (SOIPA): 26–29 June 2018. Italy: Milano. 45–48.
Simonato G, Danesi P, Frangipane di Regalbono A, Dotto G, Tessarin C, Pietrobelli M, Pasotto D. Surveillance of Zoonotic Parasites in Animals Involved in Animal-Assisted Interventions (AAIs). Int J Environ Res Public Health. 2020;17(21):7914.
La Torre F, Di Cesare A, Simonato G, Cassini R, Traversa D, Frangipane di Regalbono A. Prevalence of zoonotic helminths in Italian house dogs. J Infect Dev Ctries. 2018;12(8):666–72.
Traversa D, Di Cesare A, Simonato G, Cassini R, Merola C, Diakou A, Halos L, Beugnet F, Frangipane di Regalbono A. Zoonotic intestinal parasites and vector-borne pathogens in Italian shelter and kennel dogs. Comp Immunol Microbiol Infect Dis. 2017;51:69–75.
Simonato G, Frangipane di Regalbono A, Cassini R, Traversa D, Beraldo P, Tessarin C, Pietrobelli M. Copromicroscopic and molecular investigations on intestinal parasites in kenneled dogs. Parasitol Res. 2015;114(5):1963–70.
Štrkolcová G, Mravcová K, Mucha R, Mulinge E, Schreiberová A. Occurrence of Hookworm and the First Molecular and Morphometric Identification of Uncinaria stenocephala in Dogs in Central Europe. Acta Parasitol. 2022;67(2):764–72.
Mulinge E, Njenga SM, Odongo D, Magambo J, Zeyhle E, Mbae C, Kagendo D, Kanyi H, Traub RJ, Wassermann M, Kern P, Romig T. Molecular identification of zoonotic hookworms in dogs from four counties of Kenya. J Helminthol. 2019;94:e43.
Merino-Tejedor A, Nejsum P, Mkupasi EM, Johansen MV, Olsen A. Molecular identification of zoonotic hookworm species in dog faeces from Tanzania. J elminthol. 2019;93(3):313–8.
Ngcamphalala PI, Lamb J, Mukaratirwa S. Molecular identification of hookworm isolates from stray dogs, humans and selected wildlife from South Africa. J Helminthol. 2019;94: e39.
Ng-Nguyen D, Hii SF, Nguyen VA, Van Nguyen T, Van Nguyen D, Traub RJ. Re-evaluation of the species of hookworms infecting dogs in Central Vietnam. Parasit Vectors. 2015;8:401.
George S, Levecke B, Kattula D, Velusamy V, Roy S, Geldhof P, Sarkar R, Kang G. Molecular Identification of Hookworm Isolates in Humans, Dogs and Soil in a Tribal Area in Tamil Nadu, India. PLoS Negl Trop Dis. 2016;10(8):e0004891.
De Silva TK, Rajakaruna RS, Mohotti KM, Rajapakse RPVJ, Perera PK. First Molecular Identification of Ancylostoma Species in Dogs in a Rural Tea Estate Community in Sri Lanka and the Detection of Other Zoonotic Gastro-intestinal Parasites. Acta Parasitol. 2022;67(3):1086–96.
Oliveira-Arbex AP, David EB, Oliveira-Sequeira TC, Katagiri S, Coradi ST, Guimarães S. Molecular identification of Ancylostoma species from dogs and an assessment of zoonotic risk in low-income households, São Paulo State. Brazil J Helminthol. 2017;91(1):14–9.
Górski P, Radowańska A, Jaros D, Wiśniewski M. Morfologiczne i molekularne porównanie nicieniz rodzaju Uncinaria pasozytujacychu lisa (Vulpes vulpes) i psa (Canis familiaris) [Molecular and morphological comparison of hookworms from genus Uncinaria invading red fox (Vulpes vulpes) and dog (Canis familiaris)]. Wiad Parazytol. 2006;52(4):317–20.
Lucio-Forster A, Liotta J, Yaros J, Briggs K, Mohammed H, Bowman D. Morphological Differentiation of Eggs of Ancylostoma caninum, Ancylostoma tubaeforme, and Ancylostoma braziliense from Dogs and Cats in the United States The Journal of parasitology. 2012; 98.
Singh RP, Roy BC, Begum N, Talukder MH. Prevalence of hookworm infections among stray dogs and molecular identification of hookworm species for the first time in Bangladesh. Vet Parasitol Reg Stud Reports. 2022;30:100719.
Zibaei M, Nosrati MRC, Shadnoosh F, Houshmand E, Karami MF, Rafsanjani MK, Majidiani H, Ghaffarifar F, Cortes HCE, Dalvand S, Badri M. Insights into hookworm prevalence in Asia: a systematic review and meta-analysis. Trans R Soc Trop Med Hyg. 2020;114(3):141–54.
Mahdy MA, Lim YA, Ngui R, Siti Fatimah MR, Choy SH, Yap NJ, Al-Mekhlafi HM, Ibrahim J, Surin J. Prevalence and zoonotic potential of canine hookworms in Malaysia. Parasit Vectors. 2012;5:88.
Idrissi H, Khatat SEH, Duchateau L, Kachani M, Daminet S, El Asatey S, Tazi N, Azrib R, Sahibi H. Prevalence, risk factors and zoonotic potential of intestinal parasites in dogs from four locations in Morocco. Vet Parasitol Reg Stud Reports. 2022;34: 100775.
Kim J, Lucio-Forster A, Ketzis JK. Ancylostoma in dogs in the Caribbean: a review and study from St. Kitts, West Indies. Parasit Vectors. 2022;15(1):139.
Massetti L, Wiethoelter A, McDonagh P, Rae L, Marwedel L, Beugnet F, Colella V, Traub RJ. Faecal prevalence, distribution and risk factors associated with canine soil-transmitted helminths contaminating urban parks across Australia. Int J Parasitol. 2022;52(10):637–46.
Palmer CS, Thompson RC, Traub RJ, Rees R, Robertson ID. National study of the gastrointestinal parasites of dogs and cats in Australia. Vet Parasitol. 2008;151(2–4):181–90.
Stronen AV, Molnar B, Ciucci P, Darimont CT, Grottoli L, Paquet PC, Sallows T, Smits JEG, Bryan HM. Cross-continental comparison of parasite communities in a wide-ranging carnivore suggests associations with prey diversity and host density. Ecol Evol. 2021;11(15):10338–52.
Dybing NA, Fleming PA, Adams PJ. Environmental conditions predict helminth prevalence in red foxes in Western Australia. Int J Parasitol Parasites Wildl. 2013;2:165–72.
Al-Sabi MNS, Rääf L, Osterman-Lind E, Uhlhorn H, Kapel CMO. Gastrointestinal helminths of gray wolves (Canis lupus lupus) from Sweden. Parasitol Res. 2018;117(6):1891–8.
Bindke JD, Springer A, Böer M, Strube C. Helminth Fauna in Captive European Gray Wolves (Canis lupus lupus) in Germany. Front Vet Sci. 2017;4:228.
Morelli S, Colombo M, Traversa D, Iorio R, Paoletti B, Bartolini R, Barlaam A, Di Cesare A. Zoonotic intestinal helminthes diagnosed in a 6-year period (2015–2020) in privately owned dogs of sub-urban and urban areas of Italy. Vet Parasitol Reg Stud Reports. 2022;29:100689.
European Scientific Counsel Companion Animal Parasites (ESCCAP). Worm control in dogs and cats, guideline 1, 2021. https://www.esccap.org/uploads/docs/oc1bt50t_0778_ESCCAP_GL1_v15_1p.pdf. Accessed 4 January 2023.
Gates MC, Nolan TJ. Endoparasite prevalence and recurrence across different age groups of dogs and cats. Vet Parasitol. 2009;166(1–2):153–8.
Drake J, Carey T. Seasonality and changing prevalence of common canine gastrointestinal nematodes in the USA. Parasites Vectors. 2019;12:430.
Drake J, Parrish R. Dog importation and changes in canine intestinal nematode prevalence in Colorado, USA, 2013–2017. Parasit Vectors. 2020;13(1):404.
Jimenez Castro PD, Howell SB, Schaefer JJ, Avramenko RW, Gilleard JS, Kaplan RM. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12(1):576.
Traub RJ, Robertson ID, Irwin P, Mencke N, Thompson RC. Application of a species-specific PCR-RFLP to identify Ancylostoma eggs directly from canine faeces. Vet Parasitol. 2004;123(3–4):245–55.
Fu Y, Huang Y, Abuzeid AMI, Hang J, Yan X, Wang M, Liu Y, Sun Y, Ran R, Zhang P, Li G. Prevalence and potential zoonotic risk of hookworms from stray dogs and cats in Guangdong. China Vet Parasitol Reg Stud Reports. 2019;17:100316.
Hill RL Jr, Roberson EL. Differences in lipid granulation as the basis for a morphologic differentiation between third-stage larvae of Uncinaria stenocephala and Ancylostoma caninum. J Parasitol. 1985;71(6):745–50.
Gibbs HC. Studies on the life cycle and developmental morphology of Dochmoides stenocephala (Railliet 1884) (Ancylostomatidae: Nematoda). Can J Zool. 1961;39:325–48.
Cringoli G, Rinaldi L, Maurelli MP, Utzinger J. FLOTAC: new multivalent techniques for qualitative and quantitative copromicroscopic diagnosis of parasites in animals and humans. Nat Protoc. 2010;5(3):503–15.
Cringoli G, Rinaldi L, Maurelli MP, Morgoglione ME, Musella V, Utzinger J. Ancylostoma caninum: calibration and comparison of diagnostic accuracy of flotation in tube, McMaster and FLOTAC in faecal samples of dogs. Exp Parasitol. 2011;128(1):32–7.
Acknowledgements
Not applicable.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, SI, LC, LR; Methodology, SI, LC; Validation, LR, MPM, MP; Formal Analysis, SI, LC, AB, VC, SP; PP; RA; Investigation, SI, LC; Data Curation, SI, LC, AB, VC, SP PP; RA Writing—Original Draft Preparation, SI, LC; Writing—Review and Editing, LR, LC, MPM; Supervision, LR, LC, MPM, MP. All authors reviewed the manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In the current study, the dog’s faecal samples were provided following owner consent. In addition, ethical approval for this study was granted by the University of Naples Federico II Ethical Review Committee (Approval No. 49234–2023). All methods were performed in accordance with the guidelines and regulations (Dlgs 26/2014) of the Italian Ministry of Health. Written informed consent was obtained from the owner for the participation of the animal in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Illiano, S., Ciuca, L., Maurelli, M.P. et al. Epidemiological and molecular updates on hookworm species in dogs from southern Italy. BMC Vet Res 19, 204 (2023). https://doi.org/10.1186/s12917-023-03765-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-023-03765-3