Abstract
Background
Porcine deltacoronavirus (PDCoV) is a swine enteropathogenic coronavirus that affects young pigs, causing vomiting, acute diarrhea, dehydration, and even death. There is growing evidence that PDCoV can undergo cross-species as well as zoonotic transmissions. Due to the frequent outbreaks of this deadly virus, early detection is essential for effective prevention and control. Therefore, developing a more convenient and reliable method for PDCoV detection is the need of the hour.
Results
This study utilized a high-affinity monoclonal antibody as the capture antibody and a horseradish peroxidase labeled polyclonal antibody as the detection antibody to develop an enzyme-linked immunosorbent assay (DAS-ELSA) for PDCoV detection.Both antibodies target the PDCoV nucleocapsid (N) protein. The findings of this study revealed that DAS-ELISA was highly specific to PDCoV and did not cross-react with other viruses to cause swine diarrhea. The limit of detection of the virus titer using this method was 103 TCID50/mL of PDCoV particles. The results of a parallel analysis of 239 known pig samples revealed a coincidence rate of 97.07% (κ = 0.922) using DAS-ELISA and reverse transcriptase PCR (RT-PCR). The DAS-ELISA was used to measure the one-step growth curve of PDCoV in LLC-PK cells and the tissue distribution of PDCoV in infected piglets. The study found that the DAS-ELISA was comparable in accuracy to the TCID50 method while measuring the one-step growth curve. Furthermore, the tissue distribution measured by DAS-ELISA was also consistent with the qRT-PCR method.
Conclusion
The developed DAS-ELISA method can be conveniently used for the early clinical detection of PDCoV infection in pigs, and it may also serve as an alternative method for laboratory testing of PDCoV.
Similar content being viewed by others
Background
The existence of porcine deltacoronavirus (PDCoV) has been reported in several countries, including the United States, China, Vietnam [1,2,3,4]. Belonging to the family Coronaviridae of the order Nidovirales, PDCoV is an enveloped, single-stranded, and positive-sense RNA virus [5, 6]. PDCoV has been reported to cause dehydration, diarrhea, and even death in piglets. However, recent studies indicate that turkey chicks, calves, chickens, and children are also susceptible to this deadly virus [7,8,9]. The nucleocapsid (N) protein of PDCoV is made up of 342 amino acids and has the most conserved sequence and immunogenicity [10]. The N protein is the first protein produced when a coronavirus infects the host. During viral invasion, it can regulate the immune system and signal transduction of the host. Thus, the N protein can be detected in the early phase of virus infection and is often used as an early diagnostic marker for PDCoV infection.
Generally, pathogen isolation and PCR can be used for the detection of PDCoV. However, these methods have the disadvantages of long time and low sensitivity. In recent times, several PDCoV detection methods have been developed. These include TaqMan probe-based multiplex real-time PCRs [11, 12], SYBR green-based real-time RT-PCR, and multiplex qRT-PCR assay [13, 14]. Due to the instability of RNA samples and possible contamination with other nucleic acids, these molecular methods require expensive tools and special handling of RNA samples [15]. Indirect fluorescence assay (IFA), enzyme-linked [1, 15, 16] immunosorbent assay (ELISA) and gold immunochromatography assay are the most commonly used serological methods for detecting PDCoV [1, 15]. However, the IFA method is not suitable for detecting a large number of clinical samples at the same time, and the gold immunochromatography assay has high cost. In contrast, ELISAs are a cost-effective, simple, and convenient method of detecting a wide range of clinical samples.
This study developed a double antibody sandwich enzyme-linked immunosorbent assay (DAS-ELISA) for detecting swine PDCoV. The method used mouse-derived monoclonal antibodies as capture antibodies, while rabbit-derived polyclonal antibodies (pAbs) labeled with horseradish peroxidase (HRP) were used as detection antibodies. Both antibodies exhibited high binding affinity to the PDCoV N protein. The DAS-ELISA method reported in this study showed high sensitivity, specificity, and agreement with RT-PCR for rapid and reliable detection of PDCoV in swine samples.
Materials and methods
Cells and viruses
LLC-PK cell (cell line CL-101; ATCC) derived from porcine kidneys were used to propagate the PDCoV. The cells were cultured in Modified Eagle's Medium (MEM) supplemented with 5% fetal bovine serum (FBS), 1% antibiotic–antimycotic solution, 1% nonessential amino acids (NEAA), and 1% HEPES. The cell cultures were grown in a 5% CO2 incubator at 37 °C.
The following viruses were obtained from the Key Laboratory for Animal-derived Food Safety of Henan Province (Zheng Zhou, China): PDCoV HNZK-02 (GenBank:MH708123.1), transmissible gastroenteritis virus (TGEV), porcine sapelovirus (PSV), Porcine epidemic diarrhea virus (PEDV), porcine parvovirus (PPV), and Mammalian Orthoreovirus (MRV).
Sample collection and processing
Between 2018 and 2022, 239 clinical swine diarrhea samples (containing blood, intestinal content, intestinal tissue, and feces) were randomly collected from the pig farms in Henan Province. The blood samples were collected in 10mL anticoagulant tubes (Osset, ShanDong, China) and centrifuged at 3500 × ɡ for 15 min at 4 °C. The intestinal content or feces were added to Dulbecco’s Modified Eagle’s Medium (DMEM, Gibco, USA) in a ratio of 1:1 by volume. The intestinal tissue was cut into segments with scissors and mixed with DMEM in a ratio of 1g tissue to 9ml DMEM. After complete crushing and mixing, the samples were centrifuged at 3500 × ɡ for 15 min at 4 °C. And then, only the supernatant was collected.
Negative and positive controls for the DAS-ELISA
For the DAS-ELISA experiments, a mock-infected LLC-PK cell line served as the negative control, while PDCoV-infected LLC-PK cells served as the positive control. LLC-PK cells were cultured in 6-well plates until they were 80–90% confluent. A multiplicity of infection (MOI) of 0.1 was used to inoculate the PDCoV. After adsorption for 1h, 2 mL of the culture medium was supplemented with 5 mg/mL of trypsin and added to each well. Next, the samples were incubated incubated at 37 °C in 5% CO2 until a cytopathic effect (CPE) was observed. The plates were frozen at -80 °C. The virus titer was detected by the TCID50 method after thawing the plates twice [16].
Indirect immunofluorescence assay (IFA)
24 h post-PDCoV infection, the cells were washed twice with phosphate-buffered saline (PBS, Solarbio, Beijing, China) fixed with anhydrous ethanol (Merck, Darmstadt, Germany). After washing, the fixed cells were blocked with 5% (w/v) bovine serum albumin (BSA, Sigma, USA) in PBS for 2 h. Subsequently, the cells were incubated for 1h with an in-house generated PDCoV polyclonal antibody diluted in a ratio of 1:100. The PDCoV polyclonal antibody was derived from the serum of a healthy piglet that was infected with PDCoV. Next, the cells were incubated for 1h with a fluorescent-labeled polyclonal goat anti-pig IgG antibody (Sigma, Burlington, MA, USA, 1:1000 dilution) for 1 h. This was followed by counterstaining with DAPI (Sigma, Burlington, MA, USA) for 10 min. Finally, the culture plate was observed under a fluorescence microscope (Zeiss, Oberkochen, Germany).
Western Blot
After 24 h of PDCoV infection, the LLC-PK cell samples were lysed with Western blot (WB) and Radio Immunoprecipitation Assay Lysis buffers (RIPA) (Solarbio, China). The proteins were loaded on SDS-PAGE and transferred to PVDF membranes (Millipore, USA) using a Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, USA). Post-transfer, the membranes were blocked with 5% skim milk (BioFroxx, Germany) and incubated overnight at 4°C with the following antibodies: HRP-goat anti-rabbit IgG (H + L) (Sigma, Burlington, MA, USA) or HRP-goat anti-mouse IgG (H + L) (Sigma, Burlington, MA, USA). Finally, the protein bands were visualized using the ChemiDoc™ MP Imaging System (Bio-Rad, USA).
Preparation of monoclonal capture antibody and polyclonal detection antibody against PDCoV N protein
This research group had previously prepared and purified PDCoV N protein [17] in an attempt to produce the mouse monoclonal antibody (mAb-N) and rabbit polyclonal antibody (pAb-N). The preparation and purification of PDCoV mAb-N were performed according to previously published protocols [18]. The purified PDCoV-N protein was used to immunize rabbits thrice to induce the production of polyclonal antibodies (pAb-N). When the antibody levels in the blood increased, blood samples were collected from the ear vein. The collected blood sample was centrifuged at 3000 × ɡ for 10 min to separate the serum. Next, the octanoic acid-ammonium sulfate precipitation method was utilized to purify the PAb-N from the serum. After purification, the PAb-N was labeled with horseradish peroxidase (HRP) via the sodium periodate method [19, 20]. The prepared antibodies were characterized by their binding to PDCoV N protein in WB. A NanoDrop 2000C spectrophotometer (Thermo Scientific, Germany) was used to determine the concentration of the PDCoV mAb-N. With rPDCoV-N as the coating antigen, the PDCoV pAb-N titer was tested against PDCoV N protein by indirect ELISA.
Optimization of DAS-ELISA
The DAS-ELISA was optimized by adjusting each condition individually. The optimal concentrations of the capture antibody mAb-N and the HRP-labeled detection antibody pAb-N were determined using checkerboard titrations. The type and concentration of the blocking solution, sample incubation time, HRP-labeled pAb-N, and the single-component Substrate solution (TMB) were also optimized. The coating concentration of mAb-N was tested between 0.125 to 4μg/mL (two-fold serial dilutions). The dilution ratio of pAb-N was examined between 1:1000 to 1:32,000 (two-fold serial dilutions). Four blocking solutions of bovine serum albumin (BSA), casein buffer, skim milk, and calf serum were tested with three different concentrations (1%, 2.5%, and 5%). The incubation time for the sample and HRP-labeled pAb-N was tested from 0.5 to 2h, while TMB was tested from 5 to 30 min.
Determination of the cut-off value of DAS-ELISA
50 PDCoV-negative swine samples (feces, tissue, and intestine contents) were tested with DAS-ELISA. The critical value was calculated by the following relation: \(\overline x\) + 3SD, where \(\overline x\) represents the mean value of OD450 of 50 negative samples, and 3 SD represents three standard deviations.
Assessment of sensitivity, specificity, reproducibility, and stability of DAS-ELISA
PDCoV-positive samples with known virus titers (107TCID50/mL) were used to determine the analytical sensitivity of the DAS-ELISA. The samples were diluted tenfold (from 107 TCID50/mL to 100 TCID50/mL) and tested with DAS-ELISA. The cut-off value was used to determine the detection limit was determined.
The analytical specificity of DAS-ELISA was evaluated with PDCoV positive and negative samples. The following swine diarrhea causing viruses were selected for analysis: PEDV, TGEV, PPV, PSV, and MRV.
Different batches of the same negative and positive samples were tested simultaneously to examine the reproducibility of the DAS-ELISA. The coefficient of variation (CV) was used to determine the reproducibility of the intra-batch and inter-batch. CV was calculated using the following relation: (SD/\(\overline x\)) × 100%. All tests were repeated thrice.
This research group developed a DAS-ELISA kit based on optimized conditions and materials. The stability of the kit was determined by storing the DAS-ELISA kits at 4 °C and 37 °C for 0.5, 1, 6, 12, and 18 months. The same negative and positive PDCoV samples were measured with the kit after every time point.
Comparison of DAS-ELISA and RT-PCR for PDCoV detection
The accuracy of the DAS-ELISA kit was evaluated by measuring 239 clinical swine diarrhea samples. The results were compared with those obtained by measuring the same samples with the RT-PCR method [15]. RT-PCR specific primers were designed based on the swine PDCoV N gene sequence. The concordance rate and Kappa values(κ) were utilized to determine the correlation between DAS-ELISA and RT-PCR [1, 21].
Generation of the one-step growth curve of PDCoV infection in LLC-PK cells using DAS-ELISA
The DAS-ELISA kit was used to determine the one-step growth curve of PDCoV on LLC-PK cells. In 6-well plates, 90% confluent LLC-PK cells were inoculated with PDCoV at a MOI of 0.1. After 1 h of adsorption, the cells were washed thrice with PBS. This was followed by adding 2 mL MEM to each well. The plates were cultured in an incubator at 37 °C and 5% CO2. The supernatant and cell mixtures were collected at 6, 12, 18, 24, 36, 48 and 72 h post PDCoV infection. The virus titers were detected in these samples at different time points simultaneously using the DAS-ELISA and TCID50 assay, leading to the generation of the one-step growth curve of PDCoV infection on LLC-PK cells.
Analysis of tissue distribution of PDCoV in infected piglets by DAS-ELISA
The sample tissues were obtained from one week old PDCoV infected piglets. The piglets were humanely euthanized via intramuscular administration of Ketamine at dosages of 4–6 mg/kg prior to undergoing postmortem examination. The tissues were collected from the heart, liver, spleen, lungs, kidneys, duodenum, jejunum, ileum, cecum, colon, rectum, mesenteric lymph nodes, and submandibular lymph nodes. 0.1g of each tissue was ground and diluted five-fold with DMEM. The sample mixture was centrifuged at 3000 × ɡ for 10 min at 4 °C. After centrifugation, the supernatant was collected as a sample. Next, the samples were analyzed by DAS-ELISA to determine the tissue distribution of PDCoV in infected piglets.
Results
Phenotype of PDCoV-infected LLC-PK cells
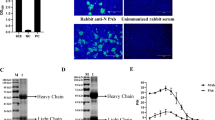
As shown in Fig. 1A, the LLC-PK cells appeared enlarged and rounded with densely packed granules following infection with PDCoV (Fig. 1A). Around 80% of the infected cells showed typical CPE after 24 h of PDCoV infection. At this point, the cells were collected and analyzed by IFA. As shown in Fig. 1B, the images revealed that the infected samples exhibited a strong green fluorescence.
Identification of anti-PDCoV N protein antibodies
The high-affinity monoclonal antibodies collected from mice were used as the capture antibody, while the polyclonal antibodies collected from rabbit serum and aged with HRP were used as the detection antibody. The purity of the antibodies and the antiserum titer of antibodies were determined by Western Blot and indirect ELISA, respectively. Both purified antibodies bound PDCoV N protein specifically, as indicated by a single 37KD band in Fig. 2A and B. The mAb-N concentration (2mg/mL) was measured by a NanoDrop 2000C spectrophotometer; The pAb-N titers against the PDCoV N protein were 1:64,000.
Identification of mAb-N and pAb-N. A Western Blot showing the specific binding of rabbit mAb-N with PDCoV N protein. (M: Marker; Lane 1: Protein sample from mock LLC-PK cells; Lane 2: Protein sample from LLC-PK cells infected with PDCoV). B Western Blot showing the specific binding of mouse pAb-N with PDCoV N protein. (M: Marker; Lane 1: Protein sample from mock LLC-PK cells; Lane 2: Protein sample from LLC-PK cells infected with PDCoV)
Optimization and development of DAS-ELISA
As shown in Table 1, the results of the checkerboard titration revealed that the P/N value was the highest when the mAb-N dilution was 1μg/mL and the pAb-N dilution was 1:8000. Thus, these concentrations were selected for DAS-ELISA.
Screening various blocking solutions revealed that blocking by BSA had a higher P/N value compared to other blocking agents. Furthermore, 1% and 2.5% BSA showed similar blocking effects, while 5% BSA showed a slightly higher P/N value (Fig. 3A). Thus, 1% BSA was selected as the blocking buffer for the subsequent experiments to reduce the cost of DAS-ELISA.
The optimal binding time of PDCoV antigen and antibody detection were evaluated under the abovementioned conditions. The results revealed that the P/N value gradually decreased with an increase in the antigen incubation time (0.5-2h) due to a rise in background noise (Fig. 3B). Therefore, an antigen incubation time of 45 min was selected. As shown in Fig. 3c, the highest P/N value was observed at a detection antibody incubation time of 45 min. Finally, the effect of the TMB reaction time (10–30 min) revealed that the P/N ratio was the highest at 10 min (Fig. 3D).
Determination of cut-off value for the DAS-ELISA
The cut-off value for DAS-ELISA was determined using 50 PDCoV negative samples with a mean of 0.170 and a standard deviation (SD) of 0.084. The cut-off value of PDCoV detection was 0.196 using the formula + 3SD. Therefore, the sample was considered PDCoV positive when its OD450 absorbance > 0.196.
Sensitivity, specificity, reproducibility, and stability of the DAS-ELISA
In order to evaluate the sensitivity of the DAS-ELISA, a PDCoV-positive sample with a known virus titer of 107 TCID50/mL was diluted by tenfold dilution, from 107 TCID50/mL to 100 TCID50/mL. These samples were then detected by DAS-ELISA. As shown in Fig. 4A, the minimum detection limit of the DAS-ELISA assay was determined to be 103 TCID50/mL. Subsequently, the reproducibility of the DAS-ELISA assay was investigated using 3 PDCoV positive and 3 negative control samples. The results revealed that the ratio of intra-batch to inter-batch variation was in the range of 0.8% to 6%. Furthermore, the coefficient of variation was less than 7% (Table 2), indicating that the DAS-ELISA had high reproducibility.
The specificity of the DAS-ELISA method was evaluated by testing other swine diarrhea causing viruses, such as TGEV, PEDV, PSV, PPV, and MRV. The results revealed that the DAS-ELISA reacted only with PDCoV. There was no cross-reactivity with other viruses tested, suggesting that the DAS-ELISA was highly specific to PDCoV (Fig. 4B).
The stability of DAS-ELISA was evaluated by storing at 4 °C and 37 °C for 14 days, 21 days, as well as 1, 6, 12, and 18 months before detecting the same PDCoV positive and negative samples. As shown in Fig. 4c, even after being stored for 18 months, there was no significant decrease in the absorbance at 4 °C. However, when the DAS-ELISA was stored at 37 °C, the absorbance declined significantly with an increase in storage time (Fig. 4C).
Comparison between DAS-ELISA and RT-PCR on PDCoV detection
The study analyzed 239 clinical samples to determine the consistency between DAS-ELISA and RT-PCR. The results showed that 56 out of the 63 samples that tested positive for RT-PCR also tested positive for DAS-ELISA. Furthermore, 173 out of the 183 samples that tested negative for DAS-ELISA also tested negative for RT-PCR. Thus, the positive consistency rate between the two methods was 88.89% (56/63), while the negative consistency rate was 96.17% (176/183). Overall, both DAS-ELISA and RT-PCR show a consistency rate of 97.07% (232/239, κ = 0.922) (Table 3).
Application of DAS-ELISA
The one-step growth curve of PDCoV on LLC-PK cells at different time points was determined by TCID50 and DAS-ELISA. The DAS-ELISA results revealed that the virus content reached its peak value 24—36h after infection and then declined significantly, consistent with the TCID50 detection results (Fig. 5A and B).
The DAS-ELISA was also used to analyze the tissue distribution of PDCoV in infected piglets. The results revealed that the virus could be detected in piglets infected with PDCoV in their tissues. The ileum had the highest virus content, followed by the jejunum and spleen (Fig. 5C). Furthermore, the distribution pattern of the virus determined by DAS-ELISA was consistent with previous reports that used qRT-PCR for detection [21]. The results reconfirmed the virus showed a wide tissue tropism.
Discussion
The PDCoV is a novel enteric coronavirus that can be transmitted from pigs to humans, posing a significant threat to global health. Since the first outbreak in 2014, PDCoV has caused several outbreaks, endangering the swine industry worldwide [22, 23]. Early and rapid detection of PDCoV infection is essential for controlling the spread of the disease due to the absence of effective drugs and vaccines in the market.
The current clinical diagnosis of PDCoV mainly relies on laboratory tests. These test methods are costly, time-consuming procedures. They also require complex instruments and/or special reagents, restricting their use in clinical practice. Consequently, a simple and inexpensive method to detect PDCoV is the need of the hour. DAS-ELISA is a low cost, sensitive and accurate serological detection method. It has been used in veterinary medicine in recent years. Several studies using DAS-ELISA for virus detection have been reported. Such as porcine circovirus type 2 (PCV2) [24], PEDV and swine acute diarrhea syndrome coronavirus (SADS-CoV) [25, 26]. These results demonstrate the reliability of DAS-ELISA again, simple operation and high specifcity and sensitivity. The technique was verified in clinical samples. Therefore, a DAS-ELISA method was developed for simple and reliable detection of PDCoV in this study.
The N protein is highly immunogenic, conserved, and expressed during early viral infection, making it an ideal antigen for diagnosing PDCoV infection. Hence, monoclonal and polyclonal antibodies (mAbs and pAbs) that specifically target the PDCoV N protein were constructed and purified using the N protein from strain HNZK-02 as the immunogen. SDS-PAGE and Western blotting analysis revealed both mAbs and pAbs bind the PDCoV N protein with > 90% purity.
This study examined 239 clinical feces and intestinal samples using DAS-ELISA and RT-PCR. The results revealed that 56 of the 63 samples (88.9%) that were RT-PCR positive were also DAS-ELISA positive. Meanwhile, 176 out of the 183 samples (96.2%) that tested negative for DAS-ELISA were also negative for RT-PCR. Thus, although DAS-ELISA is less sensitive than RT-PCR, the former is still the best alternative for PDCoV detection because of its simplicity, cost-effectiveness, and high consistency.
DAS-ELISA has been reported to be more sensitive and specific than indirect ELISA, particularly when it comes to detecting antigens in blood and oral swabs, where indirect ELISA has been found to be less sensitive [27, 28]. The DAS-ELISA can accurately quantify the antigen at the viral content level and is easy to operate [29]. The findings of this study demonstrated that the established DAS-ELISA can effectively detect PDCoV, providing a reliable, specific, and sensitive way to assess the presence of the virus.
In summary, the DAS-ELISA developed in this study has demonstrated high sensitivity, specificity, reproducibility, and stability, making it a simple and reliable method for detecting PDCoV in swine samples. Furthermore, the DAS-ELISA offers a rapid and cost-effective test for PDCoV detection, benefiting the swine industry by enabling close monitoring and control of PDCoV infections in pigs. Therefore, the commercial development of DAS-ELISA is crucial to facilitate the first ELISA-based PDCoV antigen detection.
Availability of data and materials
All data supporting our findings is contained within the manuscript.
References
Hu H, Jung K, Vlasova AN, Chepngeno J, Lu ZY, Wang QH, et al. Isolation and characterization of porcine deltacoronavirus from pigs with diarrhea in the United States. J Clin Microbiol. 2015;53(5):1537–48.
Song D, Zhou X, Peng Q, Chen Y, Zhang F, Huang T, et al. Newly emerged porcine deltacoronavirus associated with diarrhoea in swine in China: Identification, prevalence and full-length genome sequence analysis. Transbound Emerg Dis. 2015;62(6):575–80.
Woo PCY, Huang Y, Lau SKP, Yuen KY. Coronavirus genomics and bioinformatics analysis. Viruses. 2010;2(8):1804–20.
Woo PCY, Lau SKP, Lam CSF, Lau CCY, Tsang AKL, Lau JHN, et al. Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J Virol. 2012;86(7):3995–4008.
Zhang HL, Han FF, Shu XL, Li QQ, Ding QW, Hao CL, et al. Co-infection of porcine epidemic diarrhoea virus and porcine deltacoronavirus enhances the disease severity in piglets. Transbound Emerg Dis. 2022;69(4):1715–26.
Zhang JQ. Porcine deltacoronavirus: Overview of infection dynamics, diagnostic methods, prevalence and genetic evolution. Virus Res. 2016;226:71–84.
Li WT, Hulswit RJG, Kenney SP, Widjaja I, Jung K, Alhamo MA, et al. Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross-species transmissibility. Proc Natl Acad Sci U S A. 2018;115(22):E5135–43.
Liang QQ, Zhang HL, Li BX, Ding QW, Wang YB, Gao WM, et al. Susceptibility of chickens to porcine deltacoronavirus infection. Viruses. 2019;11(6):573.
Lednicky JA, Tagliamonte MS, White SK, Elbadry MA, Alam MM, Stephenson CJ, et al. Independent infections of porcine deltacoronavirus among Haitian children. Nature. 2021;600(7887):133–7.
McBride R, van Zyl M, Fielding BC. The coronavirus nucleocapsid is a multifunctional protein. Viruses. 2014;6(8):2991–3018.
Pan ZZ, Lu JX, Wang NN, He WT, Zhang LT, Zhao W, et al. Development of a TaqMan-probe-based multiplex real-time PCR for the simultaneous detection of emerging and reemerging swine coronaviruses. Virulence. 2020;11(1):707–18.
Zou JW, Liu HC, Chen J, Zhang J, Li XH, Long YF, et al. Development of a TaqMan-probe-based multiplex real-time PCR for the simultaneous detection of porcine circovirus 2, 3, and 4 in east China from 2020 to 2022. Vet Sci. 2022;10(1):29.
El-Tholoth M, Bai HW, Mauk MG, Saif L, Bau HH. A portable, 3D printed, microfluidic device for multiplexed, real time, molecular detection of the porcine epidemic diarrhea virus, transmissible gastroenteritis virus, and porcine deltacoronavirus at the point of need. Lab Chip. 2021;21(6):1118–30.
Li Y, Niu JW, Zhou X, Chu PP, Zhang KL, Gou HC, et al. Development of a multiplex qRT-PCR assay for the detection of porcine epidemic diarrhea virus, porcine transmissible gastroenteritis virus and porcine deltacoronavirus. Front Vet Sci. 2023;10: 1158585.
Cao LY, Kong XY, Zhang Y, Suo XP, Li XT, Duan YY, et al. Development of a novel double-antibody sandwich quantitative ELISA for detecting SADS-CoV infection. Appl Microbiol Biotechnol. 2023;107(7–8):2413–22.
Jin XH, Zhang YF, Yuan YX, Han L, Zhang GP, Hu H. Isolation, characterization and transcriptome analysis of porcine deltacoronavirus strain HNZK-02 from Henan Province. China Mol Immunol. 2021;134:86–99.
Ren HJ, Yan XG, Liu LT, Zhang YX, Li QQ, Li XM, et al. Identification of two novel B-cell epitopes on the nucleocapsid protein of porcine deltacoronavirus. Virol Sin. 2022;37(2):303–6.
Preston RJ, McCrea RA, Chang HT, Kitai ST. Anatomy and physiology of substantia nigra and retrorubral neurons studied by extra- and intracellular recording and by horseradish peroxidase labeling. Neuroscience. 1981;6(3):331–44.
Desjardins P, Hansen JB, Allen M. Microvolume protein concentration determination using the NanoDrop 2000c spectrophotometer. J Vis Exp. 2009;33:1610.
Nakane PK, Kawaoi A. Peroxidase-labeled antibody. A new method of conjugation. J Histochem Cytochem. 1974;22(12):1084–91.
Li BX, Zheng LL, Li HY, Ding QW, Wang YB, Wei ZY. Porcine deltacoronavirus causes diarrhea in various ages of field-infected pigs in China. Biosci Rep. 2019;39(9):BSR20190676.
Lee JH, Chung HC, Nguyen VG, Moon HJ, Kim HK, Park SJ, et al. Detection and phylogenetic analysis of porcine deltacoronavirus in Korean swine farms, 2015. Transbound Emerg Dis. 2016;63(3):248–52.
Saeng-Chuto K, Lorsirigool A, Temeeyasen G, Vui DT, Stott CJ, Madapong A, et al. Different lineage of porcine deltacoronavirus in thailand, vietnam and Lao PDR in 2015. Transbound Emerg Dis. 2017;64(1):3–10.
Xu L, Chen Z, Gong H, Pei X, Zhu Y, Lu Y, Wang Y, Nan S, Yin Y, Zhao Q, et al: Development a high-sensitivity sandwich ELISA for determining antigen content of porcine circovirus type 2 vaccines. J Virol Methods. 2024;328:114954.
Fan B, Sun J, Zhu L, Zhou J, Zhao Y, Yu Z, Sun B, Guo R, He K, Li B: Development of a Novel Double Antibody Sandwich Quantitative Enzyme-Linked Immunosorbent Assay for Detection of Porcine Epidemic Diarrhea Virus Antigen. Front Vet Sci. 2020;7:540248.
Cao L, Kong X, Zhang Y, Suo X, Li X, Duan Y, Yuan C, Zheng H, Wang Q: Development of a novel double-antibody sandwich quantitative ELISA for detecting SADS-CoV infection. Appl Microbiol Biotechnol. 2023;107:2413–22.
Nagy E, Nagy G, Power CA, Badarau A, Szijarto V. Anti-bacterial monoclonal antibodies. Adv Exp Med Biol. 2017;1053:119–53.
Zhang HL, Liang QQ, Li BX, Cui XG, Wei XL, Ding QW, et al. Prevalence, phylogenetic and evolutionary analysis of porcine deltacoronavirus in Henan province, China. Prev Vet Med. 2019;166:8–15.
Wang W, Li J, Fan B, Zhang X, Guo R, Zhao Y, Zhou J, Zhou J, Sun D, Li B: Development of a Novel Double Antibody Sandwich ELISA for Quantitative Detection of Porcine Deltacoronavirus Antigen. Viruses. 2021;13(12):2403.
Acknowledgements
The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Funding
This study was funded by the Henan Province Key R&D Project, specifically for the Creation of Diagnostic Reagents and Reference Materials for Important Porcine Viral Diarrhea (Project No. 231111113100). This study also received support from the Interdisciplinary Innovation Research Group Project of the Natural Science Foundation of Henan Province of China, which focused on researching the involvement of new non-injectable vaccine adjuvants and the mechanisms of inducing immunity (Project No. 232300421001), and the China Postdoctoral Science Foundation (2021M701106), which focused on researching IL-1b secretion induced by activation of inflammasome induced by porcine Delta coronavirus infection.
Author information
Authors and Affiliations
Contributions
FFH and FS performed the experiments. YQX and FFH analyzed the data and interpreted the results. FFH, JHH, and DHG prepared the manuscript. ZYW and JY conceived and designed this study.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All 239 samples were used and processed with the consent of pig farm owners. The Animal Care and Ethics Committee at Henan Agricultural University reviewed and approved all animal experimentation has been provided.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, F., Shan, F., Hou, J. et al. Establishment and application of PDCoV antigen-specific DAS-ELISA detection method. BMC Vet Res 20, 342 (2024). https://doi.org/10.1186/s12917-024-04201-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-024-04201-w