Abstract
Background
Currently, there is conflicting information and guidance on the effective management of Alpha 1 Antitrypsin Deficiency (AATD). Establishing a consensus of assessment and disease management specific to AATD is important for achieving a standardized treatment pathway and for improving patient outcomes. Here, we aim to utilize the Delphi method to establish a European consensus for the assessment and management of patients with severe AATD.
Methods
Two rounds of a Delphi survey were completed online by members of the European Alpha-1 Research Collaboration (EARCO). Respondents were asked to indicate their agreement with proposed statements for patients with no respiratory symptoms, stable respiratory disease, and worsening respiratory disease using a Likert scale of 1–7. Levels of agreement between respondents were calculated using a weighted average.
Results
Round 1 of the Delphi survey was sent to 103 members of EARCO and 38/103 (36.9%) pulmonologists from across 15 countries completed all 109 questions. Round 2 was sent to all who completed Round 1 and 36/38 (94.7%) completed all 79 questions. Responses regarding spirometry, body plethysmography, high-resolution computed tomography, and the initiation of augmentation therapy showed little variability among physicians, but there was discordance among other aspects, such as the use of low-dose computed tomography in both a research setting and routine clinical care.
Conclusions
These results provide expert opinions for the assessment and monitoring of patients with severe AATD, which could be used to provide updated recommendations and standardized treatment pathways for patients across Europe.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Alpha 1 Antitrypsin Deficiency (AATD) is a rare genetic disorder characterized by the production of defective Alpha 1 Antitrypsin (AAT) protein or the absence of AAT production, caused by variations in the SERPINA1 gene [1]. AATD is associated with many different genotypes [2]; the Pi*ZZ and Pi*SZ genotypes affect approximately 1.5 million people worldwide [3,4,5] and it is estimated that 120,000 people in Europe have the Pi*ZZ genotype [6], which is associated with low levels of AAT and in which augmentation therapy (treatment with plasma-derived AAT) may be indicated. Furthermore, around 1/800 patients with chronic obstructive pulmonary disease (COPD) in Europe are also affected by AATD [6, 7]. However, severe AATD is a largely underdiagnosed condition with approximately only 15–30% of cases diagnosed in European countries [8].
There are multiple complications and comorbidities associated with AATD that places a significant clinical burden on patients. These include COPD, emphysema, and bronchiectasis, as well as liver complications such as fibrosis and steatosis [9]. Patients with AATD often experience a poor quality of life (QoL), while caregivers experience stress, anxiety, and loss of personal time [9]. In addition to this, AATD is associated with high medical costs and healthcare resource utilization, notably when augmentation therapy is prescribed [9]. This form of therapy with purified AAT is the only specific treatment for AATD and aims to increase patient survival, control symptoms, and prevent the progression of AATD-associated emphysema [10, 11].
Effective management of severe AATD is crucial to reduce the burden placed on patients, caregivers, and hospital resources. As clinicians rarely encounter patients with AATD, physicians treating these patients rely heavily on published guidelines on how to assess, monitor, and treat patients. Multiple guidelines have been published on AATD and are derived from COPD management recommendations, thus there is no guidance on the assessment and follow up of severe AATD-specific respiratory disease. Additionally, there is substantial variation in recommendations regarding how to manage routine clinical issues [12]. Conflicting information on how to manage AATD arises partly from the low prevalence and paucity of specific clinical trials, in addition to differences in regional prevalence, availability of augmentation therapy, and insurance environments [8, 12]. Establishing a consensus of assessment and management guidelines specific to severe AATD is, therefore, essential to achieve standardized treatment pathways to improve patient outcomes and reduce disease burden. We particularly focus on the severe end of the AATD spectrum (i.e., patients with Pi*ZZ, Pi*ZNull, and Pi*NullNull genotypes) as these patients are the most compromised and have specific treatment options available to them, such as augmentation therapy. Those with intermediate forms of AATD, such as those with the Pi*MZ, Pi*SZ, and Pi*SS genotypes, have a different set of challenges, including a lack of data that justifies this treatment (since these patients are explicitly excluded from randomized controlled trials); however, the diagnosis pathway for these patients is clear and is outlined in the most recent set of European guidelines [1]. For those with severe AATD, establishing a set of consensus guidelines specific to these patients is one of several missions of the European Reference Network for respiratory diseases (ERN-LUNG)—a network of European healthcare providers dedicated to ensuring and promoting excellence in care and research for the benefit of patients affected by rare respiratory diseases.
The European Alpha-1 Research Collaboration (EARCO) is a pan-European network committed to promoting clinical research and education in AATD [13, 14]. EARCO is an initiative of the European Respiratory Society (ERS) and was established to promote research, clinical care, and awareness of AATD in Europe. The core project of EARCO is the International AATD Registry, which is a collaboration open to all investigators from around the world caring for patients with AATD. The International AATD Registry was established in response to the unmet need for a global registry, more information regarding AATD comorbidities, the natural history of the disease, and risk factors for disease progression and poor prognosis of lung disease [13, 14].
In this current study, we utilized the experience of EARCO members to help build a European consensus for managing patients with severe AATD via a Delphi study. The Delphi method is a validated tool for developing a consensus of expert opinion where there is too little, too much, or conflicting information, and it can be adapted to suit specific situations [15]. Here, the Delphi method was used to build a consensus for the optimal assessment, monitoring, and management of patients with severe AATD, leveraging its effectiveness in addressing complexity and uncertainty present in the field compared to other decision-making techniques [16].
Materials and methods
Members of EARCO were invited to participate in this Delphi study based on their expertise and experience in treating patients with AATD. The Delphi survey was conducted online, and respondents were asked to consider the initial assessment and routine follow-up/management of adults diagnosed with AATD and lung disease for those with:
-
A)
No respiratory symptoms and stable lung function with normal age-related deterioration in spirometry over time (< 50 mL/year decline in forced expiratory volume in 1 s [FEV1])
-
B)
Stable respiratory disease, with < 50 mL/year FEV1 decline and at least one of: mild-to-moderate dyspnoea, or, 1–2 exacerbations/year requiring oral corticosteroids and/or antibiotics (not requiring hospitalization)
-
C)
Worsening respiratory disease, with one or more of: moderate-to-severe dyspnoea, 1–2 exacerbations/year requiring hospitalization, or a FEV1 decline of ≥ 50 mL/year
Respondents were asked to indicate how much they agreed with proposed statements using a Likert scale of 1–7 (where 1 was strongly disagree; 7 was strongly agree). Round 1 was sent to EARCO members via email in February 2022 and consisted of 109 questions on the following topics: spirometry, body plethysmography (body box), diffusion capacity, arterial blood gases, fractional exhaled nitric oxide (FeNO), oxygen saturation, high-resolution computed tomography (HRCT), low-dose computed tomography (LDCT), chest x-ray, QoL assessment, 6-min walking distance (6MWD), Medical Research Council (MRC) dyspnoea score, cardiopulmonary exercise test, exacerbation diary cards, liver tests, other monitoring/interventions and smoking status, and initiation of augmentation therapy. Responses were calculated using a weighted average to determine the level of agreement between respondents for each statement.
Weighted averages were calculated by multiplying each response option (1–7) for a given statement by its frequency (the number of times it was chosen), summing these products, and then dividing this sum by the total number of responses provided. Weighted average thresholds for the levels of agreement were as follows: consensus in negative (≤ 2); agreement in negative (≤ 3); no consensus or agreement (3.1–4.9); agreement (≥ 5); consensus (≥ 6). For questions where no positive consensus was reached in Round 1, questions were asked again in Round 2 with the omission of answer choices that reached consensus in negative in Round 1. Round 2 of the survey consisted of 79 questions and was sent to all individuals who fully completed Round 1 in November 2022. For questions where no positive consensus was reached in Round 2, results are presented from either Round 1 or Round 2, depending on which showed the least variability.
Institutional review board approval was not applicable.
Results
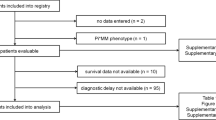
Round 1 of the Delphi survey was sent to 103 members of EARCO and 38/103 (36.9%) pulmonologists from across 15 countries completed all 109 questions (Fig. 1). Round 2 was sent to all who completed Round 1 and 36/38 (94.7%) completed all 79 questions. The questions and responses of Round 1 and 2 of the survey are shown in Additional file 1 and Additional file 2, respectively. Tables S1–S3 include details of areas that exhibited trends toward agreement, but for which no conclusions could be drawn.
Patients with no respiratory symptoms
Consensus and agreement levels achieved for patients with no respiratory symptoms are outlined in Table 1. For spirometry, consensus was reached for the frequency of assessment as well as the recording of FEV1, forced vital capacity (FVC), and FEV1/FVC, whilst the recording of other parameters and the measurement of spirometry pre-/post-bronchodilator (BD) reached agreement. Consensus was also reached for the use of body plethysmography to record total lung capacity (TLC), residual volume (RV), and RV/TLC, whilst the recording of airway resistance (Raw), specific airway resistance (sRaw), functional residual capacity (FRC), and FRC/TLC, as well as the frequency of body plethysmography assessment, reached agreement. There was no consensus or agreement on whether body plethysmography should be assessed pre-/post-BD (Table S1).
Consensus was obtained on the frequency of diffusion capacity and there was agreement for the utilization of HRCT (Table 1). However, there was no consensus or agreement for the frequency of LDCT and 6MWD, and responses were highly variable for these categories in comparison to others. For LDCT, there was no consensus or agreement on the frequency for which to assess lung densitometry in patients with no respiratory symptoms, in a research setting, or as part of routine clinical care (Fig. S1A). For 6MWD, there was no consensus or agreement on whether this assessment was applicable for patients with no respiratory symptoms; however, there was agreement that the 6MWD was not necessary to assess suitability for augmentation therapy or to assess the response of this therapy (Fig. S2A). Regarding 6MWD assessment frequency, there was no consensus or agreement (Table S1).
Results regarding arterial blood gasses, FeNO, oxygen saturation, chest x-ray, QoL assessment, MRC dyspnoea score, cardiopulmonary exercise test, exacerbation diary cards, and vaccinations for patients with no respiratory symptoms are shown in Table S1.
Patients with stable respiratory disease
Table 2 outlines the consensus and agreement levels achieved for patients with stable respiratory disease. Consensus was achieved for the spirometric recording of FEV1, FVC, and FEV1/FVC, whilst the recording of other spirometry parameters reached agreement, as did the spirometry assessment frequency and the measurement of spirometry in relation to BD. For body plethysmography, there was consensus for the recording of TLC, RV, and RV/TLC, and agreement for the recording of other parameters, as well as body plethysmography assessment frequency. However, as for patients with no respiratory disease, the assessment of body plethysmography pre-/post-BD did not reach consensus or agreement (Table S2).
Consensus was also achieved for the annual assessment of diffusion capacity and for the use of HRCT in patients with stable respiratory disease (Table 2). Again, responses were highly variable for questions regarding LDCT and the 6MWD assessment. There was agreement that LDCT scans were applicable for patients with stable respiratory disease, but no consensus or agreement was reached on how frequently they should be used to assess lung densitometry in a research setting, or as part of routine clinical care (Fig. S1B). There was agreement that the 6MWD was applicable for patients with stable respiratory disease, but no consensus or agreement on whether it should be used to assess suitability for augmentation therapy or to assess the response to this therapy (Fig. S2B and Table S2).
Regarding the initiation of augmentation therapy in patients with stable respiratory disease, there was consensus that it should be carefully evaluated to ensure AATD-related lung disease is present and that AAT levels should be measured prior to commencing the therapy (Table 2). It was agreed that prior to initiating augmentation therapy, patients should abstain from smoking for > 6 months, that the therapy should only be initiated when AAT levels are < 11 µM, and that the patient’s age should be considered prior to initiating. There was also agreement that augmentation therapy should only be initiated after emphysema is confirmed by computed tomography (CT) and that the patient’s deterioration in FEV1 should determine whether to initiate the therapy. Extended interval dosing was agreed as an acceptable augmentation therapy strategy and there was agreement that when monitoring AAT levels, they should be monitored after a change of therapy dose or interval and recorded at trough level (Table 2).
Results regarding arterial blood gasses, FeNO, oxygen saturation, chest x-ray, QoL assessment, MRC dyspnoea score, cardiopulmonary exercise test, exacerbation diary cards, and vaccinations for patients with stable respiratory disease are shown in Table S2.
Patients with worsening respiratory disease
Statements that achieved consensus or agreement for patients with worsening respiratory disease are shown in Table 3. Consensus was achieved for recording the following spirometric parameters: FEV1, FVC, and FEV1/FVC, and for the following body plethysmography parameters: TLC, RV, and RV/TLC. For spirometry, there was also agreement for assessment frequency and whether assessment should be pre-/post-BD. For body plethysmography, there was no consensus or agreement for assessment frequency or assessment in relation to BD and no consensus or agreement for diffusion capacity (Table S3).
For HRCT, consensus was achieved for utilization in patients with worsening respiratory disease (Table 3). As for patients with no respiratory symptoms and those with stable disease, responses were highly variable for questions regarding LDCT and the 6MWD assessment compared to other categories for patients with worsening respiratory disease. For LDCT, there was consensus that these scans were applicable, but no consensus or agreement on how frequently they should be used to assess densitometry in a research setting, or as part of routine clinical care (Fig. S1C). For 6MWD, there was also consensus that the assessment was applicable, but no consensus or agreement on whether it should be used to assess suitability for treatment or to assess treatment response (Fig. S2C).
Regarding the initiation of augmentation therapy in patients with worsening respiratory disease, only one statement reached consensus; AAT levels should be measured before commencement (Table 3). Respondents agreed on all other statements on the initiation of augmentation therapy. Regarding the use of this therapy in patients who have had a successful lung transplant, there was agreement in negative for augmentation therapy in this instance, i.e., there was agreement that the therapy should not be initiated in patients who have had a successful lung transplant (Table 3).
Results regarding arterial blood gasses, FeNO, oxygen saturation, chest x-ray, QoL assessment, MRC dyspnoea score, cardiopulmonary exercise test, exacerbation diary cards, and vaccinations for patients with worsening respiratory disease are shown in Table S3.
All patients with AATD
Table 4 outlines statements that reached consensus or agreement for all patients with AATD. There was agreement regarding liver function tests and that patients should be referred to a liver specialist following a diagnosis of AATD (Table 4), but there was no consensus or agreement on when patients should undergo liver elastography tests. Consensus was reached that patients with AATD should be asked about the use of antibiotics or corticosteroids, the occurrence of pneumonia, emergency room visits, changes in smoking status, and exposure to tobacco smoke or other environmental factors. There was also agreement that the Global Initiative for Chronic Obstructive Lung Disease strategy should be followed in all patients with AATD and lung disease.
Most survey respondents believed that body plethysmography should be prioritized for patients in whom the results will change the course of treatment (25/33; 75.8%; Fig. S3A) and that it should be performed to aid in the determination of clinical phenotype or the risk of disease progression (31/33; 93.9%; Fig. S3B). However, it was noted that body plethysmography may not always be feasible in some countries due to service limitations (Table S4).
Survey respondents also noted other important pieces of information, including that spirometry is essential to evaluate the natural history of the disease in patients with AATD and that appropriate liver disease screening tests should be performed regularly for patients with polymerizing AAT variants (Table S4). Furthermore, it was noted that aside from arterial puncture and the ear lobe technique, arterial blood gases can also be measured by pulse oximetry and that oxygen saturations should be assessed at every consultation. Before the initiation of augmentation therapy, patients must be genotyped, there must be clear evidence of a decline in health status, baseline AAT levels must be < 11 µM, and augmentation therapy should not be implemented in patients with stable disease. All patients with AATD should stop smoking regardless of disease severity and smoking cessation should be regularly confirmed, as smoking is a signal to stop augmentation therapy (Table S4).
Discussion
Discordance currently exists among published guidelines for the assessment and management of patients with severe AATD [12]. Many published guidelines contain country-specific recommendations and have been developed through literature reviews or expert panels [12]; to our knowledge, this is the first study that attempts to establish a consensus for the treatment and monitoring of patients with AATD using Delphi consensus methodology. Here, we provide current expert opinion on the assessment and monitoring of patients with severe AATD and break these down for those with stable disease and those with worsening disease. Despite the lack of uniformity in AATD assessment and discrepancies in physician opinion, consensus has been achieved in several aspects of the clinical assessment of AATD, such as spirometry, body plethysmography, HRCT, and the initiation of augmentation therapy. Furthermore, there were several areas that almost reached agreement; these areas are ones of current controversy, where hypothesis-based research may now be possible since there are now trends in ways of thinking. Areas with no consensus or agreement at all remain exploratory in research design.
The high concordance for the use of spirometry demonstrates its value in the assessment of lung disease associated with AATD. Due to its ease of use, availability, and reproducible and objective measurement of lung function, spirometry is one of the most widely used follow-up methods for AATD. In alignment with established clinical practice guidelines, our findings advocate for the regular use of spirometry assessment in the management of patients with severe AATD [17]. However, it is important to note that a nuanced approach to the scheduling of lung function assessment may be warranted. While the consensus leans towards annual spirometry assessment, results show that for patients experiencing worsening symptoms, spirometric parameters should be assessed more frequently depending on the deterioration pattern of each patient. One survey respondent commented that the frequency of spirometry assessment should not be uniformly prescribed but rather determined on an individual basis. This personalized approach acknowledges the heterogeneity in AATD manifestations among patients; however, while spirometry is a readily accessible diagnostic tool, it does not offer a comprehensive reflection of the extent of parenchymal damage associated with AATD-related pulmonary emphysema [17, 18]. This limitation implies that spirometric parametric measurements alone should not serve as the sole diagnostic criterion for AATD-related lung disease [19, 20] and that physicians should consider supplementary diagnostic methods and clinical indicators to gain a more comprehensive understanding of disease progression and its impact on the individual patient. In accordance with clinical guidelines, physicians are advised to incorporate additional measures, such as body plethysmography and diffusion capacity to obtain a comprehensive assessment of lung function [1, 17]. The agreement among respondents favouring these measures likely stems from their recognition that they provide valuable insights into the extent of lung function decline [17].
Our findings emphasize the value of full lung function assessment in managing patients with AATD; however, it is essential to recognize that there are still areas of discordance among other aspects of clinical guidance. Notably, there was lack of agreement regarding the use of LDCT in both research settings and in routine clinical care. HRCT allows visual identification of early-stage emphysema [21, 22], which is key to the management of AATD. However, non-inferior image quality and similar anatomical information can be achieved with LDCT protocols, which may be more suited to the serial scanning and density analysis required to monitor disease progression in AATD, as well as the effect of treatment regimens [21]. Other limitations in the use of lung density analysis for routine clinical practice include that specific software and expertise are required for analysis and interpretation of data, there is variability of reference values among scanners and software manufacturers, and lack of standardization and validated CT methodology and analysis protocols [23]. Thus, CT densitometry is a useful imaging tool for AATD-induced emphysema, but more research is needed before it can be used routinely in clinical practice [23].
There was also lack of agreement among survey respondents regarding the use of the 6MWD to assess suitability for augmentation therapy or response to this therapy in patients with stable or worsening respiratory disease associated with severe AATD. Pulmonary rehabilitation (PR) has been shown to be effective for patients with AATD [24, 25], and the 6MWD is a valid and reliable measure of exercise capacity [26]. PR has been shown to benefit patients with COPD, with and without AATD [27]; however, the authors of the study that demonstrated the benefits of PR in patients with AATD could not determine whether patient comorbidities, which can have profound effects on QoL, may have influenced results [27, 28]. Nevertheless, data did show that 6MWD is not improved by augmentation therapy alone and only improves when the patients are undergoing PR [27], which may explain why there was no agreement regarding the use of 6MWD to assess response to this therapy in the current study. A potential explanation for the lack of agreement regarding the use of 6MWD to assess suitability for augmentation therapy and to assess response to this therapy may be that, despite the most recent AATD guidelines stating the need to monitor all patients with AATD to assess treatment options using parameters such as FEV1, diffusion capacity of the lung for carbon monoxide, 6MWD, and health-related QoL parameters, access to augmentation therapy is highly variable across Europe [1]. Therefore, physicians without access to the therapy may not be familiar with current guidelines regarding methods to assess suitability for augmentation therapy and to assess response to the therapy.
Currently, augmentation therapy is reimbursed in Czechia within Eastern Europe, and is covered by public health insurance in Austria, Belgium (only for patients who started the therapy before 2010), France, Germany, Italy, Portugal, and Spain within Western Europe, highlighting the absence of harmonized access to augmentation therapy [1]. This geographical inequality in access to this optimal AATD healthcare ultimately results in different standards of care for AATD in Europe [1]. The present results indicate a high level of concordance regarding the initiation of augmentation therapy in patients with stable and worsening respiratory disease and highlights the importance of AAT levels (which relates closely to genotype) in determining eligibility. The utility of the 11 µM protective threshold as a determinant of clinical risk and as an indicator for commencing augmentation therapy is now considered questionable [29,30,31]; nevertheless, it was included in this study since plasma-derived AAT preparations are currently only licensed for patients who have AAT levels below this threshold. Furthermore, the 11 µM threshold (~ 57.2 mg/dL) are still included in several national guidelines as the threshold to define severe AATD and indicate augmentation therapy. However, whether AAT levels are above or below this 11 µM threshold should not guide treatment decisions alone; instead, this should be based on a patient's AATD genotype and other risk factors [29,30,31].
It is worth noting that a prior EARCO study highlights significant variability in the criteria for initiating augmentation therapy among European experts [32]. Greulich et al. revealed that factors other than AAT serum levels have a significant influence on the decision to initiate augmentation therapy when patients’ genotypes are considered [32]. For instance, in Pi*ZZ individuals, multivariate analysis identified younger age, reduced FEV1 (%), FEV1 decline, and the presence of emphysema by CT as significant in the decision for initiating augmentation therapy [32]. In Pi*SZ patients, age, FEV1 (%), and emphysema presence on CT were reported as significant in the decision making for the initiation of augmentation therapy [32]. In the present survey, results underscore that FEV1 and the presence of emphysema by CT are important aspects to consider when initiating augmentation therapy in both patients with stable and worsening respiratory disease. The high level of agreement on the initiation of this therapy here is in stark contrast with the great discordances shown in the study by Greulich et al., which is likely due to the differences in study design. Here, the agreement is on general statements, but when clinicians are confronted with real cases (as in the Greulich et al. paper) their decisions to treat with augmentation therapy may differ greatly and take into consideration several variables that are not included in the current recommendations described in guidelines.
Regarding the lack of consensus or agreement in some areas, there are several reasons why consensus or agreement was not reached. A limitation of this study is that due to size, there were only two survey rounds. Further rounds could have increased the number of statements that reached consensus or agreement in order to provide more refined recommendations. Additionally, there are few studies and evidence concerning some of the topics of this survey. AATD is a rare disease; therefore, it is likely that many respiratory physicians do not have a vast amount of experience in providing treatment for patients with AATD, and most knowledge of the management of AATD is derived from experience of managing COPD. The size of the survey could also have been a contributing factor to the low participation rate among EARCO members, though it is more likely that due to the timing, some countries were still battling the COVID-19 emergency and justifiably, the respiratory physicians of EARCO had more pressing matters and responsibilities.
Selecting EARCO members to participate in the study ensured expert opinions were gathered from physicians with extensive expertise and interest in the field of AATD, as well as experience with administration of augmentation therapy. However, the geographical spread of the participants was not equally distributed throughout Europe; approximately a quarter of all respondents were from Spain, which may have impacted the results. While our study builds upon guidelines previously published for the assessment and monitoring of patients AATD [12], it largely complements guidelines from the Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) [33], which is not surprising given the distribution of participating physicians. However, the SEPAR guidelines were published in 2015 and so an update is needed. Though, for a more accurate European consensus, more input is greatly needed from physicians of other European countries. The common membership and shared knowledge and adherence to the same guidelines amongst Spanish respondents may have contributed to a heightened level of consensus and agreement compared to physicians practicing in different regions. Furthermore, as most participants were from countries with AAT reimbursement policies, this could also have affected the results on this topic. Physicians may be influenced by their knowledge of the specific guidelines that they use, and country-specific regulations means that all countries do not have access to the same testing and/or treatment options; nevertheless, our results provide a good framework for physicians to approach their regulators to highlight the recommended treatments, and advocate for change, if required, in their area.
Conclusion
There is currently a lack of consistent guidance on the diagnosis, treatment, and management of patients with AATD in Europe. The present study provides updated expert opinions for the assessment and monitoring of patients with severe AATD, for those with stable and worsening disease, which were developed from the views of European respiratory physicians. Continuing to build the evidence base for the management of AATD is essential to support access to treatment and ensuring optimal access to effective care in AATD is something that EARCO is deeply committed to.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Abbreviations
- 6MWD:
-
6-Minute walking distance
- AAT:
-
Alpha 1 Antitrypsin
- AATD:
-
Alpha 1 Antitrypsin Deficiency
- BD:
-
Bronchodilator
- COPD:
-
Chronic obstructive pulmonary disease
- EARCO:
-
European Alpha-1 Research Collaboration
- ERS:
-
European Respiratory Society
- FeNO:
-
Fractional exhaled nitric oxide
- FEV1 :
-
Forced expiratory volume in 1 s
- FRC:
-
Functional residual capacity
- FVC:
-
Forced vital capacity
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- HRCT:
-
High-resolution computed tomography
- IC:
-
Inspiratory capacity
- ICS:
-
Inhaled corticosteroids
- LABA:
-
Long-acting beta-agonist
- LAMA:
-
Long-acting muscarinic antagonist
- LDCT:
-
Low-dose computed tomography
- MEF:
-
Maximum expiratory flow
- MRC:
-
Medical Research Council
- PR:
-
Pulmonary rehabilitation
- QoL:
-
Quality of life
- RV:
-
Residual volume
- TLC:
-
Total lung capacity
- VC:
-
Vital capacity
References
Miravitlles M, Dirksen A, Ferrarotti I, Koblizek V, Lange P, Mahadeva R, et al. European Respiratory Society statement: diagnosis and treatment of pulmonary disease in alpha1-antitrypsin deficiency. Eur Respir J. 2017;50(5):1700610.
Foil KE. Variants of SERPINA1 and the increasing complexity of testing for alpha-1 antitrypsin deficiency. Ther Adv Chronic Dis. 2021;12:20406223211015954.
Blanco I, Bueno P, Diego I, Perez-Holanda S, Casas-Maldonado F, Esquinas C, Miravitlles M. Alpha-1 antitrypsin Pi*Z gene frequency and Pi*ZZ genotype numbers worldwide: an update. Int J Chron Obstruct Pulmon Dis. 2017;12:561–9.
Blanco I, Bueno P, Diego I, Pérez-Holanda S, Lara B, Casas-Maldonado F, et al. Alpha-1 antitrypsin Pi*SZ genotype: estimated prevalence and number of SZ subjects worldwide. Int J Chron Obstruct Pulmon Dis. 2017;12:1683–94.
de Serres F, Blanco I. Role of alpha-1 antitrypsin in human health and disease. J Intern Med. 2014;276(4):311–35.
Blanco I, Diego I, Bueno P, Pérez-Holanda S, Casas-Maldonado F, Miravitlles M. Prevalence of α(1)-antitrypsin PiZZ genotypes in patients with COPD in Europe: a systematic review. Eur Respir Rev. 2020;29(157):200014.
Blanco I, Diego I, Castañón C, Bueno P, Miravitlles M. Estimated worldwide prevalence of the PI*ZZ alpha-1 antitrypsin genotype in subjects with chronic obstructive pulmonary disease. Arch Bronconeumol. 2023;59(7):427–34.
Horváth I, Canotilho M, Chlumský J, Chorostowska-Wynimko J, Corda L, Derom E, et al. Diagnosis and management of α(1)-antitrypsin deficiency in Europe: an expert survey. ERJ Open Res. 2019;5(1):00171–2018.
Miravitlles M, Herepath M, Priyendu A, Sharma S, Vilchez T, Vit O, et al. Disease burden associated with alpha-1 antitrypsin deficiency: systematic and structured literature reviews. Eur Respir Rev. 2022;31(163):210262.
McElvaney NG, Burdon J, Holmes M, Glanville A, Wark PA, Thompson PJ, et al. Long-term efficacy and safety of α1 proteinase inhibitor treatment for emphysema caused by severe α1 antitrypsin deficiency: an open-label extension trial (RAPID-OLE). Lancet Respir Med. 2017;5(1):51–60.
Wewers MD, Casolaro MA, Sellers SE, Swayze SC, McPhaul KM, Wittes JT, Crystal RG. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987;316(17):1055–62.
Attaway A, Majumdar U, Sandhaus RA, Nowacki AS, Stoller JK. An analysis of the degree of concordance among international guidelines regarding alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2019;14:2089–101.
Miravitlles M, Chorostowska-Wynimko J, Ferrarotti I, McElvaney NG, O’Hara K, Stolk J, et al. The European Alpha-1 Research Collaboration (EARCO): a new ERS clinical research collaboration to promote research in alpha-1 antitrypsin deficiency. Eur Respir J. 2019;53(2):1900138.
Miravitlles M, Turner AM, Torres-Duran M, Tanash H, Rodríguez-García C, López-Campos JL, et al. Clinical and functional characteristics of individuals with alpha-1 antitrypsin deficiency: EARCO international registry. Respir Res. 2022;23(1):352.
Nasa P, Jain R, Juneja D. Delphi methodology in healthcare research: how to decide its appropriateness. World J Methodol. 2021;11(4):116–29.
Mukherjee N, Zabala A, Huge J, Nyumba TO, Adem Esmail B, Sutherland WJ. Comparison of techniques for eliciting views and judgements in decision-making. Methods Ecol Evol. 2018;9(1):54–63.
Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman K, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis. 2016;3(3):668–82.
Fraughen DD, Ghosh AJ, Hobbs BD, Funk GC, Meischl T, Clarenbach CF, et al. Augmentation therapy for severe alpha-1 antitrypsin deficiency improves survival and is decoupled from spirometric decline-a multinational registry analysis. Am J Respir Crit Care Med. 2023;208(9):964–74.
Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: cross sectional survey. BMJ. 2003;327(7416):653–4.
Stockley JA, Stockley RA, Sapey E. There is no fast track to identify fast decliners in alpha-1 antitrypsin deficiency by spirometry: a longitudinal study of repeated measurements. Int J Chron Obstruct Pulmon Dis. 2021;16:835–40.
Shaker SB, Stavngaard T, Stolk J, Stoel B, Dirksen A. Alpha1-antitrypsin deficiency. 7: computed tomographic imaging in alpha1-antitrypsin deficiency. Thorax. 2004;59(11):986–91.
Sverzellati N, Molinari F, Pirronti T, Bonomo L, Spagnolo P, Zompatori M. New insights on COPD imaging via CT and MRI. Int J Chron Obstruct Pulmon Dis. 2007;2(3):301–12.
Campos MA, Diaz AA. The role of computed tomography for the evaluation of lung disease in alpha-1 antitrypsin deficiency. Chest. 2017;153(5):1240–8.
Jarosch I, Gehlert S, Jacko D, Koczulla RA, Wencker M, Welte T, et al. Different training-induced skeletal muscle adaptations in COPD patients with and without alpha-1 antitrypsin deficiency. Respiration. 2016;92(5):339–47.
Kenn K, Gloeckl R, Soennichsen A, Sczepanski B, Winterkamp S, Boensch M, Welte T. Predictors of success for pulmonary rehabilitation in patients awaiting lung transplantation. Transplantation. 2015;99(5):1072–7.
Singh SJ, Puhan MA, Andrianopoulos V, Hernandes NA, Mitchell KE, Hill CJ, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1447–78.
Jarosch I, Hitzl W, Koczulla AR, Wencker M, Welte T, Gloeckl R, et al. Comparison of exercise training responses in COPD patients with and without alpha-1 antitrypsin deficiency. Respir Med. 2017;130:98–101.
Barjaktarevic I, Campos M. Management of lung disease in alpha-1 antitrypsin deficiency: what we do and what we do not know. Ther Adv Chronic Dis. 2021;12:20406223211010172.
McElvaney GN, Sandhaus RA, Miravitlles M, Turino GM, Seersholm N, Wencker M, Stockley RA. Clinical considerations in individuals with α(1)-antitrypsin PI*SZ genotype. Eur Respir J. 2020;55(6):1902410.
Franciosi AN, Fraughen D, Carroll TP, McElvaney NG. Alpha-1 antitrypsin deficiency: clarifying the role of the putative protective threshold. Eur Respir J. 2022;59(2):2101410.
Miravitlles M, Anzueto A, Barrecheguren M. Nine controversial questions about augmentation therapy for alpha-1 antitrypsin deficiency: a viewpoint. Eur Respir Rev. 2023;32(170):230170.
Greulich T, Albert A, Cassel W, Boeselt T, Peychev E, Klemmer A, et al. Opinions and attitudes of pulmonologists about augmentation therapy in patients with alpha-1 antitrypsin deficiency. A survey of the EARCO group. Int J Chron Obstruct Pulmon Dis. 2022;17:53–64.
Casas F, Blanco I, Martínez MT, Bustamante A, Miravitlles M, Cadenas S, et al. Indications for active case searches and intravenous alpha-1 antitrypsin treatment for patients with alpha-1 antitrypsin deficiency chronic pulmonary obstructive disease: an update. Arch de Bronconeumol. 2015;51(4):185–92.
Acknowledgements
The authors would like to thank the following individuals for taking the time to complete the Delphi surveys. From Belgium: Stephanie Everaerts (Department of Pulmonary Diseases, University Hospitals Leuven, Leuven), Eric Derom (Department of Respiratory Medicine, Ghent University Hospital and University of Ghent, Ghent), and Wim Janssens (Department of Respiratory Diseases, University Hospitals Leuven, Leuven). From Bulgaria: Yavor Ivanov (Clinic for Pneumonology and Phthisiatry, UMHAT “Dr G Stranski” Pleven). From Croatia: Ana Hećimović (Clinic for Respiratory Diseases, University Hospital Center Zagreb, Zagreb). From Czechia: Jan Chlumský (Department of Pneumology, Thomayer Hospital, First Faculty of Medicine, Charles University, Prague). From Denmark: Jens-Ulrik Jensen (Section of Respiratory Medicine, Department of Medicine, Herlev-Gentofte Hospital, Herlev). From Estonia: Alan Altraja (Department of Pulmonology, University of Tartu, Tartu). From Germany: Robert Bals (Department of Internal Medicine V, Pneumology and Intensive Care Medicine, Saarland University, Homburg/Saar), and Franziska Trudzinski (Department of Pneumology and Critical Care Medicine, Thoraxklinik University of Heidelberg, Heidelberg). From Italy: Angelo Corsico (Center for Diagnosis of Inherited Alpha1-antitrypsin Deficiency, Department of Internal Medicine and Therapeutics, University of Pavia, and Pneumology Unit IRCCS San Matteo Hospital Foundation, University of Pavia, Pavia), Mario Malerba (Department of Translational Medicine, University of Piemonte Orientale, Novara), Davide Piloni (Centre for Diagnosis of Inherited Alpha-1 Antitrypsin Deficiency, Laboratory of Biochemistry and Genetics, Pulmonology Unit, Fondazione IRCCS Policlinico San Matteo, Pavia), and Simone Scarlata (Unit of Internal Medicine, Campus Bio-Medico University Hospital, Rome). From Malta: Caroline Gouder (Department of Respiratory Medicine, Mater Dei Hospital, Malta). From Portugal: Joana Gomes (Serviço de Pneumologia, Centro Hospitalar Universitário de Santo António, Porto), Teresa Martin (Pulmonology Department, Hospital Beatriz Ângelo, Loures), Cristina Santos (Centro Hospitalar Universitário Lisboa Norte, Lisboa), and Maria Joana Reis Amado Maia da Silva (Pulmonology Department, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos). From Romania: Ana Maria Zaharie and Ruxandra Ulmeanu ("Marius Nasta" Institute of Pneumophthisiology, Bucharest). From Serbia: Branislava Milenkovic (Department of Pneumonology Clinic for Pulmonology Diseases, University Clinical Centre of Serbia, Belgrade). From Spain: Miriam Barrecheguren (Pneumology Department, Hospital Universitari Vall d'Hebron, Barcelona), Ana Bustamante (Pneumology Section, Hospital Sierrallana-TresMares, Cantabria), Francisco Casas-Maldonado (Servicio de Neumología, Hospital Clínico Universitario San Cecilio, Departamento de Medicina, Facultad de Medicina, Universidad de Granada, Granada), Maria Estela Gonzalez Castro (Pneumology Department, Hospital Universitario Torrecardenas, Almería), José María Hernández-Pérez (Departamento de Neumología. Hospital Universitario Nuestra Señora de Candelaria, Santa Cruz de Tenerife), Virginia Almandana Pacheco (Respiratory Department, Virgen Macarena University Hospital, Seville), Juan Luis Rodríguez-Hermosa (Research Institute of Hospital Clínico San Carlos [IdISSC], Madrid), Eva Tabernero (Respiratory Department, Hospital Universitario Cruces, Biocruces-Bizkaia, Barakaldo), and María Torres-Durán (Servicio de Neumología. Hospital Álvaro Cunqueiro. NeumoVigo I+I Research Group, IIS Galicia Sur, Vigo). From Switzerland: Christian Clarenbach (Pulmonary Medicine, University Hospital Zurich, Zurich). From the United Kingdom: John Hurst (University College London, UCL Respiratory, London), and Robert Stockley (Lung Investigation Unit, Queen Elizabeth Hospital Birmingham, University Hospitals Birmingham, NHS Foundation Trust, Birmingham). The authors would also like to thank Amy Adlard, Ben McDermott, and Katie Dogan of Meridian HealthComms Ltd (part of the Bioscript group) for editorial assistance, which was funded by CSL Behring.
Funding
Editorial assistance was funded by CSL Behring. The funder had no role in study design, data collection, decision to publish, or preparation of the manuscript. AMT is also funded by the National Institute for Health and Care Research (NIHR) Midlands Patient Safety Research Collaboration (PSRC) and West Midlands Applied Research Collaboration (ARC), as well as the NIHR Efficacy and Mechanism Evaluation (EME) Programme and Health Technology Assessment (HTA) Programme. The views expressed are those of the author and not necessarily those of the NIHR or the Department of Health and Social Care.
Author information
Authors and Affiliations
Contributions
MM, AMT, MS, J-FM, TG, MW, and NGM designed the survey, contributed to acquiring, analyzing and interpreting the data, drafted the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study involved physicians providing their agreement on statements regarding the treatment of patients only and no patient data was utilized; therefore, institutional review board approval was not applicable. To maintain confidentiality, the physician responses were anonymized and aggregated for analysis. Participation in the survey was voluntary, and all participating physicians provided informed consent prior to their involvement.
Consent for publication
Not applicable.
Competing interests
Marc Miravitlles has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Menarini, Kamada, Takeda, Zambon, CSL Behring, Specialty Therapeutics, Janssen, Grifols, and Novartis, consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, BEAM Therapeutics, Chiesi, GlaxoSmithKline, CSL Behring, Inhibrx, Ferrer, Menarini, Mereo Biopharma, Spin Therapeutics, Specialty Therapeutics, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Novo Nordisk, Sanofi, Zambon, and Grifols and research grants from Grifols. Alice M Turner has received either grants or speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, CSL Behring, Takeda, Vertex, and Grifols. Maria Sucena has received speaker fees from AstraZeneca Bial, Boehringer Ingelheim, CSL Behring, and Grifols, and consulting fees from Bial and CSL Behring. Jean-François Mornex has received consulting fees from CSL Behring, Grifols, LFB, and Takeda, payment or honoraria and support for attending meetings from CSL Behring, Grifols, and LFB, and is a member of the scientific board of ADAAT. Timm Greulich has received personal fees from AstraZeneca, Berlin-Chemie, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, and Novartis, and grants and personal fees from Grifols. Marion Wencker has received consulting fees from CSL Behring and GlaxoSmithKline, has received support for attending meetings from VisionHealth, and is the Chief Medical Officer for Sterna Biologicals. N. Gerrard McElvaney has received research grants and honoraria from Grifols, has received consulting fees from CSL Behring and Vertex, and has participated in an advisory board for Vertex.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
12931_2024_2929_MOESM3_ESM.docx
Supplementary material 3: Table S1 Further recommendations for patients with no respiratory symptoms. Table S2 Further recommendations for patients with stable respiratory disease. Table S3 Further recommendations for patients with worsening respiratory disease. Table S4 Additional comments from survey respondents. Fig. S1 Responses regarding the use of LDCT scanning. Fig. S2 Variability of responses regarding the utility of the 6MWD assessment. Fig. S3 Utility of body box assessment in AATD.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Miravitlles, M., Turner, A.M., Sucena, M. et al. Assessment and monitoring of lung disease in patients with severe alpha 1 antitrypsin deficiency: a european delphi consensus of the EARCO group. Respir Res 25, 318 (2024). https://doi.org/10.1186/s12931-024-02929-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-024-02929-5