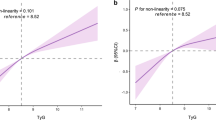
Abstract
Background
The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio and triglyceride-glucose (TyG) index are novel indexes for insulin resistance (IR). We aimed to evaluate associations of TG/HDL-C and TyG with arterial stiffness risk.
Methods
We enrolled 1979 participants from the Rural Chinese Cohort Study, examining arterial stiffness by brachial-ankle pulse wave velocity (baPWV). Logistic and linear regression models were employed to calculate effect estimates. For meta-analysis, we searched relevant articles from PubMed, Embase and Web of Science up to August 26, 2023. The fixed-effects or random-effects models were used to calculate the pooled estimates. We evaluated dose-response associations using restricted cubic splines.
Results
For cross-sectional studies, the adjusted ORs (95%CIs) for arterial stiffness were 1.12 (1.01–1.23) and 1.78 (1.38–2.30) for per 1 unit increment in TG/HDL-C and TyG. In the meta-analysis, the pooled ORs (95% CIs) were 1.26 (1.14–1.39) and 1.57 (1.36–1.82) for per 1 unit increment of TG/HDL-C and TyG. Additionally, both TG/HDL-C and TyG were positively related to PWV, with β of 0.09 (95% CI 0.04–0.14) and 0.57 (95% CI 0.35–0.78) m/s. We also found linear associations of TG/HDL-C and TyG with arterial stiffness risk.
Conclusions
High TG/HDL-C and TyG were related to increased arterial stiffness risk, indicating TG/HDL-C and TyG may be convincing predictors of arterial stiffness.
Similar content being viewed by others
Introduction
Cardiovascular diseases (CVDs), particularly stroke and ischemic heart disease (IHD) [1], constitute the top-ranking causes of premature death globally, and are the main contributors to the global burden of health [2]. Arterial stiffness, occurring with aging and a variety of pathological conditions, not only has a profound impact on the cardiovascular system but contributes significantly to the worldwide burden of CVDs [3]. In particular, arterial stiffness has been widely acknowledged as a valuable biomarker for identifying populations at higher risk of CVDs and mortality [4,5,6,7]. Given the genesis of arterial stiffness as a long-term pathological process, early identification of subjects at increased arterial stiffness risk and intervention with appropriate preventive strategies are both crucial to reducing the global health burden.
Insulin resistance (IR) is generally defined as reduced sensitivity to physiological insulin levels in insulin-targeting tissues [8]. It is likely to result in arterial stiffness by promoting dyslipidemia, a pro-inflammatory state, and causing endothelial damage [9,10,11]. Classically, the hyper-insulinemic euglycemic clamp is widely accepted as the gold standard for determining IR, but it is laborious, high-priced, and time-consuming [12], limiting its applicability in clinical settings. Interestingly, the triglyceride to high-density lipoprotein cholesterol ratio (TG/HDL-C) and triglyceride-glucose index (TyG), derived from fasting plasmsa glucose (FPG) and triglycerides [13, 14], have been recognized as convincing indictors of IR. Unfortunately, the relationships of TG/HDL-C and TyG with arterial stiffness risk are still being debated. Despite previous studies having evaluated associations of TG/HDL-C and TyG with arterial stiffness risk [15,16,17], conclusions were inconsistent across studies. Several studies reported positive relationships between TG/HDL-C and TyG and arterial stiffness risk [15, 17,18,19], but the associations disappeared in other studies [16, 20,21,22]. A review published in 2020 argued that TG/HDL-C and TyG may be accurate indicators of arterial stiffness [23], but it failed to quantitatively clarify the associations.
This study therefore aimed to explore the relationships of TG/HDL-C and TyG with arterial stiffness risk among rural Chinese adults and to summarize the dose-response associations of TG/HDL-C and TyG with arterial stiffness.
Methods
Cross-sectional study
Study design and population
This study was based on the Rural Chinese Cohort Study (RCCS) which was conducted to assess disease patterns and risk factors for non-communicable diseases in the rural population [24]. In all, 17,641 participants were re-examined in the second follow-up survey from July to August in 2018–2019. Among these, we enrolled a total of 2092 subjects who underwent an examination for arterial stiffness. Participants with missing data on TG, HDL-C, or FPG were excluded (n = 103). We further excluded participants with bilateral ankle-brachial indexes (ABIs) < 0.9 (n = 10). Ultimately, 1979 individuals were included in the current analysis (Supplemental Fig. 1).
In addition, we calculated the minimum sample size based on the proportion of participants with arterial stiffness (p) of 16.51% and the standard normal variable (Z1−α/2) of 1.96 [25]. Due to the 10% non-response response, the final sample size was 1471. This study therefore enrolled a sufficient number of participants.
Data collection and definition
Socio-demographic data (age, gender, education level, and marital status), lifestyle information (physical activity, smoking, and alcohol consumption), and medical records were obtained using structured questionnaires in in-person interviews. In physical examinations, we measured participants’ weight, height, waist circumference (WC), and blood pressure (BP) according to established and standardized procedures, as previously described [24]. Body mass index (BMI) was defined as weight divided by height squared (Kg/m2). Systolic and diastolic blood pressure (SBP and DBP) were assessed using an electronic sphygmomanometer (HEM-770AFuzzy, Omron). Overnight fasting blood samples were collected to measure biochemical parameters, including the levels of FPG, total cholesterol (TC), TG, HDL-C, and low-density lipoprotein cholesterol (LDL-C) using an automatic biochemical analyzer (HITACHI, model 7060, Tokyo). TG/HDL-C and TyG were defined as TG (mmol/L) divided by HDL-C (mmol/L) and Ln [fasting TG (mg/dL) *FPG (mg/dL)/2], respectively [26].
Ascertainment of arterial stiffness
The baPWV and ABI were tested simultaneously by trained investigators using an arteriosclerosis device (BP-203 RPE III, Omron). After participants had been resting for over 5 min in the supine position, the pressure waveform and transmission distance bilateral brachial and ankle arteries were automatically recorded by 4 cuffs with volume pulse and oscillating pressure sensors. We calculated baPWV values by the formula (La-Lb)/Tba. La and Lb are defined as the length from the heart to the ankle and brachium, and Tba is the transit time between the ankle and brachial waveforms. Accordingly, ABI was determined as ankle SBP divided by brachial SBP. As the reliability of baPWV values may be diminished in participants with severe atherosclerosis [27], we excluded participants with bilateral ABIs < 0.9. The contralateral baPWV was taken if individuals had unilateral ABI < 0.9, and the higher baPWV values of the right and left sides were considered if no ABI < 0.9. Additionally, we defined arterial stiffness as baPWV value ≥ 1800 cm/s [28].
Statistical analysis
The characteristics of study populations were described as frequency (percentage) for categorical variables and median (interquartile range) for continuous variables. The differences in participants without or with arterial stiffness were compared by the Kruskal-Wallis or χ2 test.
Logistic regression was used to assess relationships of TG/HDL-C and TyG with arterial stiffness, while linear trends were assessed by applying the median values of quartiles as continuous variables. Linear regression was used to assess associations of TG/HDL-C and TyG with baPWV levels, with beta coefficient (β) and 95% CI. Given possible confounders, we examined three models: model 1 included age and gender; model 2 included model 1 plus marital status, education level, smoking, alcohol drinking, and physical activity; and model 3 included model 2 plus BMI, WC, SBP, DBP. Subgroup analyses were conducted for age (< 65 and ≥ 65 years), sex (men and women), smoking (never and ever/current), alcohol drinking (no and yes), and BMI (< 24 and ≥ 24 Kg/m2). We further explored the interaction effects of blood pressure and fasting blood glucose levels with TG/HDL-C and TyG for arterial stiffness. Sensitivity analyses were conducted with the standard for arterial stiffness of 1400 cm/s. Additionally, we explored the relationships between TG/HDL-C and TyG with healthy vascular aging (HVA), normal vascular aging (NVA), and early vascular aging (EVA), defined as < baPWV-percentile < 10th, 10th ≤ baPWV-percentile ≤ 90th, and baPWV-percentile > 90th of the population stratified by age and sex [29].
SAS v9.4 was taken for all analyses (SAS Institute Inc., Cary, NC, USA), with a two-sided P value < 0.05 considered statistically significant.
Dose-response meta-analysis
Search strategy
We comprehensively searched PubMed, EMBASE and Web of Science up to August 26, 2023 for all relevant records in the English-language, using combinations of MeSH and free-text terms. Detailed search strategies are shown in Supplemental Table 5. Extra eligible publications were manually screened for the bibliographical references.
This dose-response meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [30]. The registration number on the International Prospective Register of Systematic Reviews (PROSPERO) is CRD42022325395.
Study selection
We included studies if: (1) they were cross-sectional, case-control, or cohort studies; (2) their populations were aged ≥ 18; (3) the exposure included TG/HDL-C or TyG index; (4) the outcome included arterial stiffness (defined as PWV) or PWV levels; and (4) they provided quantitative estimates and 95% CIs or standard errors (or relevant data to compute these). If multiple publications derived from the same study, the included data was taken from studies with the largest sample size or the most informative report. Reviews, editorials, and letters were excluded. In addition, two authors (W.Z. and Y.K.) separately searched relevant literature, reviewed titles, and abstracts, and screened full texts using the same selection criteria.
Data extraction and quality assessment
Two authors (W.Z. and Y.K.) separately collected information regarding first author, publication year, country, study design, sex, age, study size, number of cases, follow-up years if cohort studies, measurement of exposure, assessment of outcome, confounding factors, and most adjusted estimates (ORs, risk ratios [RRs], hazard ratios [HRs], or β) with 95% CIs. If a study presented both central (carotid-femoral or aortic pulse wave velocity [cfPWV or aPWV]) and peripheral measures (baPWV), we extracted results of central measures, in accordance with the gold standard approach for evaluating arterial stiffness [31].
The Agency for Healthcare Research and Quality scale (AHRQ), including 11 aspects with three answers (0, no or unclear; 1, yes), was taken to evaluate the quality of cross-sectional studies [32]. Studies ware categorized as poor (0–3), general (4–7), or high quality (8–11), respectively [33]. The Newcastle-Ottawa Scale (NOS) was applied to assess the quality of cohort studies with scores ranging from 0 to 9 across 8 items [34]. Any uncertainty was resolved in consultation with the third reviewer (D.H.).
Data synthesis and analysis
Our effect estimates incorporated both binary data (arterial stiffness) and continuous data (PWV levels) in this meta-analysis. ORs and β values with corresponding 95% CIs were taken as general effect measurements for associations of TG/HDL-C and TyG with arterial stiffness risk and PWV levels. We assumed that the RRs/HRs given in eligible records were about equal to ORs [35]. Any results in original articles that stratified by different gender or exposure types were regarded as independent studies. If the number of cases in each group was not published, we determined it via the provided RRs/HRs and the count of total cases [36]. When data on exposed subjects or person-years in a category was missing, we assumed it to be equal in size [36]. The midpoint was taken as the mean TG/HDL-C or TyG if the exposure category was reported as a range [36]. If the lowest or highest category was open-ended, the range was assumed to be the same width as the nearest group, and the midpoint was determined accordingly [37].
Heterogeneity was evaluated through Cochran’s Q and I2 statistics [38]. For the Q statistic, P < 0.1 was considered as statistical significance. Regarding I2 statistics, I2 values of 75%, 50%, and 25% were taken to be high, general, and poor heterogeneity, respectively [38]. Fixed-effects models were selected to pool ORs or β values with 95% CIs if I2 < 50%; otherwise, random-effects models were applied. Further, we conducted generalized least-squares regression to evaluate dose-response relationships [39]. We also explored potential linear or nonlinear trends through modelling TG/HDL-C and TyG with restricted cubic splines, with 3 knots located at the 75th, 50th, and 25th percentiles of distribution [40]. Only studies with at least 3 categories of TG/HDL-C or TyG were included in the dose-response analysis.
Subgroup analyses were performed between sex (men, women, and both), average age (≤ 50 and > 50 years), region (Asia, and Europe/United States [US]), sample size (≤ 1000 and > 1000), study design (cross-sectional and cohort studies), study quality (high and medium quality), and PWV assessment site (baPWV, cfPWV, and aPWV), then adjusted for some major potential confounders (BMI, smoking, alcohol drinking, FPG, and SBP) in the study-specific dose-response analysis. Moreover, we conducted meta-regression analyses among different subgroups [41], and performed sensitivity analyses through excluding one study at a time. If there were 8 or more available studies, publication bias (small-study effect) was examined with the Egger’s test and funnel plots [42]. The trim and fill method was employed for adjustment when publication bias was found.
Stata 14.0 was taken for all analyses (Stata Corp, College Station, TX, USA). Statistical significance was defined as two-sided P < 0.05.
Results
Cross-sectional study
The characteristics of our study’s populations, without and with arterial stiffness, are shown in Table 1. Significant differences were found in the distributions of age, marital status, alcohol consumption, physical activity, BMI, SBP, DBP, FPG, TG, TyG index, and baPWV (all P < 0.05).
We further observed positive and linear associations of TG/HDL-C and TyG with arterial stiffness in the present study (Table 2). In the multivariable adjusted model 3, the ORs (95% CIs) for arterial stiffness across TG/HDL-C quartiles 1, 2, 3, 4 were 1.00 (reference), 1.43 (0.95–2.15), 1.70 (1.13–2.55), and 1.81 (1.18–2.78) (Ptrend = 0.005), respectively, while the ORs (95% CIs) for arterial stiffness were 1.00 (reference), 1.57 (1.04–2.37), 1.91 (1.25–2.92), and 2.73 (1.75–4.26) across TyG quartiles (Ptrend < 0.001), respectively. Similarly, the ORs (95% CIs) for arterial stiffness with per 1 unit increment in TG/HDL-C and TyG were 1.12 (1.01–1.23) and 1.78 (1.38–2.30) in model 3, respectively. Additionally, multivariable linear regressions illustrated that per 1 unit increment in TG/HDL-C and TyG were related to 0.11 (0.03–0.19) and 0.58 (0.39–0.79) m/s increase in baPWV. When subjects were stratified by potential risk factors, consistent results were observed (Fig. 1). As shown in Supplemental Table 2, no significant interaction was discovered between TG/HDL-C and arterial stiffness (all Pinteraction > 0.05), but we found a significant interaction between SBP and TyG for arterial stiffness (Pinteraction = 0.025).
In addition, with the standard for arterial stiffness of 1400 cm/s, the results were consistent with the main results (Supplemental Table 1). The relationships of TG/HDL-C and TyG with HVA, NVA and EVA were similar to those of arterial stiffness (Supplemental Tables 3 and 4).
Dose-response meta-analysis
Literature search and study characteristics
Our initial search found 6343 relevant records. Of those, 22 articles (comprising 40 studies) were selected for meta-analysis [14,15,16,17,18,19,20,21,22, 25, 29, 43,44,45,46,47,48,49,50,51,52,53]. Finally, this meta-analysis included 41 eligible studies, including the current study. The selection and exclusion details are illustrated in a flow diagram (Supplemental Fig. 2).
Details of included articles are provided in Supplemental Table 5. Overall, we included 18 cross-sectional and 5 cohort study articles, comprising 66,676 individuals. Of those, 38 studies assessed the associations between surrogate estimates of IR (16 for TG/HDL-C and 22 for TyG) and arterial stiffness risk [15,16,17,18,19,20,21,22, 25, 29, 43,44,45,46, 48,49,50,51,52], while 15 examined the associations of surrogate estimates of IR with PWV levels [14, 17, 22, 25, 29, 47, 49, 51, 53], including 5 for TG/HDL-C and 10 for TyG. The mean AHRQ of cross-sectional studies was 7.8 (Supplemental Table 7), and the average NOS score of cohort studies was 8.4 (Supplemental Table 8).
TG/HDL-C and arterial stiffness risk
Our meta-analysis included 9 studies exploring the correlation of arterial stiffness with the highest versus lowest TG/HDL-C levels [15, 16, 20, 43, 44]. The summary OR of the highest group was 1.54 (95% CI 1.32–1.80, I2 = 42.0%, Pheterogeneity = 0.087; Fig. 2). When conducting the sensitivity analysis, the pooled OR was robust. No publication bias was revealed by the Egger’s tests (P = 0.051) and funnel plots (Supplemental Fig. 3A).
Moreover, we discovered a positive linear relationship between TG/HDL-C and arterial stiffness risk [15, 16, 19,20,21, 29, 43,44,45] (Pnon−linear = 0.376; Fig. 3A). With per 1 unit increase in TG/HDL-C, arterial stiffness risk increased by 26% (OR 1.26, 95% CI 1.14–1.39, I2 = 61.8%, Pheterogeneity = 0.002; Fig. 4). With sensitivity analysis, no individual studies altered the summary OR significantly. Publication bias was revealed through the Egger’s tests (P = 0.001) and funnel plots (Supplemental Fig. 4A). After adjusting for publication bias, the summary finding was robust (OR 1.16, 95% CI 1.05–1.28).
Additionally, TG/HDL-C was positively related to PWV (β 0.09, 95% CI 0.04–0.14, I2 = 38.4%, Pheterogeneity = 0.165; Supplemental Fig. 4) [14, 29, 47].
TyG and arterial stiffness risk
The meta-analysis included 13 studies to evaluate associations of arterial stiffness with the highest versus lowest TyG [16,17,18, 22, 25, 46, 49, 51, 52]. The pooled OR of the highest TyG level was 1.86 (95% CI 1.53–2.25, I2 = 73.7%, Pheterogeneity < 0.001; Fig. 2). With sensitivity analysis, the summary results were consistent with original estimates. The Egger’s tests (P = 0.111) and funnel plots did not reveal publication bias (Supplemental Fig. 4B).
Further, with 21 records included in the dose-response meta-analysis, we discovered a positive linear relationship of TyG with arterial stiffness risk [16, 17, 19, 22, 25, 29, 46, 48,49,50,51,52] (Pnon−linear = 0.289; Fig. 3B). With per 1 unit increase of TyG, the pooled arterial stiffness risk increased by 57% (OR 1.57, 95% CI 1.36–1.82, I2 = 94.1%, Pheterogeneity < 0.001; Fig. 4). With sensitivity analysis, the size and direction of pooled results also remained similar. The Egger’s tests (P < 0.001) and funnel plots all detected publication bias (Supplemental Fig. 4B). After adjustment, the major findings were not affected substantially (OR 1.10, 95% CI 0.97–1.26).
Moreover, TyG was positively related to PWV levels with the pooled β value of 0.57 (95% CI 0.35–0.78, I2 = 97.2%, Pheterogeneity < 0.001; Supplemental Fig. 5) [14, 17, 22, 25, 29, 49, 51, 53].
Subgroup analysis
Given the restricted number of studies, subgroup analysis was only performed between TG/HDL-C and TyG and arterial stiffness in the dose-response analysis. The overall findings of subgroup analyses confirmed that the main findings were robust, though we discovered potential sources of heterogeneity (Supplemental Table 9). For TG/HDL-C, we found that age, study quality, and an adjusted variable (FPG) may be sources of heterogeneity (all P < 0.05) by univariable meta-regression. For TyG, the heterogeneity was reduced among subgroups stratified by gender, sample size, and an adjustment factor (SBP), all of which may be potential sources of heterogeneity (all P < 0.05).
Subgroup analysis of associations between TG/HDL-C and TyG index (per 1 unit increment) and arterial stiffness Data are shown as OR (95% CI). Note: Adjusted for age, gender, marital status, education, smoking, alcohol drinking, physical activity, body mass index, waist circumference, systolic and diastolic blood pressure, total cholesterol, a for TG/HDL-C, b for TyG index
Discussion
In the cross-sectional analysis, both TG/HDL-C and TyG index were positively correlated with arterial stiffness and PWV levels. The validity and generalizability of the findings were confirmed by the subsequent meta-analysis, indicating TG/HDL-C and TyG could be reliable predictors of arterial stiffness.
Although the correlations of IR with arterial stiffness have been discussed for decades, the relationships of IR with arterial stiffness are still being debated. Several studies have illustrated that TG/HDL-C and TyG are related to arterial stiffness [17,18,19]. We observed consistent positive and linear associations between TG/HDL-C and TyG and arterial stiffness. Meanwhile, significant interaction between SBP and TyG for arterial stiffness was observed, which is consistent with a recent meta-analysis [54].
Our meta-analysis included 40 studies, along with the current study, to assess associations of TG/HDL-C and TyG with arterial stiffness. The results suggested that TG/HDL-C and TyG were positively related to arterial stiffness risk and PWV levels, in line with previous reviews [23, 55]. Moreover, this meta-analysis revealed linear associations of TG/HDL-C and TyG with arterial stiffness risk, reinforcing the validity and generalizability of our findings.
To investigate the source of heterogeneity, a series of subgroup analyses were conducted in the dose-response analysis. Despite the high heterogeneity, the findings of subgroup and sensitivity analyses generally supported our major results. For TG/HDL-C, the heterogeneity may be due to variations in age, study quality, and a confounding factor (FPG). The study of Ungvari et al. found that age-induced pathological alterations in vascular structure and function may result in the genesis of arterial stiffness [56]. Moreover, it is well-documented that diabetes, measured by FPG, plays a crucial role in increasing arterial stiffness risk [57]. Regarding TyG, the high heterogeneity might result from differences in sex, sample size, and an adjusted variable (SBP). It seems reasonable to assume that sex-related discrepancies in hormonal status and adipose tissue distribution could explain the differences between sexes [58, 59]. The difference in SBP may be explained by SBP being associated with progression of arterial stiffness [54].
Several biological mechanisms may explain relationships of TG/HDL-C and TyG with arterial stiffness and PWV levels. To date, emerging studies have demonstrated that both excessive activation of insulin receptor and vascular mineralocorticoid receptors (MR), as occurs in a state of IR, are now recognized as playing a critical role in increasing Na+ channel (EnNaC) activity and expression via mTOR and SGK-1-dependent mechanisms [60]. Further, current data from epidemiological and experimental studies support the notion that over-activation of the EnNaC is associated with a series of negative consequences, including decreased NO bio-availability [61, 62], endothelial cell stiffening [63, 64], impaired vasodilator function [65, 66], oxidative stress, and stimulation of an inflammatory environment [62, 64], all of which contribute to the genesis of vascular fibrosis and stiffness [55, 60, 62,63,64]. In addition, IR may directly lead to vascular endothelial dysfunction [67], which has been implicated in the pathogenesis of arterial stiffness [55].
The current study has some strengths. First, the study provided the most up-to-date pooled estimates, and it performed dose-response analysis. Second, the study combined cross-sectional study with dose response meta-analysis, which provided more robust evidence. Moreover, we found positive linear associations which support the finding that higher TG/HDL-C and TyG are related to increased arterial stiffness risk. Finally, this study was conducted with TG/HDL-C and TyG analyzed separately as categorical variables and continuous variables, with similar results observed, further validating the stability of our main findings.
The present study has certain limitations, however, that should be acknowledged when interpreting its results. First, although baPWV was the commonly-used measure of arterial stiffness due to its simplicity and non-invasiveness, it could be affected by stiffness of peripheral arteries, making it less effective [68]. Second, although our findings indicated a more profound relationship between the TyG index and arterial stiffness compared to TG/HDL-C, the difference was not significant. Further research is needed to explore the potential of this finding. Third, although medication may be a confounding factor, we failed to adjust for it due to a number of missing values in medication history. The relationship can be clarified in a future study. Fourth, several studies were based on populations with diabetes or hypertension, which may be another cause of potential bias, although results of subgroup analysis were robust. Eventually, there was evidence of heterogeneity or publication bias; however, the overall findings were unaffected significantly after the trim and fill adjustment. In light of the above factors, more relevant studies are needed to clarify the relationships of TG/HDL-C and TyG with arterial stiffness risk and PWV levels.
Conclusion
Taken together, our results indicate that TG/HDL-C and TyG may be reliable predictors of arterial stiffness risk, while TyG is positively associated with PWV levels. Given the convenience of measuring TG/HDL-C and TyG in clinical settings, further research should focus on determining whether inclusion of TG/HDL-C and TyG measures can improve the efficacy of current arterial stiffness prediction tools.
Highlights.
-
1.
1. TG/HDL-C and TyG index were positively associated with risk of arterial stiffness and PWV levels.
-
2.
2. TG/HDL-C and TyG index may be convincing predictors of arterial stiffness that could be used in clinical practice.
-
3.
3. This study refined and expanded upon findings of associations of TG/HDL-C and TyG index with arterial stiffness risk.
Data availability
No datasets were generated or analysed during the current study.
References
Global burden. Of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, et al. Global Burden of Cardiovascular diseases and Risk factors, 1990–2019: Update from the GBD 2019 study. J Am Coll Cardiol. 2020;76:2982–3021.
Chirinos JA, Segers P, Hughes T, Townsend R. Large-artery stiffness in Health and Disease: JACC State-of-the-art review. J Am Coll Cardiol. 2019;74:1237–63.
Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605.
Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27.
Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–11.
Sheng CS, Li Y, Li LH, Huang QF, Zeng WF, Kang YY, Zhang L, Liu M, Wei FF, Li GL, et al. Brachial-ankle pulse wave velocity as a predictor of mortality in elderly Chinese. Hypertension. 2014;64:1124–30.
Lee SH, Park SY, Choi CS. Insulin resistance: from mechanisms to therapeutic strategies. Diabetes Metab J. 2022;46:15–37.
Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–85.
Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112:2193–200.
Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293–302.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26.
Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. 2014;13:146.
Wu Z, Zhou D, Liu Y, Li Z, Wang J, Han Z, Miao X, Liu X, Li X, Wang W, et al. Association of TyG index and TG/HDL-C ratio with arterial stiffness progression in a non-normotensive population. Cardiovasc Diabetol. 2021;20:134.
Chung TH, Shim JY, Kwon YJ, Lee YJ. High triglyceride to high-density lipoprotein cholesterol ratio and arterial stiffness in postmenopausal Korean women. J Clin Hypertens (Greenwich). 2019;21:399–404.
Poon AK, Meyer ML, Tanaka H, Selvin E, Pankow J, Zeng D, Loehr L, Knowles JW, Rosamond W, Heiss G. Association of insulin resistance, from mid-life to late-life, with aortic stiffness in late-life: the atherosclerosis risk in communities Study. Cardiovasc Diabetol. 2020;19:11.
Li M, Zhan A, Huang X, Hu L, Zhou W, Wang T, Zhu L, Bao H, Cheng X. Positive association between triglyceride glucose index and arterial stiffness in hypertensive patients: the China H-type hypertension Registry Study. Cardiovasc Diabetol. 2020;19:139.
Zhao S, Yu S, Chi C, Fan X, Tang J, Ji H, Teliewubai J, Zhang Y, Xu Y. Association between macro- and microvascular damage and the triglyceride glucose index in community-dwelling elderly individuals: the Northern Shanghai Study. Cardiovasc Diabetol. 2019;18:95.
Nakagomi A, Sunami Y, Kawasaki Y, Fujisawa T, Kobayashi Y. Sex difference in the association between surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complications. 2020;34:107442.
Zhao W, Gong W, Wu N, Li Y, Ye K, Lu B, Zhang Z, Qu S, Li Y, Yang Y, Hu R. Association of lipid profiles and the ratios with arterial stiffness in middle-aged and elderly Chinese. Lipids Health Dis. 2014;13:37.
Kılıç A, Baydar O, Elçik D, Apaydın Z, Can MM. Role of dyslipidemia in early vascular aging syndrome. Turk J Med Sci. 2021;51:727–34.
Su Y, Wang S, Sun J, Zhang Y, Ma S, Li M, Zhang A, Cheng B, Cai S, Bao Q, Zhu P. Triglyceride glucose Index Associated with arterial stiffness in Chinese Community-Dwelling Elderly. Front Cardiovasc Med. 2021;8:737899.
Mazidi M. Surrogate markers of insulin resistance and arterial stiffness. J Diabetes Complications. 2020;34:107491.
Zhang M, Zhao Y, Sun L, Xi Y, Zhang W, Lu J, Hu F, Shi X, Hu D. Cohort Profile: the rural Chinese cohort study. Int J Epidemiol. 2021;50:723–l724.
Wu S, Xu L, Wu M, Chen S, Wang Y, Tian Y. Association between triglyceride-glucose index and risk of arterial stiffness: a cohort study. Cardiovasc Diabetol. 2021;20:146.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Lu Y, Pechlaner R, Cai J, Yuan H, Huang Z, Yang G, Wang J, Chen Z, Kiechl S, Xu Q. Trajectories of age-related arterial stiffness in Chinese men and women. J Am Coll Cardiol. 2020;75:870–80.
Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, et al. The relationship of brachial-ankle pulse wave velocity to future cardiovascular disease events in the general Japanese population: the Takashima Study. J Hum Hypertens. 2014;28:323–7.
Gómez-Sánchez L, Gómez-Sánchez M, Lugones-Sánchez C, Tamayo-Morales O, González-Sánchez S, Rodríguez-Sánchez E, García-Ortiz L, Gómez-Marcos MA. On Behalf of the Eva I: Association of Insulin Resistance with vascular ageing in a general caucasian Population: an EVA Study. J Clin Med 2021, 10.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8.
Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, Niu Y, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8:2–10.
Hu J, Dong Y, Chen X, Liu Y, Ma D, Liu X, Zheng R, Mao X, Chen T, He W. Prevalence of suicide attempts among Chinese adolescents: a meta-analysis of cross-sectional studies. Compr Psychiatry. 2015;61:78–89.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175:66–73.
Bekkering GE, Harris RJ, Thomas S, Mayer AM, Beynon R, Ness AR, Harbord RM, Bain C, Smith GD, Sterne JA. How much of the data published in observational studies of the association between diet and prostate or bladder cancer is usable for meta-analysis? Am J Epidemiol. 2008;167:1017–26.
Jin X, Chen H, Shi H, Fu K, Li J, Tian L, Teng W. Lipid levels and the risk of hemorrhagic stroke: a dose-response meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31:23–35.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9.
Greenland S. Dose-response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–65.
Santiago de Araújo Pio C, Marzolini S, Pakosh M, Grace SL. Effect of Cardiac Rehabilitation Dose on Mortality and Morbidity: a systematic review and Meta-regression analysis. Mayo Clin Proc. 2017;92:1644–59.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Wen JH, Zhong YY, Wen ZG, Kuang CQ, Liao JR, Chen LH, Wang PS, Wu YX, Ouyang CJ, Chen ZJ. Triglyceride to HDL-C ratio and increased arterial stiffness in apparently healthy individuals. Int J Clin Exp Med. 2015;8:4342–8.
Wen J, Zhong Y, Kuang C, Liao J, Chen Z, Yang Q. Lipoprotein ratios are better than conventional lipid parameters in predicting arterial stiffness in young men. J Clin Hypertens (Greenwich). 2017;19:771–6.
Li G, Wu HK, Wu XW, Cao Z, Tu YC, Ma Y, Wang WQ, Cheng J, Zhou ZH. Small dense low density lipoprotein-cholesterol and cholesterol ratios to predict arterial stiffness progression in normotensive subjects over a 5-year period. Lipids Health Dis. 2018;17:27.
Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17:41.
Chen C, Dai JL. Triglyceride to high-density lipoprotein cholesterol (HDL-C) ratio and arterial stiffness in Japanese population: a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018;17:130.
Guo W, Zhu W, Wu J, Li X, Lu J, Qin P, Zhu C, Xu N, Zhang Q. Triglyceride glucose index is Associated with arterial stiffness and 10-Year Cardiovascular Disease Risk in a Chinese Population. Front Cardiovasc Med. 2021;8:585776.
Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, Hong J, Zhang Y, Wang W. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20:82.
Pan Y, Zhong S, Zhou K, Tian Z, Chen F, Liu Z, Geng Z, Li S, Huang R, Wang H et al. Association between Diabetes Complications and the Triglyceride-Glucose Index in Hospitalized Patients with Type 2 Diabetes. J Diabetes Res 2021, 2021:8757996.
Ji W, Gao L, Sun P, Jia J, Li J, Wang X, Fan F, Zhang Y. Association of the triglyceride-glucose index and vascular target organ damage in a Beijing community-based population. Front Cardiovasc Med. 2022;9:948402.
Yang X, Gao Z, Huang X, Zhang M, Chen Z. The correlation of atherosclerosis and triglyceride glucose index: a secondary analysis of a national cross-sectional study of Japanese. BMC Cardiovasc Disord. 2022;22:250.
Muhammad IF, Bao X, Nilsson PM, Zaigham S. Triglyceride-glucose (TyG) index is a predictor of arterial stiffness, incidence of diabetes, cardiovascular disease, and all-cause and cardiovascular mortality: a longitudinal two-cohort analysis. Front Cardiovasc Med. 2022;9:1035105.
Wilson J, Webb AJS. Systolic blood pressure and longitudinal progression of arterial stiffness: a quantitative Meta-analysis. J Am Heart Assoc. 2020;9:e017804.
Hill MA, Yang Y, Zhang L, Sun Z, Jia G, Parrish AR, Sowers JR. Insulin resistance, cardiovascular stiffening and cardiovascular disease. Metabolism. 2021;119:154766.
Ungvari Z, Tarantini S, Sorond F, Merkely B, Csiszar A. Mechanisms of vascular aging, a geroscience perspective: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:931–41.
Piché ME, Tchernof A, Després JP. Obesity phenotypes, diabetes, and Cardiovascular diseases. Circ Res. 2020;126:1477–500.
DuPont JJ, Kenney RM, Patel AR, Jaffe IZ. Sex differences in mechanisms of arterial stiffness. Br J Pharmacol. 2019;176:4208–25.
van den Munckhof ICL, Holewijn S, de Graaf J, Rutten JHW. Sex differences in fat distribution influence the association between BMI and arterial stiffness. J Hypertens. 2017;35:1219–25.
Hill MA, Jaisser F, Sowers JR. Role of the vascular endothelial sodium channel activation in the genesis of pathologically increased cardiovascular stiffness. Cardiovasc Res. 2022;118:130–40.
Sowers JR, Habibi J, Aroor AR, Yang Y, Lastra G, Hill MA, Whaley-Connell A, Jaisser F, Jia G. Epithelial sodium channels in endothelial cells mediate diet-induced endothelium stiffness and impaired vascular relaxation in obese female mice. Metabolism. 2019;99:57–66.
Jia G, Habibi J, Aroor AR, Hill MA, Yang Y, Whaley-Connell A, Jaisser F, Sowers JR. Epithelial Sodium Channel in Aldosterone-Induced Endothelium stiffness and aortic dysfunction. Hypertension. 2018;72:731–8.
Jeggle P, Callies C, Tarjus A, Fassot C, Fels J, Oberleithner H, Jaisser F, Kusche-Vihrog K. Epithelial sodium channel stiffens the vascular endothelium in vitro and in Liddle mice. Hypertension. 2013;61:1053–9.
Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, et al. Endothelial mineralocorticoid receptor mediates Diet-Induced aortic stiffness in females. Circ Res. 2016;118:935–43.
Tarjus A, Maase M, Jeggle P, Martinez-Martinez E, Fassot C, Loufrani L, Henrion D, Hansen PBL, Kusche-Vihrog K, Jaisser F. The endothelial αENaC contributes to vascular endothelial function in vivo. PLoS ONE. 2017;12:e0185319.
Sternak M, Bar A, Adamski MG, Mohaissen T, Marczyk B, Kieronska A, Stojak M, Kus K, Tarjus A, Jaisser F, Chlopicki S. The deletion of endothelial Sodium Channel α (αENaC) impairs endothelium-dependent Vasodilation and Endothelial Barrier Integrity in Endotoxemia in vivo. Front Pharmacol. 2018;9:178.
Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–68.
Miyata M. Noninvasive Assessment of arterial stiffness using oscillometric methods: baPWV, CAVI, API, and AVI. J Atheroscler Thromb. 2018;25:790–1.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 82073646, 81973152, 82103940, 82273707 and 82304228), the Postdoctoral Research Foundation of China (grant no. 2021M692903), the Key R & D and promotion projects in Henan Province (grant no.232102311017), Guangdong Basic and Applied Basic Research Foundation (grant nos. 2021A1515012503, and 2022A1515010503), and the Shenzhen Science and Technology Program (grant nos. JCYJ20210324093612032 and JCYJ20220818095818040).
Author information
Authors and Affiliations
Contributions
W.Z. D.H. and Y.Z. substantially contributed to the design and drafting of the study. W.Z. analyzed the data and wrote the manuscript. W.H., H.H., T.L., L.Y., J.Z., Y.F., Y.W., X.F., Y.K., M.W., L.W., Y.C., Y.G., X.L., Y.Z., L.S., J.P.,Z.Z., F.H., M.Z., Y.L., D.H., and Y.Z. revised the manuscript critically. All authors were involved in the collection of data and approval of the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no conflicts of interest.
Original publication
The manuscript has not been published and is not being considered for publication elsewhere, in whole or in part, in any language.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, W., Huo, W., Hu, H. et al. Dose-response associations of triglyceride to high-density lipoprotein cholesterol ratio and triglyceride–glucose index with arterial stiffness risk. Lipids Health Dis 23, 115 (2024). https://doi.org/10.1186/s12944-024-02095-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-024-02095-z