Abstract
Background
Ectopic pregnancy is more common amongst assisted reproduction cycles and is a cause of significant maternal morbidity. Few predictive markers exist to help identify and modify risk of ectopic pregnancy in preparing for embryo transfer. The relationship between serum and AMH and ectopic pregnancy rate is unknown.
Methods
This was a retrospective cohort study investigating women who underwent fresh embryo transfer cycles from January 2017 to December 2019 in Peking University Third Hospital. The primary outcome was ectopic pregnancy. Restricted cubic splines with four knots for AMH concentration (0-3, 3-6, 6-12, 12-max) were used to map out the non-linear relationship between the predicted ectopic pregnancy rate and the serum AMH concentration. Log binomial regression was used to test the crude risk ratio (cRR) and the adjusted risk ratio (aRR) after adjustment for confounders with 95% confidence intervals (CI) to determine the difference across various groups.
Results
A total of 13,718 cycles in women undergoing fresh embryo transfer were eligible for analysis. The ectopic pregnancy rate was 1.3% per embryo transfer cycle initiated and 3.3% per pregnancy. Serum AMH concentrations were higher amongst women with ectopic pregnancy than in women with a confirmed intrauterine pregnancy or heterotopic pregnancy or who did not become pregnant (Mean levels: 4.0 ng/ml vs 3.2 ng/ml, 1.7 ng/ml, and 2.8 ng/ml). An AMH concentration of 7 ng/ml represented the best cut-off value to predict ectopic pregnancy. The ectopic pregnancy rate was 3.4% per cycle and 7.5% per pregnancy in women with AMH levels ≥ 7 ng/ml; and 1.2% per cycle and 2.9% per pregnancy in women with AMH levels < 7 ng/ml. Serum AMH concentration ≥ 7 ng/ml was associated with an increased risk of ectopic pregnancy in all fresh embryo transfer cycles started (aRR = 2.35 (1.45, 3.58)) as well in women who became pregnant (aRR = 2.23 (1.49, 3.33).
Conclusions
Baseline AMH concentration ≥ 7 ng/ml is associated with an increased risk of ectopic pregnancy in fresh embryo transfer cycles.
Similar content being viewed by others
Background
Assisted reproductive technology (ART) has assisted millions of couples with subfertility since its advent in 1978 [1, 2]. However, the risk of adverse outcomes, such as ectopic pregnancy, placenta previa, preterm birth, low birth weight, et.al. are significantly increased in ART pregnancies [3, 4].
Ectopic pregnancy is a life-threatening complication of early pregnancy characterized by embryo implantation outside the uterus [5]. A good understanding of the factors that increase the risk of ectopic pregnancy is essential to improving pregnancy outcomes as well as preserving future fertility [6]. Accumulating evidence suggests that ART is an independent risk factor for ectopic pregnancy, despite the embryo being transferred directly into the uterus [7, 8]. The rate of ectopic pregnancy varies from 1.4 - 3.5% in ART pregnancies, with variation across countries [7,8,9,10,11]. Risk factors for ectopic pregnancy in women undergoing in vitro fertilization (IVF) include: tubal factor infertility, excessive ovarian response, previous miscarriage or ectopic pregnancy and thin endometrium [11,12,13]. Controlled ovarian hyperstimulation in IVF is associated with an increased risk of ectopic pregnancy as compared with women undergoing natural cycles [10, 12, 14]. Furthermore, the rate of ectopic pregnancy in frozen embryo transfer cycles is much lower than in fresh embryo transfer cycles [9, 15,16,17], suggesting that the altered tubal-uterine environment caused by the ovarian stimulation contributes to abnormal implantation of the transferred embryo.
The relation between baseline biomarkers and ectopic risk are poorly described. Serum AMH is derived from pre-antral and small antral follicles in the ovary and thus serum AMH is proportional to the size of the follicular pool [18]. Recent studies suggest an association between serum AMH levels > 7 ng/ml and decreased live birth rates in fresh transfer cycles [19, 20], indicating that high AMH levels may be associated with adverse pregnancy outcomes. Previous studies suggest that increased oocyte yield was associated with a significantly increased rate of ectopic pregnancy [21, 22]. Given that serum AMH concentration is considered the gold-standard biomarker to predict oocyte yield from ovarian stimulation [23, 24], we hypothesized that serum AMH concentration might represent a better predictor for ectopic pregnancy when compared to oocyte yield. This study aimed to investigate whether higher serum AMH concentration were associated with an increase in ectopic pregnancy rates in fresh embryo transfer cycles.
Methods
This retrospective cohort study was approved by the Ethics Committee of Peking University Third Hospital. A total of 35,617 women who underwent their first IVF cycles that resulted in fresh embryo transfer in Peking University Third Hospital from January 2017 to December 2019 were eligible for this study. Freeze- all cycles, donor oocyte cycles, pre-implantation genetic test (PGT) cycles and cycles where measurement of AMH was omitted or no embryo was available for transfer were excluded for analysis. We also excluded cycles where the stage of embryo development at transfer and the number of embryos transferred could not be determined.
The IVF protocol in our center has been described in previous studies [19]. Briefly, follicular stimulating hormone (FSH) (Gonal-F; Serono, Geneva, Switzerland) and/or human menopausal gonadotrophin (HMG) (Pergonal; Serono) was used for ovarian stimulation in conjunction gonadotrophin-releasing hormone (GnRH) agonist (GnRH-a, Decapeptyl; Ferring, Lausanne, Switzerland) or GnRH antagonist (Centrotide; Serono) to prevent ovulation. A dose of 5000-10,000 U of human chorionic gonadotrophin (HCG, Livzon, China) was administered when two or more follicles reached 18-mm mean diameter. Oocytes were transvaginally collected under ultrasound guidance 36-38 h after HCG administration. Conventional IVF or intracytoplasmic sperm injection (ICSI) was used for fertilization and up to two embryos were transferred at the cleavage stage or blastocyst stage. Thin endometrium was diagnosed where the endometrial thickness was at or less than 7 mm on the day of trigger. Embryo quality was assessed by a well-trained embryologist according to previously described criteria [25].
All women had a baseline serum AMH measurement by an ultrasensitive two-site ELISA (AnshLabs, Webster, TX, USA) as previously described [24]. All AMH measurements were taken within 6 months of commencing a treatment cycle. Serum estradiol concentration was measured on the day of trigger (Siemens Immulite 2000 immunoassay system, Siemens Healthcare Diagnostics, Shanghai, P. R. China). The inter-assay and intra-assay coefficients of variation were less than 8% and less than 10% for AMH and estradiol concentrations respectively [24].
Ectopic pregnancy was defined as the implantation of an embryo at any site other within the endometrium and was diagnosed on transvaginal ultrasound scan or at laparoscopy. Intrauterine pregnancy was defined by the presence of gestational sac(s) on ultrasound at around 7 weeks gestation with the detection of heartbeat activity within the uterine. Heterotopic pregnancy was diagnosed when at least one embryo was found to have implanted in the uterine cavity and at least one embryos was simultanesouly implanted outside of the uterine cavity. Clinical pregnancy included ectopic pregnancy, heterotopic pregnancy, and intrauterine pregnancy in this study.
An ANOVA test or Chi-square test was used to compare variables between groups as appropriate. Log binomial regression was used to test the crude risk ratio (cRR) and the adjusted risk ratio (aRR) after adjustment for confounders with 95% confidence intervals (CI). The linear relationship between AMH concentration and the risk of ectopic pregnancy was also analyzed using log binomial regression. Directed Acyclic Graphs (DAGs) were used to identify potential confounders in the multivariable analysis [26,27,28] (Supplemental Fig. 1). Restricted cubic splines with four knots for AMH levels (0-3, 3-6, 6-12, 12-max) were used to map out the non-linear relationship between the predicted ectopic pregnancy rate and the serum AMH level as a continuous variable before and after adjustment for covariates [29]. We then stratified women into groups by AMH level to further confirm the results from the restricted cubic splines. Stratification and the restricted cubic splines were used to determine the best cut-off value for AMH concentration to predict ectopic pregnancy. Female age, BMI, primary infertility, parity, ectopic history, infertility diagnosis (tubal factor, ovulatory dysfunction) were considered to be confounders and were adjusted in Model 1. In Model 2, gonadotrophin (Gn) dose, number of retrieved oocytes, peak serum estradiol concentraction, endometrial thickness, cycle type (antagonist), fertilization with intracytoplasmic sperm injection (ICSI), double embryo transfer, blastocyst transfer and the availability of at least one poor-quality embryo for transfer were added to the list of cavariates in Model 1 to further detect potential risk factors for ectopic pregnancy. We first analyzed the association of AMH and ectopic pregnancy in women undergoing a first embryo transfer cycle. We then examined the association between AMH and ectopic pregnancy in women who achieved a clinical pregnancy. Heterotopic pregnancy was excluded in the primary analysis but included in sensitivity analysis. All statistical analysis was performed using Stata version 15.1 (StataCorp LLC, Texas, USA). A p value < 0.05 was considered to represent a statistically significant difference.
Results
Baseline characteristics of women in fresh embryo transfer cycles
A total of 13,718 fresh embryo transfer cycles were eligible for analysis. Of these, 8144 did not result in pregnancy, 5378 resulted in an intrauterine pregnancy, 182 resulted in an ectopic pregnancy and 14 heterotopic pregnancies were diagnosed (Supplemental Fig. 2). The ectopic pregnancy rate was 1.3% per embryo transfer cycle and 3.3% per pregnancy.
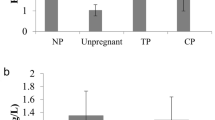
Women who had an ectopic pregnancy were more likely to have a previous ectopic pregnancy or a history of ovulatory dysfunction when compared with women who had an intrauterine pregnancy (14% vs 9, 18% vs 10%). They were also less likely to have a primary infertility diagnosis (49% ectopic pregnancy vs 57% intrauterine). Ectopic pregnancy occurred more frequently in GnRH agonist cycles (70% vs 50%). Women who resulted in an ectopic pregnancy had a thinner endometrial thickness and a higher rate of thin endometrium (10.1 mm vs 11.1 mm, 4% vs 1%). A diagnosis of male factor infertility and ICSI fertilization cycles were less common amongst women with an ectopic pregnancy (43% vs 51, 23% vs 33%). The total Gn dose and days of stimulation were lower in women with an ectopic pregnancy (2556 IU vs 2773 IU, 10.9 days vs 11.4 days). Male and female age, BMI, duration of infertility, number of retrieved oocytes, peak serum estradiol concentration, parity, endometriosis or tubal factor infertility diagnoses, donor sperm cycles, blastocyst transfer, double embryo transfer and the availability of at least one poor-quality embryo for transfer were not significantly different between women with intrauterine pregnancy and women with ectopic pregnancy (Table 1). The mean serum AMH concentration was higher in women with transfer cycles resulting in ectopic pregnancy than in women with no resulting pregnancy, intrauterine pregnancy or heterotopic pregnancy (4.0 ng/ml vs 2.8 ng/ml, 3.2 ng/ml, and 1.7 ng/ml) (Fig. 1).
Association of serum AMH levels and ectopic pregnancy
By using restricted cubic splines to map out the non-linear relationship between the predicted ectopic pregnancy rate and the serum AMH level as a continuous variable before and after adjustment for covariates, we found a “linear” relationship between serum AMH levels and predicted ectopic pregnancy rate per transfer cycle and predicted ectopic pregnancy rate per clinical pregnancy (Supplemental Fig. 3). The association remained in the sensitivity analysis where the heterotopic pregnancy was considered as the ectopic pregnancy (Supplemental Fig. 3).
We then stratified women into ten groups according to serum AMH levels (0-0.9 ng/ml; 1.0-1.9 ng/ml; 2.0-2.9 ng/ml; 3.0-3.9 ng/ml; 4.0-4.9 ng/ml; 5.0-5.9 ng/ml; 6.0-6.9 ng/ml; 7.0-7.9 ng/ml; 8.0-9.9 ng/ml; > = 10.0 ng/ml). Ectopic pregnancy rate was increased in women with AMH levels higher than 7 ng/ml (7.0-7.9 ng/ml; 8.0-9.9 ng/ml; > = 10.0 ng/ml) (2.9, 2.9, 4.2% per cycle and 6.2, 6.2, 10.2% per pregnancy, respectively) (Fig. 2). Serum AMH concentration greater than 7 ng/ml (7.0-7.9 ng/ml; 8.0-9.9 ng/ml; > = 10.0 ng/ml) was associated with an increased rate of ectopic pregnancy as compared to serum AMH levels at the range of 0-0.9 ng/ml (aRR = 2.39 (1.09-5.21), aRR = 2.43 (1.12-5.28), aRR = 3.16 (1.56-6.42) in embryo transfer cycles; aRR = 1.67 (0.78-3.57), aRR = 1.71 (0.80-3.66), aRR = 2.54 (1.28-5.03)) in clinical pregnancies, Table 2). The results were further confirmed in the sensitivity analysis (Supplemental Table 1). Thus, AMH concentration of 7 ng/ml was considered as the best cut-off value to predict ectopic pregnancy. The ectopic pregnancy rate per transfer cycle in women with baseline AMH concentration ≥ 7 ng/ml and < 7 ng/ml was 7.5 and 3.4% per clinical pregnancy and 2.9 and 1.2% per started cycle, respectively.
We also examined the association between serum AMH concentration and ectopic pregnancy in single embryo transfer cycles. Ectopic pregnancy occurred more frequently when baseline AMH was higher than 6 ng/ml (Supplemental Fig. 4) but did not reach statistical significance due to the small case number (19 in total).
Risk factors of ectopic pregnancy
In Model 1, we demonstrated an association between serum AMH ≥ 7 ng/mL and ectopic pregnancy in fresh transfer cycles (aRR = 2.35 (1.45, 3.58)) as well as amongst clinical pregnancies (aRR = 2.23 (1.49, 3.33)) (Fig. 3, Model 1, no heterotopic pregnancy). To test whether the association was mediated by the number of collected oocytes, peak serum estradiol concentration, endometrial thickness, agonist/antagonist cycle type, Gn dose, fertilization with ICSI, embryo quality, blastocyst transfer or double embryo transfer, we added these variables in Model 2. We demonstrated that AMH concentration ≥ 7 ng/ml was still associated with an increased risk of ectopic pregnancy (transfer cycle: aRR = 2.05 (1.31, 3.20); pregnancy: aRR = 1.92 (1.25, 2.95). Figure 3, Model 2, no heterotopic pregnancy). When we included women with heterotopic pregnancy as ectopic pregnancy, the results remained in Model 1 and Model 2 (Fig. 3, with heterotopic pregnancy). The use of GnRH antagonist protocol and thin endometrium were alsorisk factors for ectopic pregnancy in women who had a clinical pregnancy (aRR = 2.00 (1.39, 2.89); aRR = 2.88 (1.35, 6.13). Figure 3, Model 2, no heterotopic pregnancy).
Risk factors for ectopic pregnancy in women who underwent fresh embryo transfer cycles and women who resulted in pregnancy. Left: heterotopic pregnancy was excluded; Right: heterotopic pregnancy was included as ectopic pregnancy. Model 1: female age, BMI, parity, primary infertility, ectopic pregnancy history, ovulatory dysfunction, tube factor and serum AMH levels ≥ 7 ng/ml were included in the regression analysis. Model 2: female age, BMI, parity, primary infertility, ectopic pregnancy history, ovulatory dysfunction, tube factor, serum AMH levels ≥ 7 ng/ml, Gn doses, the use of ICSI, blastocyst transfer, double embryo transfer, transfer of at least one poor quality embryo, the number of oocyte yeild, estradiol levels, thin endometrium, and the use of GnRH antagonist were included in the regression analysis
Discussion
This large retrospective cohort study of 13,718 fresh embryo transfer cycles demonstrated that women with baseline AMH concentration ≥ 7 ng/ml were more likely to have an ectopic pregnancy. To the best of our knowledge, this is the first study to demonstrate an association between AMH levels and ectopic pregnancy in fresh embryo transfer cycles.
AMH is a glycoprotein secreted by the granulosa cells of the pre-antral and small antral follicles in female ovaries [18, 30]. As an ovarian reserve marker, AMH is used to predict ovarian response, the impact of chemoradiotherapy on ovarian function, and the success rate from IVF [19, 30]. To date, only one type II receptor named AMHR2 has been found for AMH in humans [31]. AMHR2 is present in several organs, including the prostate, lungs, brains, and the endometrium, suggesting that the biological effects of AMH may be much broader than initially thought [32,33,34]. AMHR2 is upregulated in the endometrium of women with endometriosis when compared to women without the disease [35]. Additionally, increased AMH levels in cultured human endometrial stromal cells can negatively affect cell viability and increase cell apoptosis [33, 36]. These results strongly suggest that increased AMH levels can directly affect the physiological conditions in the endometrium, which may further affect embryo implantation. However, the reason underlying the increase in ectopic pregnancy in women with high serum AMH remains unexplained. Serum AMH is positively associated with ovarian response, which may negatively affect endometrial receptivity. Accumulating evidence suggests that ovarian stimulation can alter the gene expression profile of the endometrium and negatively affect embryo implantation [37,38,39,40,41]. Furthermore, excessive ovarian response is an independent risk factor for ectopic pregnancy [10, 12, 22]. To summarize, both the direct and indirect effects of higher AMH concentration may contribute to the increased rate of ectopic pregnancy.
Although previous studies have suggested that oocyte yield and/or peak serum estradiol are associated with a significant increase in ectopic pregnancy [21, 22], our study did not find these markers to be significantly different between women with intrauterine pregnancy and women with ectopic pregnancy. Women with the > 20 collected oocytes and/or high peak serum estradiol concentration would routinely be converted to a freeze all cycle in our center, therefore the true impact of increase in ectopic pregnancy may be underestimated. Additionally, we found that GnRH antagonist protocol and endometrial thickness were associated with higher ectopic pregnancy rates (Fig. 3). The association between ectopic pregnancy risk and thin endometrium has been previously described [42]. Recent studies suggest that serum AMH level > 7 ng/ml in a fresh transfer cycles is associated with a decrease in live birth [19, 20], therefore the relationship between AMH and adverse pregnancy outcome requires further exploration. Consistent with these studies, we demonstrated that serum AMH ≥ 7 ng/ml is associated with a significant increase in ectopic pregnancy rate, which reaches 10% per pregnancy when AMH is ≥ 10 ng/ml. Given that ectopic pregnancy poses a substantial risk of morbidity and mortality to a pregnant woman, consideration should be given to the relative risks and benefits of a freeze all cycle in these women.
The study is strengthened by the large cohort size and the 100% follow-up. Because all data came from a single center, the clinical and laboratory practices did not substantially change over the course of the study. We analysed the effect of AMH per embryo transfer cycle as well as per clinical pregnancy, which may help apply these findings to clinical decision making. We used very “small” stratifications and restricted cubic splines to determine the best cutoff of AMH concentration for ectopic pregnancy. Furthermore, we conducted the sensitivity analysis to rule out the effect from heterotopic pregnancies. Our study has several limitations. Due to the retrospective design, residual confounding factors may be neglected, such as smoking history or social-economic factors. Our dataset did not allow us to analyze other identified risk factors for ectopic pregnancy, such as pelvic infection, systematic infection, oviduct damage, volume of transfer fluid and transfer depth [8]. The results in our study should be confirmed by future prospective cohort studies. Our study found an ectopic pregnancy rate of 2.9% per pregnancy in women with baseline serum AMH levels < 7 ng/ml. Women with AMH levels < 7 ng/ml represent 92% of total pregnant women. To demonstrate a 3% higher (5.9%) ectopic pregnancy rate per pregnancy in women with AMH levels ≥ 7 ng/ml, recruitement of 348 pregnant women with AMH ≥ 7 ng/ml and 3993 pregnant women with AMH < 7 ng/ml would be required (with a power of 80% and alpha-error of 5%).
Conclusions
Baseline serum AMH concentration ≥ 7 ng/ml is associated with an increased risk of ectopic pregnancy in fresh embryo transfer cycles.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- hCG:
-
human chorionic gonadotropin
- rLH:
-
recombinant LH
- GnRH:
-
ovarian Gonadotrophin-Releasing Hormone
- FSH:
-
follicle-stimulating hormone
- LH:
-
luteinizing hormone
- ART:
-
assisted reproductive technology
- AMH:
-
anti-Müllerian hormone
- CI:
-
confidence intervals
- aRR:
-
adjusted risk ratio
- cRR:
-
crude risk ratio
- IVF:
-
in vitro fertilization
- PGT:
-
pre-implantation genetic test
- HMG:
-
human menopausal gonadotrophin
- ICSI:
-
intracytoplasmic sperm injection
- DAGs:
-
Directed Acyclic Graphs
References
Hilson D, Bruce RL, Sims DG. Successful pregnancy following in-vitro fertilisation. Lancet. 1978;2(8087):473.
Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978;2(8085):366.
Qin JB, Wang H, Sheng X, Xie Q, Gao S. Assisted reproductive technology and risk of adverse obstetric outcomes in dichorionic twin pregnancies: a systematic review and meta-analysis. Fertil Steril. 2016;105(5):1180–92.
Li C, Zhao WH, Zhu Q, Cao SJ, Ping H, Xi X, et al. Risk factors for ectopic pregnancy: a multi-center case-control study. BMC Pregnancy Childbirth. 2015;15:187.
Kirk E, Bottomley C, Bourne T. Diagnosing ectopic pregnancy and current concepts in the management of pregnancy of unknown location. Hum Reprod Update. 2014;20(2):250–61.
Bhattacharya S, McLernon DJ, Lee AJ, Bhattacharya S. Reproductive outcomes following ectopic pregnancy: register-based retrospective cohort study. PLoS Med. 2012;9(6):e1001243.
Clayton HB, Schieve LA, Peterson HB, Jamieson DJ, Reynolds MA, Wright VC. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet Gynecol. 2006;107(3):595–604.
Perkins KM, Boulet SL, Kissin DM, Jamieson DJ. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001-2011. Obstet Gynecol. 2015;125(1):70–8.
Londra L, Moreau C, Strobino D, Garcia J, Zacur H, Zhao Y. Ectopic pregnancy after in vitro fertilization: differences between fresh and frozen-thawed cycles. Fertil Steril. 2015;104(1):110–8.
Bu Z, Xiong Y, Wang K, Sun Y. Risk factors for ectopic pregnancy in assisted reproductive technology: a 6-year, single-center study. Fertil Steril. 2016;106(1):90–4.
Santos-Ribeiro S, Tournaye H, Polyzos NP. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: a 12-year nationwide analysis including 160 000 pregnancies. Hum Reprod. 2016;31(2):393–402.
Liu H, Zhang J, Wang B, Kuang Y. Effect of endometrial thickness on ectopic pregnancy in frozen embryo transfer cycles: an analysis including 17,244 pregnancy cycles. Fertil Steril. 2020;113(1):131–9.
Farquhar CM. Ectopic pregnancy. Lancet. 2005;366(9485):583–91.
Jwa SC, Seto S, Takamura M, Kuwahara A, Kajihara T, Ishihara O. Ovarian stimulation increases the risk of ectopic pregnancy for fresh embryo transfers: an analysis of 68,851 clinical pregnancies from the Japanese assisted reproductive technology registry. Fertil Steril. 2020;114(6):1198–206.
Shapiro BS, Daneshmand ST, De Leon L, Garner FC, Aguirre M, Hudson C. Frozen-thawed embryo transfer is associated with a significantly reduced incidence of ectopic pregnancy. Fertil Steril. 2012;98(6):1490–4.
Ishihara O, Kuwahara A, Saitoh H. Frozen-thawed blastocyst transfer reduces ectopic pregnancy risk: an analysis of single embryo transfer cycles in Japan. Fertil Steril. 2011;95(6):1966–9.
Decleer W, Osmanagaoglu K, Meganck G, Devroey P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization-embryo transfer cycles: a retrospective cohort study. Fertil Steril. 2014;101(1):162–5.
Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–85.
Hu KL, Liu FT, Xu H, Li R, Qiao J. Association of serum anti-Müllerian hormone and other factors with cumulative live birth rate following IVF. Reprod Biomed Online. 2020;40(5):675–83.
Hu KL, Yang R, Xu H, Mol BW, Li R, Wang R. Anti-Müllerian hormone in guiding the selection of a freeze-all versus a fresh embryo transfer strategy: a cohort study. J Assist Reprod Genet. 2022;39(10):2325–33.
Acharya KS, Acharya CR, Provost MP, Yeh JS, Steward RG, Eaton JL, et al. Ectopic pregnancy rate increases with the number of retrieved oocytes in autologous in vitro fertilization with non-tubal infertility but not donor/recipient cycles: an analysis of 109,140 clinical pregnancies from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2015;104(4):873–8.
Weiss A, Beck-Fruchter R, Golan J, Lavee M, Geslevich Y, Shalev E. Ectopic pregnancy risk factors for ART patients undergoing the GnRH antagonist protocol: a retrospective study. Reprod Biol Endocrinol. 2016;14:12.
Fleming R, Seifer DB, Frattarelli JL, Ruman J. Assessing ovarian response: antral follicle count versus anti-Mullerian hormone. Reprod Biomed Online. 2015;31(4):486–96.
Xu H, Zeng L, Yang R, Feng Y, Li R, Qiao J. Retrospective cohort study: AMH is the best ovarian reserve markers in predicting ovarian response but has unfavorable value in predicting clinical pregnancy in GnRH antagonist protocol. Arch Gynecol Obstet. 2017;295(3):763–70.
Hu KL, Hunt S, Zhang D, Li R, Mol BW. The association between embryo storage time and treatment success in women undergoing freeze-all embryo transfer. Fertil Steril. 2022;118(3):513–21.
Textor J, Hardt J, Knüppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745.
Correia KF, Dodge LE, Farland LV, Hacker MR, Ginsburg E, Whitcomb BW, et al. Confounding and effect measure modification in reproductive medicine research. Hum Reprod. 2020;35(5):1013–8.
Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol. 2017;217(2):167–75.
Marrie RA, Dawson NV, Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62(5):511–7.e1.
Broer SL, Broekmans FJ, Laven JS, Fauser BC. Anti-Mullerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701.
Mishina Y, Rey R, Finegold MJ, Matzuk MM, Josso N, Cate RL, et al. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10(20):2577–87.
Barbotin AL, Peigné M, Malone SA, Giacobini P. Emerging roles of anti-Müllerian hormone in hypothalamic-pituitary function. Neuroendocrinology. 2019;109(3):218–29.
Wang J, Dicken C, Lustbader JW, Tortoriello DV. Evidence for a Müllerian-inhibiting substance autocrine/paracrine system in adult human endometrium. Fertil Steril. 2009;91(4):1195–203.
Paulson M, Sahlin L, Hirschberg AL. Endometrial expression of anti-Müllerian hormone and its type II receptor in women with polycystic ovary syndrome. Reprod Biomed Online. 2020;41(1):128–37.
Carrarelli P, Rocha AL, Belmonte G, Zupi E, Abrão MS, Arcuri F, et al. Increased expression of antimüllerian hormone and its receptor in endometriosis. Fertil Steril. 2014;101(5):1353–8.
Namkung J, Song JY, Jo HH, Kim MR, Lew YO, Donahoe PK, et al. Mullerian inhibiting substance induces apoptosis of human endometrial stromal cells in endometriosis. J Clin Endocrinol Metab. 2012;97(9):3224–30.
Martínez-Conejero JA, Simón C, Pellicer A, Horcajadas JA. Is ovarian stimulation detrimental to the endometrium? Reprod Biomed Online. 2007;15(1):45–50.
Horcajadas JA, Mínguez P, Dopazo J, Esteban FJ, Domínguez F, Giudice LC, et al. Controlled ovarian stimulation induces a functional genomic delay of the endometrium with potential clinical implications. J Clin Endocrinol Metab. 2008;93(11):4500–10.
Haouzi D, Assou S, Mahmoud K, Tondeur S, Rème T, Hedon B, et al. Gene expression profile of human endometrial receptivity: comparison between natural and stimulated cycles for the same patients. Hum Reprod. 2009;24(6):1436–45.
Haouzi D, Assou S, Dechanet C, Anahory T, Dechaud H, De Vos J, et al. Controlled ovarian hyperstimulation for in vitro fertilization alters endometrial receptivity in humans: protocol effects. Biol Reprod. 2010;82(4):679–86.
Devroey P, Bourgain C, Macklon NS, Fauser BC. Reproductive biology and IVF: ovarian stimulation and endometrial receptivity. Trends Endocrinol Metab. 2004;15(2):84–90.
Rombauts L, McMaster R, Motteram C, Fernando S. Risk of ectopic pregnancy is linked to endometrial thickness in a retrospective cohort study of 8120 assisted reproduction technology cycles. Hum Reprod. 2015;30(12):2846–52.
Acknowledgments
We thank Rui Wang and Ben W. Mol in Monash University for providing valuable suggestions on the data analysis. We also thank Lixue Chen in Peking University Third Hospital for helping us to collect the data.
Funding
This study was supported by the National Natural Science Foundation of China (81925013, 82288102), National Key Research and Development Project of China (2022YFC2702502), China Postdoctoral Science Foundation (2022M720295).
Author information
Authors and Affiliations
Contributions
KLH conceived the idea, reviewed the literature, designed the study, designed the figures and tables, and wrote the manuscript. SL, SH, HX and RY critically revised the manuscript. RL revised the manuscript. All authors participated in the analysis and interpretation of data in this article. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Institutional Review Board (IRB) approval for this retrospective cohort study was obtained from the Ethics Committee of Peking University Third Hospital on 19th February 2019 (reference: 2019SZ-041) without the need for informed consent.
Consent for publication
All authors approved the final version of the manuscript and consent for publication.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
DAGs was used to identify potential confounders in the multivariable analysis.
Additional file 2: Fig. S2.
Flow chart.
Additional file 3: Fig. S3.
Predicted probability for ectopic pregnancy in women who underwent fresh embryo transfer cycles and women who resulted in clinical pregnancy against serum AMH levels.
Additional file 4: Supplemental Table 1.
Analysis of the association between different AMH stratifications and ectopic pregnancy (including heterotopic pregnancy) in women who underwent fresh embryo transfer as well as women who resulted in clinical pregnancy.
Additional file 5: Fig. S4.
Ectopic pregnancy rate in women who underwent single embryo transfer.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Hu, KL., Li, S., Hunt, S. et al. High anti-Müllerian hormone (AMH) is associated with increased risks of ectopic pregnancy in women undergoing fresh embryo transfer cycle, a cohort study. Reprod Biol Endocrinol 21, 18 (2023). https://doi.org/10.1186/s12958-022-01038-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-022-01038-6