Abstract
Background
After receiving neoadjuvant chemoradiation, the number of examined lymph nodes in resectable gastroesophageal cancer (GEC) will decrease, this may not accurately determine the N staging. So our study evaluates the clinical significance of a new staging model based on the logarithmic odds of positive lymph nodes (LODDS) in patients with GEC after receiving neoadjuvant chemoradiation.
Methods
A total of 1 130 patients with pathologically diagnosed GEC who received neoadjuvant chemoradiation from 2004 to 2019 included in the National Cancer Institute Surveillance, Epidemiology, and Results (SEER) database were selected for analysis. Lymph nodes were staged according to the AJCC TNM staging system (eighth edition) and LODDS. Patient prognosis across the two systems were evaluated by the Kaplan–Meier method, differences in node staging were evaluated by the Akaike information criterion and Bayesian information criterion. In addition, 914 patients from our center were externally validated.
Results
Compared to the traditional TNM staging system, the new TLODDSM staging system was comprised of stage I, stage II, stage IIIA, stage IIIB, and stage IVA, and decision curve analysis showed that the new staging system had higher benefits for different decision thresholds than the old staging system. The Akaike information criterion and Bayesian information criterion of the new staging system was lower than those of the old staging system, indicating the sensitivity of the TLODDSM staging system for predicting the prognosis of patients was higher. In addition, stage-IIIB or -IVA patients in the new staging system benefited from adjuvant chemotherapy. The externally validated data from our center supported this conclusion.
Conclusions
Compared to the TNM staging system, the TLODDSM staging system has significant advantages in predicting prognosis of patients with GEC who have completed neoadjuvant chemoradiation, guiding the adjuvant chemotherapy for patients.
Similar content being viewed by others
Background
The latest global cancer statistics reveal that gastroesophageal cancer (GEC) is the ninth most common malignancy and the sixth most common cause of cancer-related death [1]. Primary esophageal and GEC can be classified as either adenocarcinoma or squamous cell carcinoma. Caucasians in western countries mainly have adenocarcinoma, whereas Caucasians in eastern countries mostly have squamous cell carcinoma. Presently, GEC is treated by surgery, and although significant progress has been made in the treatment of GEC, the overall 5-year survival rate of patients remains low, which may be due to the lack of an accurate staging system before treatment and a standard treatment protocol [2, 3].
Different stages of GEC require different treatments. Patients with early stages of the disease can be treated with surgery, those with locally advanced stages can be treated with preoperative radiotherapy and chemotherapy, and those with distant metastases can receive palliative care to improve symptoms and quality of life. The prognosis of patients with GEC at different stages varies greatly [4, 5]. Cunningham et al. reported that among patients with resectable GEC, those receiving neoadjuvant chemotherapy had better overall survival (OS) (hazard ratio [HR] = 0.75; 95% CI 0.60–0.93; P < 0.01) and progression-free survival (PFS) (HR = 0.66; 95% CI 0.53–0.81; P < 0.01) compared to those treated with surgery alone [6]. As neoadjuvant chemoradiation replaces EGC resection, the pathological staging of advanced cancer is losing its clinical relevance, although it can still be used as a reference for staging early disease and predicting patient survival. Moreover, neoadjuvant chemoradiation associates with a decreased number of examined lymph nodes (ELNs). The eighth edition of NCCN includes TNM staging for GEC patients who received neoadjuvant chemoradiation; however, there are disadvantages to using this staging system because of the decreased number of ELNs [7]. In addition, it is unclear whether this population can benefit from adjuvant chemotherapy (ACT). Therefore, we propose a new TNM staging system based on the logarithmic odds of positive lymph nodes (LODDS), which provides more accurate pathological staging of patients with GEC who have received neoadjuvant treatment and selects the population who can truly benefit from ACT. The currently recognized comprehensive treatment for GEC still recommends ACT as the recommended treatment method. Nano-activated carbon can play the role of lymph node tracer in the operation of gastric cancer, thyroid cancer, pancreatic cancer and other malignant tumors. Nano-activated carbon also has the same adsorption characteristics as carbon, has a larger specific surface area. Therefore, it can be used as a carrier to adsorb chemotherapy drugs for lymphatic system targeted chemotherapy. Green nanomaterials are the direction of future treatment [8,9,10].
This study included data from the SEER database of patients with resectable GEC who received neoadjuvant chemoradiation. The patients were divided into five groups according to the LODDS in order to evaluate the accuracy and relevance of the new staging system compared to the old staging system in predicting the prognosis of patients. The benefits of the new staging system were validated through external data, and the population that could benefit from ACT was confirmed.
Methods
Patient cohort
SEER*Stat (version 8.4.0) software was used to identify 1 444 patients with resectable GEC confirmed between 2004 and 2019. The inclusion criteria were as follows: (1) patients with pathologically confirmed GEC (ICD-O-3: C16.0), (2) patients with complete follow-up and survival data, (3) patients who received neoadjuvant chemotherapy or radiotherapy, (4) patients who underwent radical surgery, and (5) patients with resectable primary GEC. The variables were age, gender, race, grade, tumor size, tumor stage, ELN, positive examined lymph nodes (PLN), and survival information. Patients were excluded if data were missing. The externally validated data consisted of 914 patients with resectable GEC who received neoadjuvant chemoradiation and underwent surgery in our department from 2011 to 2018. The staging of all tumors was according to the TNM staging system (eighth edition).
Statistical analysis
The distribution of pathological factors was examined, and the TNM staging system was replaced with the LODDS staging system, which was defined as log [(PLN + 0.5)/(ELN-PLN + 0.5)]. LODDS values ranged from − 2.1 to 1.8, with an inter-group spacing of 0.2. Patients were divided into 17 groups, and the HR (range, − 2.1 ≤ LODDS ≤ − 2.0) was defined as 1 and sorted from low to high. The OS of the adjacent two groups was subjected to log rank test, and 4 chi square values with significant differences were selected from 16 chi square values. The 17 groups were divided into five stages. LODDS1 (− 2.1–1.8) consisted of 56 individuals, LODDS2 (− 1.7–1.4) consisted of 395 individuals, LODDS3 (− 1.3–0.2) consisted of 505 individuals, LODDS4 (− 0.1–0.2) consisted of 82 individuals, and LODDS5 (0.3–1.8) consisted of 92 individuals. The survival curves were compared among the five groups, and an improved TLODDSM staging system (I-IVA) was established by replacing the N staging system in the AJCC staging system (eight edition).
When evaluating the discriminative ability of the two staging systems for prognosis, we used the Akaike information criterion (AIC) and Bayesian information criterion (BIC). We used decision curve analysis (DCA) to evaluate the net clinical benefit of the new staging system and compared it with the AJCC staging system (eight edition). We used chi-square test to evaluate the prognostic homogeneity of the two staging systems (the larger the likelihood ratio, the better the prognostic homogeneity of the staging system) and survival curve and COX analysis to analyze the prognosis of patients at different stages. All statistical analyses were conducted using SPSS (version 24.0) and R language (version 4.0.0) software packages with P < 0.05 indicating statistically significant differences.
Results
Patient demographics
A total of 1130 patients with resectable GEC who received neoadjuvant chemoradiation were included (Fig. 1). Specifically, 516 (45.7%) patients were 65 years of age or older, 168 (14.9%) patients were female, 290 (25.7%) patients had poorly differentiated tumors, 410 (36.3%) patients had tumors greater than 5 cm in diameter, and 88 (7.8%) patients had T4 tumors. The median ELN was 14, whereas the median PLN was 1 (Table 1). The median survival period was 27 months (range, 0–190 months), and the number of deaths was 590 (52.2%).
New lymph node staging system
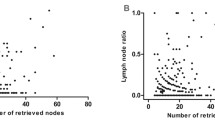
As the number of ELNs increased, the patient’s prognosis improved (Fig. 2A); however, as the LODDS increased, the patient’s prognosis worsened (Fig. 2B). The range of LODDS was − 2.1–1.8, and we divided LODDS into 17 groups at intervals of 0.2. The HR of − 2.1 ≤ LODDS ≤ − 2.0 was defined as 1, and the HR values for each stage were calculated and sorted from low to high, with the highest as 1.0 < LODDS ≤ 1.8 (HR = 7.875; 95% CI 1.672–37.095; P = 0.009). Survival analysis was performed on 17 consecutive stages, and chi square values were calculated. Four high chi square values were identified as cutoff points (4.207, 1.265, 0.392, 0.265), and patients were divided into five stages as follows (Table 2). The 5-year survival rates for LODDS1–5 were 71.87, 57.80, 35.14, 24.14, and 16.46%, respectively, with statistically significant differences, indicating that our staging system has a high discriminative ability (Fig. 2C). According to the eighth edition of staging, we used the LODDS staging system instead of the traditional staging system to divide patients into 35 groups. The HR of T0LODDS1M0 was defined as 1, and the HR values for each stage were calculated and sorted from low to high, with the highest as T4bLODDS5M0 (HR = 398.007; 95% CI 24.479–6471.206; P < 0.001; Table 3). Survival analysis was performed on 35 consecutive stages (Fig. 3A), and chi square values were calculated. Four high chi square values were identified as cutoff points (1.958, 1.007, 1.332, 0.636), and patients were divided into five stages as follows (Fig. 3B): stage I (T0LODDS1), stage II (TisLODDS1), stage IIIA (T1LODDS1, T2LODDS1), IIIB (T3LODDS1, T0LODDS2, TisLODDS2, T1LODDS2, T2LODDS2, T0LODDS3, TisLODDS3, T0LODDS4, T1LODDS3, T2LODDS3, T3LODDS2, T4aLODDS1, TisLODDS4, T0LODDS5, T3LODDS, TisLODDS5, T1LODDS4), and stage IVA (T4aLODDS2, T1LODDS5, T4bLODDS1, T2LODDS4, T4aLODDS3, T3LODDS4, T4bLODDS2, T2LODDS5, T3LODDS5, T4bLODDS3, T4aLODDS4, T4aLODDS5, T4bLODDS4, T4bLODDS5). The HR of stage I was 1. The 5-year survival rates of stage II (HR = 11.118; 95% CI 1.005–123.042; P < 0.05), stage IIIA (HR = 13.058; 95% CI 1.722–99.014; P = 0.013), stage IIIB (HR = 22.675; 95% CI 3.186–161.352; P = 0.002), and stage IVA (HR = 63.741; 95% CI 8.872–457.934; P < 0.001) were 100.00, 100.00, 67.53, 44.58, and 9.60%, respectively, with statistically significant differences (P < 0.05; Fig. 4A). However, the 5-year survival rates of stage I to stage IVA in the traditional TNM staging system were 53.98, 57.42, 41.89, 39.99, and 9.98%, respectively, with no statistically significant differences (P > 0.05; Table 4).
Comparison of prognostic effectiveness between the two staging systems
The AIC (7356.862) and BIC (7361.242) of the TLODDSM staging system were lower than the AIC (7385.357) and BIC (7389.737) of the traditional TNM staging system, and the likelihood of the TLODDSM staging system was higher than that of the traditional TNM staging system (chi square test, 113.091 versus 95.953, Table 5). The DCA showed that the TLODDSM staging system had a higher net benefit compared to the traditional TNM staging system (Fig. 4C, D). Taken collectively, these results indicate that the TLODDSM staging system is superior to the AJCC staging system.
Selection of a population that can benefit from adjuvant chemotherapy
Patients in stage I of the TLODDSM staging system did not benefit from ACT (P > 0.05; Fig. 5A). Furthermore, stage-II patients did not benefit from ACT (HR = 0.33; 95% CI 0.01–8.18; P > 0.05; Fig. 5B), stage-IIIA patients did not benefit from ACT (HR = 0.54; 95% CI 0.16–1.90; P > 0.05; Fig. 5C), stage-IIIB patients did benefit from ACT (HR = 0.55; 95% CI 0.42–0.71; P < 0.01; Fig. 5D), and stage-IVA patients did benefit from ACT (HR = 0.53; 95% CI 0.31–0.90; P < 0.01; Fig. 5E).
A OS for patients with or without ACT in TLODDSM stage I group in our training set; B OS for patients with or without ACT in TLODDSM stage II group in our training set; C OS for patients with or without ACT in TLODDSM stage IIIA group in our training set; D OS for patients with or without ACT in TLODDSM stage IIIB group in our training set; E OS for patients with or without ACT in TLODDSM stage IVA group in our training set; F OS for patients with or without ACT in TNM stage I group in our training set; G OS for patients with or without ACT in TNM stage II group in our training set; H OS for patients with or without ACT in TNM stage IIIA group in our training set; I OS for patients with or without ACT in TNM stage IIIB group in our training set; J OS for patients with or without ACT in TNM stage IVA group in our training set
Patients in stage I of the traditional TNM staging system did benefit from ACT (HR = 0.61; 95% CI 0.36–1.02; P < 0.05; Fig. 5F). Furthermore, stage-II patients did not benefit from ACT (HR = 2.07; 95% CI 0.44–9.74; P > 0.05; Fig. 5G), stage-IIIA patients did benefit from ACT (HR = 0.51; 95% CI 0.37–0.70; P < 0.01; Fig. 5H), stage-IIIB patients did not benefit from ACT (HR = 0.75; 95% CI 0.47–1.21; P > 0.05; Fig. 5I), and stage-IVA patients did benefit from ACT (HR = 0.39; 95% CI 0.15–1.04; P < 0.01; Fig. 5J).
T LODDS M staging system in the external validation group
After considering the inclusion criteria, 914 patients with resectable GEC who received neoadjuvant chemoradiation in our hospital were included. Specifically, 297 (32.5%) patients were 65 years of age or older, 268 (29.3%) patients were female, 413 (45.2%) patients had poorly differentiated tumors, 377 (41.2%) patients had tumors with dimeters greater than 5 cm, and 472 (51.6%) patients had T4 tumors. The median number of ELNs was 18, and the median number of PLNs was 2 (Table 1). The median survival period was 35 months (range, 0–80), and the number of deaths was 433 (47.4%). According to the above criteria, the patients were further divided into 35 groups and classified into five stages based on the TLODDSM staging system (Fig. 6A). Stages I, II, IIIA, IIIB, and IVA had 5-year survival rates of 100.00, 97.78, 88.91, 32.33, and 17.44%, respectively, with statistically significant differences (P < 0.05, Fig. 6B). The 5-year survival rates of stage I to IVA in the traditional TNM staging system were 55.70, 0, 73.54, 55.04, and 20.99%, respectively, with no statistically significant differences (P > 0.05; Fig. 6C).
A The Kaplan–Meier curves of OS for patients in our new LODDS staging system in our external set; B The Kaplan–Meier curves of OS for TLODDSM staging system in our external set; C The Kaplan–Meier curves of OS for traditional TNM staging system in our external set; D TLODDSM stage were compared to the TNM stage in terms of 3-year OS in our DCA in our external set; E TLODDSM stage were compared to the TNM stage in terms of 5-year OS in our DCA in our external set
The AIC (5229.220) and BIC (5245.503) of the TLODDSM staging system were lower than the AIC (5285.257) and BIC (5301.54) of the traditional TNM staging system, and the likelihood of the new staging system was higher than that of the old staging system (chi-square test, 275.798 versus 229.595, Table 5). The DCA showed that the TLODDSM staging system had a higher net benefit compared to the traditional TNM staging (Fig. 6D, E). The externally validated data from our center supported this conclusion.
The TLODDSM staging system showed that stage-I patients did not benefit from ACT (P > 0.05; Fig. 7A), stage-II patients did not benefit from ACT (P > 0.05; Fig. 7B), stage-IIIA patients did benefit from ACT (HR = 0.15; 95% CI 0.03–0.77; P < 0.05; Fig. 7C), stage-IIIB patients did benefit from ACT (HR = 0.67; 95% CI 0.47–0.96; P < 0.05; Fig. 7D), and stage-IVA patients did benefit from ACT (HR = 0.73; 95% CI 0.57–0.93; P < 0.01; Fig. 7E).
A OS for patients with or without ACT in TLODDSM stage I group in our external set; B OS for patients with or without ACT in TLODDSM stage II group in our external set; C OS for patients with or without ACT in TLODDSM stage IIIA group in our external set; D OS for patients with or without ACT in TLODDSM stage IIIB group in our external set; E OS for patients with or without ACT in TLODDSM stage IVA group in our external set; F OS for patients with or without ACT in TNM stage I group in our external set; G OS for patients with or without ACT in TNM stage II group in our external set; H OS for patients with or without ACT in TNM stage IIIA group in our external set; I OS for patients with or without ACT in TNM stage IIIB group in our external set; J OS for patients with or without ACT in TNM stage IVA group in our external set
The traditional TNM staging system showed that stage-I patients did not benefit from ACT (HR = 1.16; 95% CI 0.57–2.37; P > 0.05; Fig. 7F), stage-II patients did not benefit from ACT (HR = 1.20; 95% CI 0.40–3.55; P > 0.05; Fig. 7G), stage-IIIA patients did benefit from ACT (HR = 0.15; 95% CI 0.03–0.74; P < 0.05; Fig. 7H), stage-IIIB patients did not benefit from ACT (HR = 0.62; 95% CI 0.38–1.03; P > 0.05; Fig. 7I), and stage-IVA patients did benefit from ACT (HR = 0.68; 95% CI 0.54–0.87; P < 0.01; Fig. 7J).
Discussion
The eighth edition of the TNM Staging System for Esophageal Cancer and GEC distinguishes clinical staging (cTNM), pathological staging (pTNM), and neoadjuvant treatment staging (ypTNM), and no longer applies one staging model. Unlike cTNM and pTNM staging, ypTNM staging for the two pathological types, adenocarcinoma and squamous cell carcinoma, is identical [11,12,13]. For the staging of GEC, the seventh edition continues to be used, which emphasizes PLN as the node staging standard, without reflecting the impact of the lymph node station number on staging [14, 15]. Although many studies support the correlation between the lymph node stage number and GEC prognosis, some lymph nodes may fuse due to the invasiveness of the tumor, or some PLNs may rupture during surgical dissection, resulting in inaccurate node staging based on the number. Furthermore, neoadjuvant chemoradiation may result in a decreased number of ELNs [16,17,18]. Therefore, a new node staging system is needed to more accurately evaluate the prognosis of patients. New staging systems for lymph nodes, such as the lymph node ratio (LNR), LODDS, negative examined lymph node (NLN) number, and lymph node micro-metastases, can better predict the prognosis of patients [19,20,21]. According to Yang et al., in the context of a new TNM staging system, LODDS has better predictive value than node number, LNR, and NLN number in patients with PLNs [11].
LODDS is defined as the logarithm of the probability ratio of positive or negative lymph nodes when one lymph node is detected, which effectively balances the number of ELNs and the number of PLNs [22]. Spolverato et al. reported that the LODDS system (C index: 0.636; AIC: 4304.0) was more sensitive than the node score system (C index: 0.632; AIC: 4308.4) and the LNR system (C index: 0.631; AIC: 4225.8) in 804 patients who underwent radical gastrectomy for gastric cancer [23]. Among Siewert type II gastroesophageal adenocarcinoma patients who underwent surgery after neoadjuvant radiotherapy, the LNR was more sensitive than the PLN number, and LODDS was an independent prognostic factor (HR = 1.16; 95% CI 1.08–1.25; P < 0.01) [24]. Zhou et al. demonstrated that LODDS (C index: 0.675; AIC: 6243.740) and LNR (C index: 0.686; AIC: 6261.027) staging systems were superior to the traditional node staging system (C index: 0.658; AIC: 6355.077) in predicting the prognosis of 735 patients with gastroesophageal adenocarcinoma who completed surgery, and externally validated data also confirmed this conclusion [25]. To reduce the stage migration caused by a decreased ELN number [26,27,28], we replaced the traditional TNM staging system with the LODDS staging system (LODDS1–5). The overall survival of patients was significantly improved with the LODDS1–5 staging system, and stages I, II, IIIA, IIIB, and IVA showed statistical differences in the 5-year survival rate, whereas the traditional TNM staging system showed no statistical differences, indicating that the new staging system can better predict the prognosis of patients. Furthermore, we compared the AIC, BIC, and DC of the two staging systems and observed that the heterogeneity in the TLODDSM staging system was relatively low, making it the best staging system for evaluating patient prognosis.
Different stage combinations can lead to completely different prognoses, and it is unclear whether patients with GEC who receive neoadjuvant chemoradiation can benefit from ACT. Rahman et al. reported that patients with GEC who received neoadjuvant chemotherapy benefited from ACT (HR = 0.84; 95% CI 0.77–0.94; P = 0.001) [29]. Mokdad et al. [30] studied 10 086 patients with gastroesophageal adenocarcinoma who received neoadjuvant chemotherapy and radiotherapy in 2006 and 2013 and reported that ACT improved the OS (HR = 0.79; 95% CI 0.72–0.88; P < 0.001) and median survival (40 versus 34 months). On the other hand, Sisic et al. observed no improvement in OS (P = 0.331) and relapse-free survival (P = 0.118) in patients with GEC receiving ACT [31], suggesting not all patients require ACT. Our study shows that stage-I, -II, and -IIIA patients did not benefit from ACT, whereas stage-IIIB and -IVA patients did benefit from treatment. In terms of the traditional TNM staging system, stage- I, -IIIA, and -IVA patients did benefit from ACT, whereas stage-II and -IIIB patients did not benefit from treatment. Our study also shows that the poorer the staging, the more likely the patient is to benefit from ACT. However, traditional TNM stages do not imply that patients with poorer prognosis are more likely to benefit from ACT, indicating that our staging system can guide the postoperative monitoring, follow-up, and prognosis of patients and avoid the consequences of over-treatment and under-treatment.
This study has several limitations. First, the inconsistency of neoadjuvant radiation dosage and chemotherapy regimen can lead to certain biases. Second, different centers, different pathologists, and the lack of unified standards for lymph node detection can also lead to bias. Third, this study was a retrospective study. The optimal cut-off value for the new staging system is still difficult to reach consensus, and when referring to LODDS for staging, the calculation is not fast and convenient. Except for patients with a higher risk of lymph node staging migration, it may not significantly improve the predictive prognosis. Presently, there is no standard protocol for the staging and treatment of GEC after receiving neoadjuvant chemoradiation. However, we established the LODDS staging system to re-stage patients, which can guide treatment and predict prognosis.
Conclusion
The TLODDSM staging system is superior in staging and predicting the prognosis of patients with GEC after receiving neoadjuvant chemoradiation (radiation first). We recommend that stage-IIIB and -IVA patients should receive ACT.
Availability of data and materials
Data will be made available on request.
Abbreviations
- GEC:
-
Gastroesophageal cancer
- ELNs:
-
Examined lymph nodes
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- ACT:
-
Adjuvant chemotherapy
- LODDS:
-
Logarithmic odds of positive lymph nodes
- PLN:
-
Positive lymph nodes
- AIC:
-
Akaike information criterion
- BIC:
-
Bayesian information criterion
- DCA:
-
Decision curve analysis
- NLN:
-
Negative examined lymph node
References
Sung H, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Obermannová R, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004.
Morgan E, et al. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649-58.e2.
Grizzi G, et al. Preferred neoadjuvant therapy for gastric and gastroesophageal junction adenocarcinoma: a systematic review and network meta-analysis. Gastric Cancer. 2022;25(5):982–7.
Zhu M, et al. Pembrolizumab in combination with neoadjuvant chemoradiotherapy for patients with resectable adenocarcinoma of the gastroesophageal junction. Clin Cancer Res. 2022;28(14):3021–31.
Cunningham D, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Wang Q, et al. Impacts of neoadjuvant therapy on the number of dissected lymph nodes in esophagogastric junction cancer patients. BMC Gastroenterol. 2023;23(1):64.
Khalilov R, et al. Future prospects of biomaterials in nanomedicine. Adv Biol Earth Sci. 2023;8:5–10. https://doi.org/10.6247/abes.9s5.
Rosic G, et al. Cancer signaling, cell/gene therapy, diagnosis and role of nanobiomaterials. Adv Biol Earth Sci. 2024;9:11–34. https://doi.org/10.6247/abes9s11.
Huseynov E, et al. Novel nanomaterials for hepatobiliary diseases treatment and future perspectives. Adv Biol Earth Sci. 2024;9:81–91. https://doi.org/10.6247/abes9s81.
Yang Y, et al. Comparison of 4 lymph node staging systems for the prognostic prediction of esophagogastric junction adenocarcinoma with ≤15 retrieved lymph nodes. Eur J Surg Oncol. 2022;48(5):1017–24.
Chen J, et al. Development and validation of a survival nomogram for patients with Siewert type II/III adenocarcinoma of the esophagogastric junction based on real-world data. BMC Cancer. 2021;21(1):532.
Wang T, et al. Development and validation of a novel competing risk model for predicting survival of esophagogastric junction adenocarcinoma: a SEER population-based study and external validation. BMC Gastroenterol. 2021;21(1):38.
Liu CT, et al. A nomogram based on nutrition-related indicators and computed tomography imaging features for predicting preoperative lymph node metastasis in curatively resected esophagogastric junction adenocarcinoma. Ann Surg Oncol. 2023;30(8):185-94. https://doi.org/10.1245/s10434-023-13378-7.
Ma Z, et al. Comparison of lymph node metastasis pattern from esophagogastric junction adenocarcinoma versus very low thoracic esophageal squamous cancer: a propensity-matched analysis. J Thorac Dis. 2023;15(2):442–51.
Shannon AB, et al. Lymph node evaluation after neoadjuvant chemotherapy for patients with gastric cancer. Ann Surg Oncol. 2022;29(2):1242–53.
Lai H, et al. Lymph node evaluation after neoadjuvant therapy for patients with adenocarcinoma of esophagogastric junction. Asian J Surg. 2023;46(7):2923-25. https://doi.org/10.1016/j.asjsur.2023.02.007.
Ding B, et al. Impact of examined lymph node number on accurate nodal staging and long-term survival of resected Siewert type II-III adenocarcinoma of the esophagogastric junction: a large population-based study. Front Oncol. 2022;12:979338.
Lai H, et al. Prognostic value of a new staging system for adenocarcinoma of the esophagogastric junction after preoperative radiation therapy. Asian J Surg. 2023;46(7):2919-22. https://doi.org/10.1016/j.asjsur.2023.02.005.
Kamiya H, et al. Evaluating prognostic value and stage migration effects using a positive lymph node ratio in adenocarcinoma of the esophagogastric junction. BMC Cancer. 2023;23(1):218.
Sharma P, et al. Importance of investigating high-risk human papillomavirus in lymph node metastasis of esophageal adenocarcinoma. World J Gastroenterol. 2020;26(21):2729–39.
Che K, et al. Prognostic nomograms based on three lymph node classification systems for resected gastric adenocarcinoma: a large population-based cohort study and external validation. Ann Surg Oncol. 2021;28(13):8937–49.
Spolverato G, et al. Prognostic performance of different lymph node staging systems after curative intent resection for gastric adenocarcinoma. Ann Surg. 2015;262(6):991–8.
Liu F, et al. Proposal of a nomogram for predicting survival in patients with siewert type II adenocarcinoma of the esophagogastric junction after preoperative radiation. Ann Surg Oncol. 2019;26(5):1292–300.
Zhou Z, et al. Different lymph node staging systems for patients with adenocarcinoma of esophagogastric junction. Curr Med Res Opin. 2018;34(6):963–70.
Sekhar H, et al. Nodal stage migration and prognosis in anal cancer: a systematic review, meta-regression, and simulation study. Lancet Oncol. 2017;18(10):1348–59.
Liang W, et al. Impact of examined lymph node count on precise staging and long-term survival of resected non-small-cell lung cancer: a population study of the US SEER database and a Chinese multi-institutional registry. J Clin Oncol. 2017;35(11):1162–70.
Shi Q, et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database. J Clin Oncol. 2013;31(29):3656–63.
Rahman S, et al. Impact of postoperative chemotherapy on survival for oesophagogastric adenocarcinoma after preoperative chemotherapy and surgery. Br J Surg. 2022;109(2):227–36.
Mokdad AA, et al. Adjuvant chemotherapy vs postoperative observation following preoperative chemoradiotherapy and resection in gastroesophageal cancer: a propensity score-matched analysis. JAMA Oncol. 2018;4(1):31–8.
Sisic L, et al. The postoperative part of perioperative chemotherapy fails to provide a survival benefit in completely resected esophagogastric adenocarcinoma. Surg Oncol. 2020;33:177–88.
Acknowledgements
None.
Funding
Department of Science and Technology of Jilin Province (No YDZJ202201ZYTS668).
Author information
Authors and Affiliations
Contributions
SL conceived and supervised the study. ZMW recruited data information. YYG analyzed the data and visualized the results. SL wrote the original draft. YXZ reviewed and edited the manuscript. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the Declaration of Helsinki’s ethical standards for medical research involving human people. The protocol was reviewed and approved by the Institutional Research Ethical Committee of Second Hospital of Jilin University.
Consent for publication
All authors read the manuscript and agreed to its content and declare no competing interest in relation to their work.
Competing interests
No potential competing interests were disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, S., Wang, Z., Ge, Y. et al. Prognostic significance of an innovative staging system based on the logarithmic odds of positive lymph nodes for resectable gastroesophageal cancer after neoadjuvant chemoradiation: a population-based study with external validation of data. J Transl Med 22, 801 (2024). https://doi.org/10.1186/s12967-024-05448-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05448-5