Abstract
Background
Difficult-to-treat Rheumatoid arthritis (D2T RA) is primarily characterised by failure of at least two different mechanism of action biologic/targeted synthetic disease-modifying antirheumatic drug (DMARDs) with evidence of active/progressive disease. While a variety of drugs have been used in previous studies to treat D2T RA, there has been no systematic summary of these drugs. This study conducted a systematic review of randomized controlled trials aimed at analyzing the efficacy and safety of individual therapeutic agents for the treatment of D2T RA and recommending the optimal therapeutic dose.
Methods
The English databases were searched for studies on the treatment of D2T RA published between the date of the database’s establishment and March, 2024. This study uses R 3.1.2 for data analysis, and the rjags package runs JAGS 3.4.0.20. The study fitted a stochastic effects Bayesian network meta-analysis for each outcome measure.
Result
A total of 42 studies were included in this study. Compared with placebo, the improvement of Disease Activity Score of 28 Joints (DAS28) score is ranked from high to low as tocilizumab, baricitinib and opinercept. The improvement of American College of Rheumatology 50 response (ACR50) score in patients with drug use was ranked from good to poor as follows: olokizumab, tocilizumab, adalimumab, baricitinib, and upadacitinib, and 8 mg/4w tocilizumab demonstrated the best efficacy. Notably, rituximab is generally the safest drug. Janus kinase (JAK) inhibitors and T cell costimulation modulators are effective in D2T RA refractory to biologic DMARDs, while JAK inhibitors and interleukin-6 (IL-6) inhibitors show effectiveness in D2T RA refractory to csDMARDs.
Conclusion
Tocilizumab and rituximab have better efficacy and safety in the treatment of D2T RA, and the 8 mg/4w dose of tocilizumab may be the first choice for achieving disease remission.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a common and chronic inflammatory autoimmune disease characterized by destructive, chronic, debilitating arthritis [1]. According to the European Alliance of Associations for Rheumatology (EULAR) RA management guidelines, patients whose disease activity cannot be controlled despite the use of two or more conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biologic DMARDs (bDMARDs), or targeted synthetic DMARDs (tsDMARDs) have recently been referred to as having difficult-to-treat RA (D2T RA) [2, 3]. The prevalence of D2T RA varies by its definition and has generally been estimated to range from 5 to 20%, and it is closely related to a variety of chronic diseases [4,5,6]. Furthermore, D2T RA patients also faced various challenges, including uncontrolled disease activity, diminished quality of life, as well as economic burdens stemming from frequent healthcare utilization and recurrent admissions [2]. It is worth noting that, despite current treatment modalities, two-thirds of D2T RA patients have failed to achieve disease control, and up to 40% of D2T RA patients experienced treatment failure due to drug resistance or adverse drug reactions [7,8,9]. Therefore, exploring safe, and effective drugs for D2T RA patients is of great urgency.
At present, there is no radical treatment for D2T RA. However, a variety of pharmacologic and non-pharmacologic interventions have been shown to be effective in controlling the progress and relieving clinical symptoms of D2T RA. Non-pharmacologic interventions include motor, psychological, educational, and self-management strategies, which are capable of achieving inflammation-free outcomes, alleviating residual pain, and mitigating secondary fibromyalgia [10]. Pharmacologic interventions include not only conventional synthetic agents (csDMARDs, bDMARDs and tsDMARDs), but also targeting specifically Janus kinase (JAK), interleukin-6 (IL-6), tumor necrosis factor (TNF), high-selective Bruton tyrosine kinase (BTK) noncovalent inhibitors, CD20, and T cell costimulation modulators [3].
In previously published articles, although a variety of drugs have been used to treat D2T RA, there is no systematic summary of these drugs. The purpose of the network meta-analysis is to find the optimal treatment for D2T RA based on the efficacy and safety rankings of each drug, providing some insights for the clinical treatment of D2T RA.
Methods
Data sources and search strategy
This network meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis extension statement for network meta-analyses, and it was registered in the Prospective Register of Systematic Reviews to ensure transparency, reliability, and novelty.
A systematic literature search of PubMed, Embase, Web of Science, Cochrane Library, MEDLINE, Web of Knowledge, Clinical Trials.gov, FDA.gov, and preprint databases (SSRN, bioRxiv, and MedRxiv) was performed from the date of inception of the databases to March 2024, without restrictions on publication language or primary outcome. The PRISMA flowchart of screened studies is shown in Fig. 1. Furthermore, the reference lists of the included studies were manually checked to identify the eligible studies.
Selection criteria
We included prospective randomized controlled trials (RCTs), in line with the most recent definition of D2T RA from European League Against Rheumatism (EULAR 2021). All included RCTs were concerned with monotherapy or combination therapy approved for the treatment of D2T RA. Patients were further stratified according to their previous therapeutic regimens and the insufficient efficacy observed, including cases where there was a suboptimal response to at least two bDMARDs, csDMARDs, DMARDs, MTX/TNF-αi, or TNF-i. To ensure homogeneity among participant and trial characteristics, studies where all participants had early RA were included.
The meta-analysis excluded studies that were non-original, multiple reports of the same or overlapping data, as well as conducted without a control group. Meanwhile, we excluded studies with missing data that could not be obtained even after contacting the authors.
Outcome measures
The primary outcome included the severity of disease remission, defined as Disease Activity Score of 28 Joints (DAS28) score and American College of Rheumatology 50 response (ACR50). The secondary outcome encompassed tender joint counts (TJC), swollen joint counts (SJC), level of lymphocyte, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR). Outcome measures of safety include the incidence of adverse events (AE), serious adverse event (SAE) and deaths due to treatment.
Data collection and risk of bias assessment
After excluding the studies that failed to fulfill the criteria, two independent investigators screened each study and extracted relevant data concerning of the outcomes of this network meta-analysis. The following information was extracted for each included study: article information (first author, nations, publication year), study characteristics (study design and duration, sample size, time of study conduct, primary and secondary outcomes, adverse reactions, evaluation indicators), and baseline characteristics of patients (age, sex ratio, disease duration). Disagreements between the two investigators were resolved by consensus and discussion with a third investigator. Based on the latest Cochrane risk of bias assessment tool (RoB−2 2019), we categorized the included literature into The risk-of-bias judgments for each domain are “low risk of bias,” “some concerns,” or “high risk of bias.
Data analysis
A network meta-analysis was conducted within a frequentist framework using the netmeta package in the statistical software R (V.4.0.3). We designed a network including placebo, any intervention for D2T RA and treatment as direct comparisons. If studies included control interventions, these were grouped together and added as a further comparison. Random effects pairwise meta-analyses with the Hartung-Knapp-Sidik-Jonkman method were utilized for direct comparisons to estimate standardised mean differences (SMD) and odds ratios (OR) for continuous and dichotomous outcomes, respectively. Indirect evidence was assessed using the entire network, and a random effects netmeta model was used to control for multiarm trial effects.
For the network meta-analysis, all doses of therapies were included, with the basic parameter set to “0” (no effect) for using placebo, so that the basic parameter for the other treatments provided the treatment effect relative to use placebo. From these basic parameters, we determined the treatment effect between every pair of treatments.
The results are expressed as SMD and OR with corresponding 95% confidence intervals. We used the Cochran’s Q statistic to determine the pairwise between-study heterogeneity. I2 was used to evaluate the percentage of variance caused by between-study heterogeneity. It was assumed that heterogeneity was common across the entire network.
Due to an insufficient number of studies, subgroup and meta-regression analyses, as well as the assessment of publication bias, were not feasible to explore potential sources of heterogeneity across the network. Sensitivity analyses were conducted by excluding studies with high indirectness and risk of bias, studies with participants older than 60, studies with interventions longer than 24 months and studies using an attention/active or passive control comparison.
Results
Study selection and characteristics of included trials
We identified 11,762 citations through the literature search, excluded 10,469 titles and abstracts after initial screening and assessed 301 studies for eligibility. A final number of 42 full-text articles met all eligibility criteria (Fig. 1). In addition, we provided the details of the selected studies in supplementary Material. These included studies were published between 2013 and 2024, including 19,827 patients who were randomly assigned to 24 biological treatment and control groups. Our study involved 24 biologics: TNF blockers (secukinumab, golimumab, etanercept, adalimumab, infliximab, BI 695501, opinercept), interleukin (IL)-1 antagonist (anakinra), IL-6 antagonist (tocilizumab, sarilumab, olokizumab), anti-CD28 (abatacept), anti-CD20 (rituximab, HLX01), anti-T cell (abatacept), mRNA inhibitor micro-RNA (miR)-124 (ABX464), JAK inhibitor (upadacitinib, baricitinib, ABT-494, peficitinib), the novel cytotoxic T-lymphocyte antigen-4 fusion protein (leining), inhibitor of BTK (fenebrutinib) and histamine-4-receptor antagonist (toreforant)(Supplementary Material Table 1).
Effect of interventions
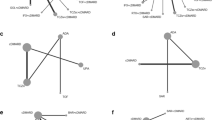
DAS28 results are derived from 3 biopharmaceuticals in 3 studies (a total of 1970 patients)(Fig. 2). The improvement of DAS28 score is ranked from high to low as tocilizumab, baricitinib and opinercept, in which tocilizumab performed best and opinercept performed worst (Table 1). The DAS28 scores of all patients treated with the drug were significantly better than those treated with placebo, but were not statistically significant (Fig. 3A). In addition, the DAS28 score was also affected by drug dose. By comparing DAS28 scores after different doses of the drug, DAS28 scores of balitinib monotherapy and tolizumab improved with increasing dose. Baricitinib at 4 mg/d and tocilizumab at 8 mg/4w are generally considered more effective. For the improvement of DAS28 scores of these 3 drugs at different doses, the top three were tocilizumab at 8 mg/4w, baricitinib at 4 mg/d, and opinercept at 25 mg/4w.
(A) Network plots of treatment comparisons for ACR50 in patients with D2T RA. (B) Network plots of treatment comparisons for SJC in patients with D2T RA. (C) Network plots of treatment comparisons for AE in patients with D2T RA. (D) Network plots of treatment comparisons for SAE in patients with D2T RA. Figure 2A shows direct comparisons between adalimumab and fenebrutinib, upadacitinib, and etanercept. Figure 2B and C, and 2D show direct comparisons between adalimumab and fenebrutinib, while the remaining drugs were only compared with placebo
The results for ACR50 score are derived from nine drugs in 23 studies (11762 patients in total). The improvement of ACR50 score in patients with drug use was ranked from good to poor as follows: olokizumab, tocilizumab, adalimumab, baricitinib, and upadacitinib. But filgotinib, ABX464, etanercept, sarilumab, rituximab, fenebrutinib, golimumab, secukinumab, RG6125 and leining were not statistically significant compared to placebo (Fig. 3B). Among them, olokizumab had the greatest effect on ACR50 score improvement, while leining had the least improvement. All drugs except RG6125 and leining improved ACR50 scores more than placebo. In addition, different doses of each drug have been shown to affect the efficacy of that drug. First, the efficacy of olokizumab, tocilizumab, baricitinib, sarilumab, rituximab and fenebrutinib increased with dose. By comparing the ACR50 scores of different doses, the ACR50 scores of patients using filgotinib, ABX464, adalimumab, upadacitinib and secukinumab improved with dose reduction. At different doses and frequencies of administration, the top three improvements in ACR50 score from high to low were olokizumab at 64 mg/4w, tocilizumab at 8 mg/4w and ABX464 at 50 mg/d.
Forest plot of biologics versus placebo efficacy in D2T RA. (A) DAS28; (B) ACR50. Figure 3 shows that for two measures of efficacy: DAS28 and ACR50. In DAS28 results, there was no statistical significance in the therapeutic effect of all drugs compared with placebo. However, olokizumab, tocilizumab, adalimumab, baricitinib, and upadacitinib performed better than placebo, when ACR50 was used as an index to evaluate efficacy
We secondarily compared various biologics’ curative effect in the field of SJC, TJC, CRP, ESR, leukomonocyte. At the end of the interventions, opinercept (SMD = − 5.2, 95% CI: -9.3 - -1.1), tocilizumab (SMD = − 4, 95% CI: -6.6 - -1.1) and sarilumab (SMD = − 3.6, 95% CI: -6.0 - -0.98) were superior in reducing SJC compared with placebo. However, in other aspects, none of the biological showed both statistically significant and effective curative effects. Ranking the efficacy of the drugs by various evaluation methods, we found that tocilizumab was the most effective drug for the ranking of ESR results, while opinercept had the best impact on the SJC score.
Safety of interventions
The results of AE are derived from 10 biologic drugs in 24 studies (3970 patients). Compared with placebo, the incidence of AE was higher with sarilumab [OR = 2, 95%CI (1.4, 2.8), P < 0.05], tocilizumab [OR = 2.5, 95%CI (1.3, 4.6), P < 0.05], golimumab [OR = 3.8, 95%CI (2.3, 6.3), P < 0.05], indicating poor safety. The incidence of AE from lowest to highest was rituximab, adalimumab, fenebrutinib, filgotinib, HLX01, opinercept, peficitinib, ABT_494, leining, etanercept, baricitinib, upadacitinib, toreforant, ABX464, sarilumab, tocilizumab, golimumab, among them, the incidence of AE of rituximab is low, and the safety is relatively good. Golimumab has a high incidence of AE and a relatively poor safety profile. The dose and interval of different drugs also affect the incidence of adverse drug outcomes. For both filgotinib and peficitinib, it is noteworthy that the incidence of AE increased with increasing drug dose. There is no significant linear relationship between the improvement of the incidence of AE and dose of sarilumab, with the best dose being 100 mg/2w and the worst dose being 100 mg/w. By ranking the AE rates of drugs using different doses, we found that the top three AE rates from low to high were figotinib at 200 mg/d, figotinib at 50 mg/d, and rituximab at 500 mg/2w.
We secondarily compared various biologics’ curative effect in the field of SAE and incidence of death. The only treatment with a reasonable probability of achieving a clinically relevant reduction in SAE as compared with placebo is upadaxitinib (OR = 3, 95%CI: 1.2–8.9). In different doses of drugs that reduce the incidence of SAE, sarilumab is the most effective drug, and with 150 mg/w is the best.
Subgroup analyses
Due to the lack of statistical significance in the comprehensive results of some of the main indicators, we conducted subgroup analysis. We further stratified patients to include those who had a poor response to at least two bDMARDs, csDMARDs, DMARDs, MTX/TNF-αi, or TNF-i. Therefore, we divide the causes of D2T RA into the following categories: bDMARDs inefficacy, csDMARDs inefficacy, DMARDs inefficacy, MTX/TNF-a i inefficacy and TNF i inefficacy. Compared with placebo, JAK i and T cell costimulation modulator are more effective in treating D2T RA refractory to bDMARDs. For D2T RA refractory to csDMARDs, JAK i and IL-6 i have higher effectiveness (Table 2).
Risk of bias within studies and heterogeneity
Judging from the trajectory diagrams, the iteration number reaches more than 5000 times, the MCMC chain fluctuates stably and overlaps well, and judging from the density diagram, the iteration number reaches 20,000 times, Bandwith tends to 0 and reaches stability, which comprehensively shows that the model converges well and its quality evaluation is high. Secondly, according to the node analysis diagram, most of the P-value between the direct, indirect and reticular comparison of various biologics are greater than 0.05, and there is no statistical difference. Thirdly, The calculated I2 in each indicator between direct comparison and indirect comparisonare similar, indicating good heterogeneity. Lastly, the calculated DIC values based on Consistency model and inconsistency model showed that in the same indicator, the DIC values of both are similar. It proved that the consistency basically perfect fit.
Discussion
The term ‘D2T RA’ has recently been defined to characterise a heterogeneous group of RA patients with persistent signs and symptoms [11]. D2T RA can cause serious harm such as gradual erosion of joints, limited mobility, and even disability. For the above characteristics, the EULAR defines two overarching principles and 11 PtCs (involving diagnostic confirmation of RA, assessment of inflammatory disease activity, pharmacological and non-pharmacological interventions, treatment compliance, functional impairment, pain, fatigue, goal setting and self-efficacy, and the impact of comorbidities), which are the latest guidelines for the treatment of D2T RA [3]. However, the treatment guideline does not rank the effectiveness and safety of relevant treatment drugs and treatment methods. This article contributes by quantifying relevant indicators and supplement the treatment guideline.
Tocilizumab shows the highest efficacy compared to other biological agents when considering all indicators. According to our study, tocilizumab ranked first for improvement in DAS28 score, second for improvement in ACR50 score, and second for improvement in SJC. Tocilizumab specifically targets the IL-6 receptor and inhibits IL-6-related signal transduction. It further reduces acute phase reactants, reduces B cell activation, bone resorption and cartilage destruction, thereby inhibiting the differentiation of T lymphocytes into Th17 cells and quickly and effectively controlling the progression of RA. At the same time, because tocilizumab can inhibit the down-regulation of the cytochrome P450 enzyme (CYP) system caused by IL6, and this down-regulation is only confirmed at high concentrations, the efficacy of 8 mg/4w of tocilizumab is better than that of 4 mg/4w of tocilizumab [12].
Tocilizumab ranks second in lower the incidence of AE but has a poor safety profile compared to placebo. To improve the safety of tocilizumab therapy in patients with RA, several measures can be taken. Firstly, careful patient selection is crucial. Tocilizumab should be used in patients who have had an inadequate response to DMARDs or biologic agents. Patients with a history of serious infections, active tuberculosis, or other contraindications should be excluded from treatment. Regular monitoring of patients on tocilizumab is necessary to detect early signs of adverse events. Besides, the dose of tocilizumab should be optimized based on the patient’s weight and response to treatment. High doses can increase the risk of side effects. Concomitant use of tocilizumab with DMARDs such as methotrexate can enhance the efficacy and safety of tocilizumab therapy. This combination can help maintain remission or low disease activity and prevent flares of RA symptom. Comorbid conditions such as cardiovascular disease, diabetes, or renal insufficiency should be managed concurrently with tocilizumab therapy to reduce the risk of exacerbating these conditions due to the immunosuppressive effects of the drug. Measures can enhance the safety and tolerability of tocilizumab therapy in patients with RA, ensuring that they benefit from its therapeutic effects while minimizing the risk of adverse events.
We found that olokizumab produced the most significant improvement in ACR50 score compared to other drugs. Olokizumab is a monoclonal antibody that targets the IL-6 receptor, which plays a key role in the pathogenesis of RA by promoting inflammation and joint damage. By blocking the IL-6 receptor, olokizumab can effectively reduce inflammation and slow down the progression of RA, leading to improved clinical outcomes such as reduced pain and swelling, improved physical function, and lower disease activity scores [13]. However, while olokizumab is effective in treating RA, it has been associated with a higher rate of AEs compared to placebo or other treatments. These AEs may include infections, neutropenia, thrombocytopenia, liver enzyme elevations, and hypersensitivity reactions [14]. The increased risk of these events could be due to the immunosuppressive effects of olokizumab, which not only reduces inflammation but also suppresses the immune system’s ability to fight off infections and other diseases. Additionally, the pharmacodynamic properties of olokizumab, such as its long half-life, might contribute to an increased risk of certain types of AEs [15]. Moreover, the occurrence of AEs with olokizumab treatment may also be influenced by patient factors, including their overall health status, concomitant medications, and comorbidities. For example, patients with pre-existing conditions such as cardiovascular disease or diabetes may be more susceptible to certain adverse events associated with olokizumab treatment [16].
Sarilumab treated patients had the lowest rate of AE, and in addition, the improvement in SJC was the third highest among the drugs. Sarilumab is a humanized monoclonal antibody that targets the interleukin-6 receptor (IL-6R), a cytokine involved in the pathogenesis of RA. It works by blocking the IL-6 signaling pathway, which is crucial for the inflammatory response characteristic of RA. This targeted approach helps to reduce inflammation and the associated symptoms of RA, such as joint pain and swelling [17, 18]. The lower probability of AEs with sarilumab treatment can be attributed to several factors. Sarilumab’s high specificity for the IL-6R allows it to exert its therapeutic effect more selectively on cells expressing IL-6R without widespread effects on other immune cell populations [19]. This selectivity may lead to fewer off-target effects and a reduced risk of certain AEs common with other immunosuppressive agents used to treat RA [20]. Posteriorly, the pharmacokinetic properties of sarilumab, including its dosing regimen and route of administration, could contribute to its safety profile. Subcutaneous injections might provide a more controlled release of the drug, potentially resulting in less fluctuation in blood levels and a reduced risk of side effects. Clinical trial data have shown that sarilumab is generally well-tolerated [21].
Compared to previous articles focusing on effectiveness and safety, our study uniquely employs indirect comparisons, offering a more direct assessment of different drugs’ advantages and disadvantages. In addition, we conducted a subgroup analysis to visually demonstrate whether the combined efficacy of drug classes across various aspects is meaningful. These provide a more referential comparison and selection.
Inevitably, the article also has certain limitations. On the whole, we’re dealing with small sample sizes of drugs, lots of doses of various drugs but small samples of each dose. It can also be seen from the results section that there is some bias and heterogeneity in the data. Especially, in our study based on drug types, although it is easier to understand, the sample size and dose of different drugs can affect the weight. It may have an impact on the accuracy of the results. While in our study based on the dosage of drugs, the results of all dose comparisons of all drugs are too many and have limited significance for clinical guidance. Just like in the index AE, rituximab was the only drug that was more effective than placebo, but its data were not statistically significant. But the clinical data provided by the only included original articles are meaningful [22]. The reason might be this paper was a network meta analysis, and the addition of other articles influence its weight then led to the change of the 95%CI of the drug. These all show that we need more articles for reference.
In conclusion, our study comprehensively analyzes the efficacy and safety of various therapeutic drugs in D2T RA and evaluates optimal therapeutic doses. Notably, rituximab is generally the safest drug, with 8 mg/4w tocilizumab demonstrating the best efficacy, followed by 200 mg/2w sarilumab and 4 mg/d baricitinib compared with placebo. This information aims to empower patients and clinicians in making informed decisions regarding RA treatment. Moreover, we found that JAK inhibitors and T cell costimulation modulators are effective in D2T RA refractory to bDMARDs, while JAK inhibitors and IL-6 inhibitors show effectiveness in D2T RA refractory to csDMARDs. However, this issue necessitates further research and clinical observations.
Data availability
The data supporting this meta-analysis are from previously reported studies and datasets, which have been cited. All data generated or analyzed during this study are included in this published article. The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
8. References
Chen Y, Wang B, Chen Y, Wu Q, Lai WF, Wei L, Nandakumar KS, Liu D. HAPLN1 affects cell viability and promotes the pro-inflammatory phenotype of Fibroblast-Like synoviocytes. Front Immunol. 2022;13:888612.
Watanabe R, Okano T, Gon T, Yoshida N, Fukumoto K, Yamada S, Hashimoto M. Difficult-to-treat rheumatoid arthritis: current concept and unsolved problems. Front Med (Lausanne). 2022;9:1049875.
Nagy G, Roodenrijs NMT, Welsing PMJ, Kedves M, Hamar A, van der Goes MC, Kent A, Bakkers M, Pchelnikova P, Blaas E, et al. EULAR points to consider for the management of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2022;81:20–33.
Roodenrijs NMT, de Hair MJH, van der Goes MC, Jacobs JWG, Welsing PMJ, van der Heijde D, Aletaha D, Dougados M, Hyrich KL, McInnes IB, et al. Characteristics of difficult-to-treat rheumatoid arthritis: results of an international survey. Ann Rheum Dis. 2018;77:1705–9.
Kearsley-Fleet L, Davies R, De Cock D, Watson KD, Lunt M, Buch MH, Isaacs JD, Hyrich KL. Biologic refractory disease in rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77:1405–12.
de Hair MJH, Jacobs JWG, Schoneveld JLM, van Laar JM. Difficult-to-treat rheumatoid arthritis: an area of unmet clinical need. Rheumatology (Oxford). 2018;57:1135–44.
Klatzmann D, Abbas AK. The promise of low-dose interleukin-2 therapy for autoimmune and inflammatory diseases. Nat Rev Immunol. 2015;15:283–94.
Herman S, Zurgil N, Machlav S, Shinberg A, Langevitz P, Ehrenfeld M, Deutsch M. Distinct effects of anti-tumor necrosis factor combined therapy on TH1/TH2 balance in rheumatoid arthritis patients. Clin Vaccine Immunol. 2011;18:1077–82.
Roeleveld DM, Koenders MI. The role of the Th17 cytokines IL-17 and IL-22 in rheumatoid arthritis pathogenesis and developments in cytokine immunotherapy. Cytokine. 2015;74:101–7.
Roodenrijs NMT, Hamar A, Kedves M, Nagy G, van Laar JM, van der Heijde D, Welsing PMJ. Pharmacological and non-pharmacological therapeutic strategies in difficult-to-treat rheumatoid arthritis: a systematic literature review informing the EULAR recommendations for the management of difficult-to-treat rheumatoid arthritis. RMD Open 2021, 7.
Nagy G, Roodenrijs NMT, Welsing PM, Kedves M, Hamar A, van der Goes MC, Kent A, Bakkers M, Blaas E, Senolt L, et al. EULAR definition of difficult-to-treat rheumatoid arthritis. Ann Rheum Dis. 2021;80:31–5.
Shetty A, Hanson R, Korsten P, Shawagfeh M, Arami S, Volkov S, Vila O, Swedler W, Shunaigat AN, Smadi S, et al. Tocilizumab in the treatment of rheumatoid arthritis and beyond. Drug Des Devel Ther. 2014;8:349–64.
Abuelazm M, Ghanem A, Mahmoud A, Brakat AM, Elzeftawy MA, Mamdouh Fayoud A, Awad AK, Abdelazeem B. The efficacy and safety of olokizumab for rheumatoid arthritis: a systematic review, pairwise, and network meta-analysis. Clin Rheumatol. 2023;42:1503–20.
Li Y, Zhang W. [IL-6: the next key target for rheumatoid arthritis after TNF-α]. Sheng Wu Gong Cheng Xue Bao. 2017;33:36–43.
Chen L, Huan H, Cheng P, ShanLe Y, Rong C. Research progress of anti-rheumatic drugs for improving disease condition by biological and targeted synthesis. Med Guide. 2022;41:997–1003.
Chun P. A IL-6 receptor blocker for rheumatoid Ar-thritis—sarilumab. Pharm Inform. 2019;08:1–8.
Onuora S. Rheumatoid arthritis: Sarilumab more effective than adalimumab. Nat Rev Rheumatol. 2017;13:2.
Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–603.
Lamb YN, Deeks ED. Sarilumab: a review in moderate to severe rheumatoid arthritis. Drugs. 2018;78:929–40.
Crotti C, Biggioggero M, Becciolini A, Favalli EG. Sarilumab: patient-reported outcomes in rheumatoid arthritis. Patient Relat Outcome Meas. 2018;9:275–84.
Emery P, Rondon J, Parrino J, Lin Y, Pena-Rossi C, van Hoogstraten H, Graham NMH, Liu N, Paccaly A, Wu R, Spindler A. Safety and tolerability of subcutaneous sarilumab and intravenous tocilizumab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2019;58:849–58.
Peterfy C, Emery P, Tak PP, Østergaard M, DiCarlo J, Otsa K, Navarro Sarabia F, Pavelka K, Bagnard MA, Gylvin LH, et al. MRI assessment of suppression of structural damage in patients with rheumatoid arthritis receiving rituximab: results from the randomised, placebo-controlled, double-blind RA-SCORE study. Ann Rheum Dis. 2016;75:170–7.
Acknowledgements
We would like to thank all the participants involved in this study. Thanks to the researchers for sharing the original clinical cohort data, which makes this meta-analysis possible. Special thanks to the careful efforts made by reviewers and editors to improve articles.
Funding
This project was supported by grants from the Natural Science Foundation of Shanxi Province (No. 202203021221269) and the National Natural Science Foundation of China (No. 82001740).
Author information
Authors and Affiliations
Contributions
Qin-Yi Su and Jing Luo designed the study. Jing Luo, Yan Zhang, Qian Li, Zhong-Qing Jiang searched the literature. Qin-Yi Su, Jing Luo, Zi-Rong Wen and Qian Li selected the data. Sheng-Xiao Zhang, Jing Luo, Mo-Ran Shi and Yi-Cong Zhao analyzed the data. Qin-Yi Su, Zhong-Qing Jiang, Yu-Ying Wang and Jing Luo wrote the manuscript. Sheng-Xiao Zhang and Qin-Yi Su contributed to manuscript revision, read, and approved the submitted version. *Sheng-Xiao Zhang is corresponding author.
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no confict of interest.
Ethics approval
This is an observational study. The Research Ethics Committee has confirmed that no ethical approval is required.
Patient consent statement
Not applicable.
Declarations
Ethics statement This study does not involve human participants or animal subjects.
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Su, QY., Luo, J., Zhang, Y. et al. Efficacy and safety of current therapies for difficult-to-treat rheumatoid arthritis: a systematic review and network meta-analysis. J Transl Med 22, 795 (2024). https://doi.org/10.1186/s12967-024-05569-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-024-05569-x