Abstract
Viral infections pose significant threats to human health, leading to a diverse spectrum of infectious diseases. The innate immune system serves as the primary barrier against viruses and bacteria in the early stages of infection. A rapid and forceful antiviral innate immune response is triggered by distinguishing between self-nucleic acids and viral nucleic acids. RNA-binding proteins (RBPs) are a diverse group of proteins which contain specific structural motifs or domains for binding RNA molecules. In the last decade, numerous of studies have outlined that RBPs influence viral replication via diverse mechanisms, directly recognizing viral nucleic acids and modulating the activity of pattern recognition receptors (PRRs). In this review, we summarize the functions of RBPs in regulation of host-virus interplay by controlling the activation of PRRs, such as RIG-I, MDA5, cGAS and TLR3. RBPs are instrumental in facilitating the identification of viral RNA or DNA, as well as viral structural proteins within the cellular cytoplasm and nucleus, functioning as co-receptor elements. On the other hand, RBPs are capable of orchestrating the activation of PRRs and facilitating the transmission of antiviral signals to downstream adaptor proteins by post-translational modifications or aggregation. Gaining a deeper comprehension of the interaction between the host and viruses is crucial for the development of novel therapeutics targeting viral infections.
Similar content being viewed by others
Introduction
RBPs are typically considered as proteins that interact with single-stranded or double-stranded RNA, thereby altering the fate or function of the RNA molecules with which they interact. Currently, thousands of different RBPs have been identified, comprising approximately 7.5% of the genes encoding proteins. RBPs extensively regulate every stage of the RNA lifecycle, including RNA splicing, mRNA synthesis and maturation, which are also essential for the life cycle of viruses. Therefore, RBPs play a critical role in host antiviral responses. The diverse functions of RBPs underscores the considerable diversity inherent in the structural elements that responsible for RNA recognition. RBPs can be categorized into canonical and non-canonical groups based on the presence or absence of RNA-binding domains (RBDs). Canonical RBPs possess well-defined RBDs, which comprise of RNA recognition motif (RRM), K homology (KH) domain, zinc-finger domains (ZNF), DEAD/DEAH helicase, double stranded RNA binding domain (dsRBD), pumilio homology domain (PUM), cold-shock domain (CSD) and others [1]. Multiple immune regulatory proteins containing well-defined RBDs play a pivotal role in the host’s innate antiviral immune response, such as RIG-I and ZNFX1 (Fig. 1). In addition, numerous non-canonical RBPs lacking recognized RBDs have been demonstrated to interact with RNA molecules and also involved in antiviral response processes, including long non-coding RNAs like Lnczc3h7a and NEAT1 [2]. In fact, non-canonical RBPs make up the majority, accounting for approximately three-quarters of the total number of RBPs. These non-canonical RBPs lack the classical RBDs; instead, they feature intrinsically disordered regions that lack characteristic motifs or may carry uncharacterized RBDs [3]. RBPs exhibit specificity in recognizing RNA sequences or structures, thereby facilitating regulation of targeted gene expression at both the transcriptional and post-transcriptional levels. RBPs also exhibit reversibility and dynamism in their interactions with RNA, facilitating versatile regulation of RNA-related processes, such as splicing, translation, and localization. These requirements ensure that the intricate and precise network of interactions between biological molecules can adapt to the dynamic cellular environment and achieve specific biological functions.
Canonical RBPs, including numerous immune regulatory proteins, possess well-defined RBDs. Illustrated here are representative examples of immune regulatory proteins that contain typical RNA-binding motifs. Some RBPs contain multiple repeated RBDs, while others possess various distinct RBDs. For example, NONO possesses two RNA recognition motifs (RRMs), whereas Mex3B contains two KH domains and one Zinc-finger-RING domain. Various domains are visually depicted within distinct colored boxes.
RBPs play a significant role in various diseases, particularly in conditions involving autoimmunity and autoinflammation, due to their involvement in RNA metabolism and stability. Dysregulation or mutations in RBPs can result in aberrant RNA processing, contributing to the development of diseases characterized by abnormal immune responses. For example, mutations in RNA-binding protein Adenosine Deaminase Acting on RNA 1 (ADAR1) cause Aicardi-Goutières syndrome (AGS), a rare autoimmune disorder that causes inflammation and leads to various neurological symptoms [4]. ADAR1, a member of the adenosine deaminase family, primarily catalyzes the conversion of adenosine (A) to inosine (I) in double-stranded RNA, subsequently affecting gene expression and protein function. Twelve AGS affected individuals from eight families harbored biallelic ADAR1 variants, which disrupted normal editing activity and lead to the accumulation of unedited or misedited RNAs generated from genomic repetitive elements [4]. RBPs also affect the maturation, activation and control of tolerance of lymphocytes, such as B cell affinity maturation and secretion, as well as T cell maturation and tolerance [5]. Therefore, RBPs are involved in the development of autoimmune pathogenesis and inflammation by regulating diverse immunological processes.
Over the past few decades, an increasing number of novel viruses and virus-related diseases have been identified. For example, acquired immunodeficiency syndrome (AIDS) is caused by human immunodeficiency virus (HIV), severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) is responsible for COVID-19, and the H1N1 virus causes H1N1 influenza (commonly known as swine flu). As underscored by the COVID-19 global pandemic, viral infections pose significant threats to global human health and economic development. RNA virus Sindbis (SINV) infection alters the activity of hundreds of RBPs by the development of RNA-interactome capture (RIC), including well-established and unconventional RBPs [6]. Virus infection activates pathways involving E3 ubiquitin ligases, kinases and chaperones, many of which are among the stimulated RBPs. Upon viral infection, the innate immune system is rapidly activated and serves as the primary force in initiating early antiviral responses [7]. The innate antiviral defense is a conserved mechanism to protect the host, which is initiated by a set of genetically encoded pattern recognition receptors (PRRs), such as RIG-I-like receptors (RLRs) [8], Toll-like receptors (TLRs) [9] and cytosolic sensors for DNA [10]. RBPs exert critical regulatory roles in the activation of PRRs and downstream signal transduction to counteract viral infections. In this review, we will focus on the role of RBPs in innate antiviral response, aiming to provide novel insight into the future research on the interactions between viruses and hosts.
Innate antiviral response
Innate immunity is the first line of defense that the host employs to counter invasion by microbial pathogens, including viruses. PRRs are activated by binding conserved pathogen-associated molecular patterns (PAMPs), such as viral RNA/DNA, which are essential for viral survival and replication. This activation of PRRs subsequently results in the activation of downstream adaptors and transcription factors such as interferon regulatory factor 3 (IRF3)/IRF7 and promotes the production and secretion of type I interferons (IFN-I) [11]. IFN-I is a family of highly conserved antiviral cytokines, which inhibit viral replication and the spread of the virus within the cell. The secreted IFN-I interacts with the IFN-I receptor (IFNAR1) on the cell surface and activates STAT1/2 by Janus kinases, which eventually leads to the expression of hundreds of IFN-stimulated genes (ISGs) and inflammatory cytokines, such as interferon-inducible RNA-dependent protein kinase R (PKR), 2’-5’-oligoadenylate synthetase (OAS), ISG15 and MxA [12]. By coordinating the expression of ISGs and the release of inflammatory cytokines, the innate immune system optimizes its capacity to detect, respond to, and block viral infections.
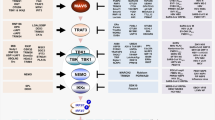
RIG-I-like receptor mediated signaling pathway
Upon being challenged with RNA viruses, cytoplasmic RNA sensors, RIG-I and MDA5, recognize and bind to viral RNA, thereby initiating innate immune response. RIG-I and MDA5 are mainly localized in the cytoplasm of cells, but they exhibit differences in their specificities for recognizing different types of viral RNA. RIG-I primarily recognizes short double-stranded RNA with a 5’ triphosphate, while MDA5 preferably detects long dsRNA, characteristic of replicative intermediates in RNA viruses [8, 13]. This diversity in their recognition capabilities enables RLRs to collectively detect a broader spectrum of viral infections, thereby contributing to the transmission of antiviral signals. Once binding to its viral RNA ligands, RIG-I and MDA5 undergo conformational changes that release the tandem caspase activation and recruitment domain (CARD), which transmit signals to the downstream mitochondrial-resident adaptor MAVS (also called VISA, IPS-1, or Cardif) [14,15,16,17]. The activation of MAVS acts as a central platform for the recruitment of ubiquitin ligases TRAF2/3/6 and kinases such as TBK1 and the IKK complex [18, 19]. Subsequently, the transcription factors IRF3 and p65 are phosphorylated and translocate from the cytoplasm into the nucleus, which turns on the transcription of IFN-I and inflammatory cytokines by binding interferon-stimulated response elements (ISRE) and NF-κB binding sites.
Cytosolic DNA sensors mediated signaling pathway
DNA sensors are widely expressed in the cytoplasm of mammalian cells and have been identified in recent years, such as DNA-dependent activator of IFN-regulatory factors (DAI, also known as ZBP1), interferon-gamma inducible protein 16 (IFI16) and 2′3′-cyclic GMP-AMP (cGAMP) synthase (cGAS) [20]. cGAS is the main DNA recognition receptor in the cytoplasm, which recognizes viral dsDNA and synthesizes 2′3′-cGAMP using ATP and GTP. The generated cGAMP functions as a second messenger and transmits the signal to the endoplasmic reticulum adaptor STING (stimulator of interferon genes, also known as MITA, ERIS and MPYS) [21,22,23,24]. Once activated, STING undergoes conformational changes and initiates oligomerization, resulting in trafficking to the Golgi apparatus and the exposure of its C-terminal tail to recruit TBK1 [25, 26]. Furthermore, the recruitment and activation of TBK1 phosphorylates IRF3, which drives the production of IFN-I and robust antiviral response. Other DNA sensors, such as DAI and IFI16, also sense intracellular DNA and transmit signals in a STING-dependent manner, but they exhibit distinct DNA recognition biases. DAI is classified among Z-DNA binding proteins and is characterized by its N-terminal Z-DNA binding domain (ZBD), which exhibits a specific affinity for left-handed double-stranded DNA helix [27]. Interestingly, DAI was also reported as an innate sensor of influenza A virus (IAV), a single-stranded RNA virus, and newly synthesized RNA of MCMV–infected cells, mediating RIPK3-dependent induction of programmed cell death [28, 29].
Toll-like receptors mediated signaling pathway
TLRs, one of the earliest discovered families of PRR, also play a pivotal role in innate antiviral immunity. Several TLRs, including TLR3, TLR7, TLR8 and TLR9, are only expressed in intracellular vesicles, such as endosomes and lysosomes. Unlike surface-positioned TLRs that recognize microbial components of extracellular pathogens, these intracellular TLRs detect nucleic acids derived from intracellular pathogens, providing a rapid and effective response to viral infections [30]. TLR3 is responsible for the recognition of dsRNA of some viral genomes and replication intermediates, such as reoviruses and respiratory syncytial virus. This recognition is enabled by its extracellular Leucine-Rich Repeat (LRR) domains, which possess a large horseshoe-like shape facilitating dsRNA interaction and detection [31]. Upon detection of viral dsRNA released during viral replication by TLR3, the receptor triggers downstream signaling through the adaptor protein TIR-domain-containing adapter-inducing interferon-β (TRIF). TRIF similarly interacts with TBK1 and promotes the activation of IRF3 and NF-κB, subsequently leading to the production of IFN-I and proinflammatory cytokines [32]. TLR7/8 are specialized in detecting single-stranded RNA from viruses, including influenza A, while TLR9 primarily recognizes unmethylated CpG motifs characteristic of bacteria and viruses.MyD88 interacts with cytoplasmic domains of TLRs and initiates a signaling cascade by involving the recruitment and activation of IRAK1/4 and TRAF6, ultimately resulting in the activation of IRF7 and NF-κB [7, 33]. TLRs are crucial for the recognition of viral nucleic acids and subsequently triggering the production of IFN-I and pro-inflammatory cytokines, which facilitates the clearance of viral infections and the robust activation of antiviral responses.
Roles of RBPs in host antiviral signal transduction
RBPs play crucial roles in regulating various aspects of RNA metabolism, including splicing, stability, and localization. Beyond these functions, emerging research underscores the pivotal role of RBPs in the intricate orchestration of host antiviral responses. This function is primarily achieved by their contributions to the recognition of viral nucleic acids or viral structural protein and the regulation of PRRs activation, which in turn facilitates the transmission of antiviral signals to downstream adaptor proteins. RBPs and PRRs share a close relationship, jointly regulating the cellular antiviral response. For example, the cytoplasmic RNA virus sensors RIG-I and MDA5 are intrinsically RBPs with classical RBDs. Similarly, the DNA sensor cGAS possesses a potential role as an RBP, primarily evidenced by its interactions with RNA and partnerships with other RBPs [34]. Many RBPs regulate the strength of host antiviral responses by targeting various PRRs (Fig. 2), such as RIG-I targeted by TRIM25, RNF135; cGAS targeted by G3BP1, ZCCHC3; TLR3 targeted by Mex3B, ZFYVE1, and so on. These RBPs are not only facilitate the recognition of viral RNA/DNA by PRRs, but also contribute to the activation and subsequent signaling transduction processes.
Once bound to viral RNA, RIG-I undergoes conformational changes and transmits signals to mitochondrial-resident adaptor MAVS. Some RBPs regulate the function of RIG-I, such as TRIM25, RNF135. cGAS recognizes viral dsDNA and synthesizes the 2′3′-cGAMP utilizing ATP and GTP, which then transmits the signal to the endoplasmic reticulum adaptor STING. Several RBPs also regulate the function of cGAS, such as G3BP1, ZCCHC3. Intracellular TLRs detect viral nucleic acids by LRR domains and combine with the adaptor TRIF for signal transduction. Several RBPs regulates TLR mediated signaling pathway, such as Mex3B, ZFYVE1. Furthermore, the recruitment and activation of TBK1 phosphorylates IRF3, which drives the production of IFN-I and robust antiviral response.
Recognition of viral PAMPs by RBPs
Recognition of viral RNA
Recognition of RNA is a pivotal mechanism utilized by RBPs, contributing significantly to the ability to distinguish and interact with pathogenic RNAs by innate PRRs. Canonical RBPs are known for their capacity to interact specifically with RNA molecules by RNA-binding domain, such as ZNF and DEAD/DEAH helicase. DEAD-box helicases are a large group of ATP-dependent RNA helicases, with 36 members discovered in humans so far. This family is highly conserved among various species, sharing the most conserved DEAD (Asp-Glu-Ala-Asp) motifs, which are critical for the helicase activity and RNA binding function. The DEAD/DEAH helicase domains are integral components of certain PRRs, including RIG-I and MDA5. Several other DEAD-box helicase family members also exert significant effects on the innate immune response to viral infections, such as DDX6, DDX60 and DDX41. DDX6, a known component of cytoplasmic mRNA-ribonucleoprotein (mRNP) granules like P-bodies and stress granules (SGs), functions as an RNA co-sensor for RIG-I by binding viral RNA and promoting the ability to induce interferon expression upon viral infection [35]. DDX60 is an IFN-inducible cytoplasmic RNA helicase that enhances the antiviral response by specifically activating RIG-I-like receptors, which binds to RIG-I and MDA5, but not to downstream signaling factors, to facilitate type I interferon production [36]. DDX41 is a key sensor in the innate immune response to retroviruses, detecting the DNA/RNA hybrid formed during reverse transcription and collaborates with cGAS to enhance the antiviral defense against murine leukemia virus (MLV) and HIV [37]. RBPs located within endosomes or mitochondria play a significant role in identifying RNA molecules and facilitating antiviral signal transduction. It has been demonstrated that zinc finger NFX1-type containing 1 (ZNFX1), employing its Armadillo-type fold and P-loop domain, operates as a mitochondria-localized sensor for double-stranded RNA (dsRNA) stimulated by interferons (IFNs) [38]. Zinc-finger FYVE domain-containing protein ZFYVE1 and Mex3B, members of the ZNF and KH families of RBPs respectively, exhibit direct binding to poly(I: C) and concurrently heightened the binding capacity of TLR3 to poly(I: C) [39, 40]. However, whether these RBPs act as co-receptors of TLR3 for viral RNA requires further experimental investigation.
On one hand, certain RBPs possess the ability to target multiple PRRs, thereby facilitating the recognition of RNA and orchestrating the regulation of the innate antiviral response, such as Protein Activator of PKR (PACT). PACT functions as a cellular activator of RIG-I by stimulating its ATPase activity, thereby facilitating innate antiviral responses response to SeV [41]. Furthermore, PACT is also indispensable for MDA5-dependent induction of IFN-β during encephalomyocarditis virus (EMCV) infection by interacting with LGP2 [42]. On the other hand, the RNA sensor function can also be achieved by forming complexes with multiple RBPs. For instance, a complex composed of three DEAD-box helicase family members, specifically DDX1, DDX21 and DHX36. The DDX1-DDX21-DHX36 complex has been identified as a key RNA sensor for dsRNA in myeloid dendritic cells (DCs) by the isolation and sequencing of poly(I: C)-binding proteins, which utilize the TRIF-dependent pathway to initiate IFN-I production [43]. Specifically, DDX1 binds to poly(I: C) through its Helicase A domain, while DHX36 and DDX21 interact with the TIR domain of TRIF by their HA2-DUF and PRK domains, respectively. Additionally, RBPs can also exert positive regulatory effects on viral replication. RBPs not only serve well-recognized roles as cofactors facilitating viral proliferation, such as DDX1 and DDX56 in HIV-1 replication [44], but also bind to viral RNA, leading to the promotion of viral replication by dampening the production of IFN-I. For instance, STAU1 binds to the genomic double-stranded RNA (dsRNA) of infectious bursal disease virus (IBDV), subsequently affecting the MDA5-dependent induction of IFN-β [45].
Recognition of viral DNA
Besides the widely recognized functions of RBPs in binding to RNA molecules, recent studies have revealed the capacity of specific RBPs to interact with DNA as well. Firstly, numerous RBPs can facilitate the interaction between viral DNA and cGAS. Employing a tandem affinity purification followed by a mass spectrometry (TAP-MS) approach, poly(rC)-binding protein 1 (PCBP1) was identified as a cGAS-associated protein. PCBP1 directly binds to viral DNA, significantly facilitating the interaction between cGAS and DNA, and thereby promoting the IFN-I signaling induced by DNA viruses [46]. ZCCHC3, a CCHC-type zinc-finger protein of RBPs, emerges as a co-sensor of cGAS that contributes to the effective detection of dsDNA and facilitates the innate immune response following DNA virus infections [47]. In addition, ZCCHC3 expands its role in the innate immune response by targeting RNA sensors RIG-I and MDA5. It potentiates the binding affinity of RIG-I and MDA5 to viral dsRNA and facilitates their activation by recruiting the E3 ubiquitin ligase TRIM25, thereby significantly promoting innate immune response to counteract RNA virus infections [48].
Furthermore, several DNA sensors exhibiting RNA-binding capabilities, such as ZBP1 and cGAS, hold a significant position in the innate antiviral response triggered by DNA viruses. ZBP1, classified as a member of the IMP family of RBPs, possesses six conventional RNA-binding domains, including two RRM domains and four hnRNP K homology (KH) domains [49]. ZBP1, also known as DAI (DNA-dependent activator of IFN-regulatory factors), is a critical cytosolic DNA sensor that binds to dsDNA and interacts with the IRF3 and TBK1 kinase, hereby enhancing the DNA-mediated activation of innate immune responses [50]. cGAS is also considered a potential RBP, and the significance of its RNA-binding activity cannot be ignored in the cGAS-mediated innate antiviral response [34]. cGAS exhibits a distinctive interaction with cia-cGAS, an exonic circular RNA possessing a paired stem region. In detail, cia-cGAS is highly expressed in murine long-term hematopoietic stem cells (LT-HSCs), which inhibits the interaction between cGAS and genomic DNA and reduces the production of IFN-I [51]. Notably, the RNA binding activity of cGAS does not serve as an RNA sensor in a manner similar to RIG-I, which substantially affects the DNA-binding activity of cGAS and the innate antiviral response induced by DNA viruses. Finally, RBPs potentially play crucial roles in sensing DNA viruses within the cell nucleus, such as hnRNPA2B1. hnRNPA2B1 is one member of the heterogeneous nuclear ribonucleoprotein (hnRNP) family of RBPs, containing two consecutive RRMs at its N-terminus. Upon DNA viral entry and release of genomic DNA into the nucleus, nuclear-localized hnRNPA2B1 recognizes viral DNA, and undergoes homodimerization and subsequently demethylation by JMJD6 [52]. This modified hnRNPA2B1 then relocates to the cytoplasm, where it plays a crucial role in activating the TBK1–IRF3 pathway, leading to the initiation of IFN-α/β production.
Recognition of viral structural protein
In addition to recognizing viral nucleic acids, RBPs also play an indispensable role in innate immune antiviral responses by binding viral structural proteins within the cytoplasm and nucleus. DDX1 is a member of the host DExD/H helicase family that acts as a coactivator role in the induction of the IFN-β response initiated by TGEV (transmissible gastroenteritis virus) infection. Among the TGEV-encoded proteins, nsp14 is identified as the most potent inducer of IFN-β by activating NF-κB. During this process, the host RNA helicase DDX1 plays a crucial role by interacting with nsp14, significantly contributing to the TGEV-induced IFN-β responses and the expression of ISGs [53]. DDX1 also inhibits FMDV (foot-and-mouth disease virus) replication by interacting with FMDV 3D, thereby participating in IFN-β activation and ISG expression [54]. TRIM5α, a well-characterized antiviral restriction factor, limits retroviral infection through a direct interaction with the viral capsid, leading to early uncoating and disassembly of the capsid [55]. This mechanism might result in the release of viral nucleic acid, which can be detected by cytosolic PRRs. In the nucleus, RBPs, which are commonly hijacked by viruses for replication, can additionally modulate the innate immune defense. NONO, an RBP containing two RRM domains, serves as a critical sensor of the HIV capsid within the cell nucleus. NONO was previously regarded as a versatile RNA- and DNA-binding protein scaffold with roles in transcription, splicing, and the DNA damage response. Lahaye et al. found that NONO directly binds to the capsid of HIV, exhibiting a stronger affinity for weakly pathogenic HIV-2 compared to highly pathogenic HIV-1. Upon HIV infection, NONO is indispensable for activating cGAS and facilitating the association of cGAS with HIV DNA in the nucleus [56].
Regulation of PRR activation
Functions of RBPs in RIG-I/MDA5 activation
The activation of PRRs is a critical step in the innate immune response, which facilitates the transmission of essential signals for a coordinated defense against infections and enables the immune system to quickly respond to virus invasion. The members of the TRIM family have garnered substantial attention in the field of immune signal transduction by E3 ubiquitin ligase activity, and have also been classified as novel RBPs owing to the presence of RNA binding activity associated with their NHL (NCL-1, HT2A, and LIN-41) or PRY/SPRY domains [57, 58]. Multiple members of the TRIM family have been reported to participate in the regulation of RIG-I activation, such as TRIM4 and TRIM25. TRIM4 associates with RIG-I and promotes its K63-linked polyubiquitination, which is crucial for the activation of RIG-I and RIG-I- mediated IFN-I production [59]. TRIM25 is characterized by a distinctive structural configuration comprising a RING domain, two B-box domains, a coiled-coil domain (CCD), and a C-terminal SPRY domain, which is notable for its pioneering role as the first TRIM protein confirmed to possess immune regulatory functions [60]. TRIM25 catalyzes the attachment of K63-linked polyubiquitin chains to RIG-I, facilitating its conformational change and activation [61]. TRIM25’s SPRY domain interacts with the first CARD of RIG-I and catalyzes K63-linked polyubiquitination at lys-172 of the second CARD. As later found by Dr. Shu’s research group, TRIM25 also catalyzes the activation of MDA5 in a similar manner, thus playing a role in the MDA5-MAVS antiviral axis [48]. TRIM25 also is a crucial cofactor for the antiviral protein ZAP, enhancing its activity against diverse viruses. TRIM25 enhances ZAP modification via K48- and K63-linked polyubiquitin, although this doesn’t directly impact ZAP’s antiviral efficacy [62]. Instead, TRIM25 is crucial for ZAP’s ability to inhibit the translation of viral genomes. Additionally, nuclear TRIM25 plays a distinct role in inhibiting the replication of IAV by directly interfering with viral RNA synthesis. TRIM25 can simultaneously bind to both the RNA and protein components of viral ribonucleoproteins (vRNPs), which prevents the movement of RNA into the polymerase complex and inhibits viral RNA synthesis [63, 64].
The activation of MDA5 is also influenced by members of the TRIM family, such as TRIM65 and TRIM38. TRIM65 preferentially associates with MDA5 rather than RIG-I, and it is indispensable for enhancing the K63-linked ubiquitination of MDA5 at K743, a modification that is vital for the oligomerization and activation of MDA5 [65]. TRIM38 functions as a positive regulator in the innate antiviral response by serving as a SUMO E3 ligase, facilitating the dynamic sumoylation of MDA5 at K43/865, as well as RIG-I at K96/888, respectively [66]. Conversely, members of the TRIM family can also inhibit the activation of RLRs, such as TRIM40. TRIM40 acts as a suppressor of the RLR signaling pathway by directly targeting MDA5 and RIG-I, which promotes the K27- and K48-linked polyubiquitination of MDA5 and RIG-I, eventually leading to their subsequent proteasomal degradation [67]. Intriguingly, TRIM13 plays a dual role in regulating the RLR-mediated signaling pathway. TRIM13 acts as a negative regulator of MDA5-induced type I IFN production by interacting with MDA5, while potentially contributing to the positive modulation of RIG-I activity [68].
There are abundant reports indicating that Zinc finger proteins (ZFPs) have diverse functions in the immune responses induced by viruses, encompassing both antiviral and proviral roles [69]. DNA/RNA-binding ZFPs, characterized by zinc finger motifs coordinating zinc ions for structural stability, constitute a significant portion of human proteins, with the C2H2 Zinc finger, RING finger proteins (C3HC4), PHD ((Plant Homeo Domain)), and LIM (Lin-11, Isl-1, and Mec-3) types being prominent subfamilies [70]. Multiple RING finger proteins play crucial roles in innate immunity through their ubiquitination activity, such as RNF135/Riplet, RNF122 and RNF125. RNF135 interacts with the C-terminal helicase and repressor domains of RIG-I, leading to K63-linked polyubiquitination of the C-terminal region of RIG-I [71]. RNF122 serves as a negative regulator of RIG-I-induced antiviral innate immune response by promoting K48-linked ubiquitination at the K115 and K146 sites within the RIG-I CARDs [72]. RNF125 is also recognized as a negative modulator of RIG-I-induced signaling, facilitating the ubiquitination-mediated proteasomal degradation of RIG-I/MDA5 [73]. Recent research has indicated that ZFP36 (also termed TTP), a member of the Zinc-finger RNA-binding protein family, is a positive regulator of IFN expression by interacting with RIG-I. ZFP36 itself lacks ubiquitin ligase activity but facilitates the K63-linked polyubiquitination of RIG-I, mainly targeting the K154/K164/K172 residues [74]. The mutation of C118 and C162 to serine in ZFP36 failed to increase polyubiquitination of RIG-I, suggesting that these sites may play a crucial role in binding to TRIM4 or other E3 ubiquitin ligase. In addition, ZFP36 demonstrates an affinity for the AU-rich element (ARE) found within the HIV-1 genomic RNA, leading to a decrease in the production of HIV-1 virions by elevating the levels of multiply-spliced viral RNAs [75]. ZFP36 decreases the synthesis of the inflammatory factors TNF-α and IL-6 by enhancing the decay of the respective mRNA, as the 3’ UTRs of Tnf mRNA and Il6 mRNA contain ARE regions [76, 77].
Functions of RBPs in cGAS activation
cGAS is a widely distributed DNA sensor responsible for initiating innate immune responses through the synthesis of cyclic GMP-AMP (cGAMP) using ATP and GTP, and subsequently activates STING [20]. In the course of antiviral signal transduction, cGAS undergoes numerous post-translational modifications, including ubiquitination and SUMOylation, some of which are reliant on modifications by RBPs.
TRIM family proteins also exert a central role in host defense against viral infection by modifying the ubiquitination of cGAS. TRIM56 acts as an RBP with E3 ligase activity to trigger monoubiquitination of cGAS at K335, which plays a critical role in enabling cytosolic DNA sensing and the subsequent production of cGAMP [78]. In vivo, TRIM56-deficient mice are unable to mount a proper defense against herpes simplex virus-1 (HSV-1) infections but remain resistant to RNA virus infections, such as influenza A virus. TRIM41, also known as RING finger protein that interacts with C kinase (RINCK), serves a crucial role in cGAS activation by mediating cGAS monoubiquitination, promoting cGAS-mediated cGAMP synthesis and IFN-I production in response to cytosolic DNA [79]. Interestingly, some TRIM family proteins, despite lacking E3 ubiquitin ligase activity, also play a crucial role in regulating cGAS activity, such as TRIM14. During the regulation of cGAS activity, K48-linked ubiquitination of cGAS serves as a signal for p62-mediated selective autophagic degradation. In response to viral infections, TRIM14 is upregulated and recruits the deubiquitinating enzyme USP14 to cleave the K48-linked ubiquitin chains of cGAS at K414, consequently bolstering cGAS stability and strengthening the antiviral response against DNA viruses [80]. RNF185 (Ring Finger Protein 185) is identified as a positive regulator of cGAS activation, which interacts with cGAS and catalyzes K27-linked poly-ubiquitination upon HSV-1 challenges [81].
In addition to ubiquitination modifications, ZFPs also influence cGAS activation through alternative post-translational modifications, such as neddylation and sumoylation. Nedd8 E3 ligase RNF111 significantly enhances DNA-triggered cGAS activation without impacting downstream signal activation induced by cGAMP, providing clear evidence of RNF111 on the neddylation of cGAS [82]. RNF111 interacts with cGAS and facilitates its poly-neddylation at the K231 and K421 sites, thereby enhancing cGAS dimerization and its capacity to bind DNA [82]. During the early phase of viral infection, TRIM38 targets cGAS for sumoylation at K231 and K479 (corresponding to K217 and K464 in m-cGAS), a process that shields cGAS from K48-linked polyubiquitination and degradation [83]. TRIM38 also facilitates the sumoylation of STING, thereby promoting its activation and stability. Additionally, cGAS binds to cytoplasmic DNA, triggering the formation of liquid-phase condensates referred to as “droplets or foci”, which amplifies its enzymatic activity and promotes elevated cGAMP production [84]. Poly(rC)-binding protein 2 (PCBP2) has been discovered to specifically interact with cGAS, significantly inhibiting cGAS enzyme activity by preventing its condensation, which in turn helps maintain the equilibrium of the innate immune response mediated by cGAS [85]. GTPase-activating protein SH3 domain–binding protein 1 (G3BP1), a ZFP previously recognized for its role in regulating the formation of RNA stress granules, serves as a co-sensor by binding dsRNA and RIG-I, thereby positively regulating the RIG-I-mediated signaling pathway [86, 87]. It is also identified as a crucial factor for the efficient activation of the cytosolic DNA sensor cGAS, interacting directly with cGAS and enhancing its DNA-binding capacity by facilitating its oligomerization [88].
Functions of RBPs in TLRs
The structures of TLRs encompass an extracellular leucine-rich repeat (LRR) domain responsible for ligand recognition and binding, along with a single transmembrane domain and a cytoplasmic Toll-IL-1 receptor (TIR) domain for triggering downstream signaling by interacting with the adaptor TRIF. RBPs also influence antiviral responses by regulating the activation of TLRs by targeting their cytoplasmic TIR domain or extracellular domains. Ring finger protein 170 (RNF170) was identified as a TLR3-binding E3 ligase, which facilitated K48-linked polyubiquitination of TLR3 at residue K766 within its cytoplasmic TIR domain, subsequently leading to TLR3 degradation [89]. As a result, the deficiency of Rnf170 mice exhibit heightened antiviral responses when infected with encephalomyocarditis virus (EMCV), a virus that produces dsRNA in the cytoplasm, as compared to their wild-type counterparts. Furthermore, it has been observed that ZFPs with E3 ligase activity can facilitate the activation of TLR3 by targeting its TIR domain, such as TRIM3. TRIM3 is primarily localized in the Golgi apparatus but relocates to early endosomes when stimulated with the poly(I: C), which mediates K63-linked polyubiquitination of TLR3 at the K831 residue [90]. Subsequently, K63-linked polyubiquitinated TLR3 is recognized and directed to endolysosomes for activation by the endosomal sorting complex required for transport (ESCRT) complexes. Mex3B (also known as RNF195), a RING-type ZFP, functions as a co-receptor of TLR3 in the endosomes by interacting with viral dsRNA, thereby boosting TLR3’s dsRNA-binding capability. Mex3B also plays a role in maintaining TLR3 stability by promoting proteolytic processing, which is a crucial step for its subsequent activation [39].
In addition to playing a critical role in cytosolic dsDNA-induced IFN production (as mentioned above), TRIM56 also plays a significant role in RNA-induced TLR signaling. TRIM56, as a positive regulator of TLR3 signaling, serves to limit the replication of bovine viral diarrhea virus (BVDV), an economically significant positive-strand RNA virus [91]. Interestingly, TRIM56 exerts its function independent of E3 ubiquitin ligase activity but instead promotes signal transduction by a physical interaction with TRIF. RNF216, also referred to as Triad3A/ZIN, plays a pivotal role in regulating the signaling of multiple TLRs, which interacts with the cytoplasmic TIR domain of TLR4, TLR9, and TLR8 [92]. RNF216 utilizes its E3 ubiquitin-protein ligase activity to facilitate K48-linked polyubiquitination of TLR4, TLR9, and TLR8, resulting in their degradation, and ultimately suppressing antiviral immune responses [93].
Conclusions
PRRs are crucial components of the innate immune system responsible for recognizing invading viruses, which primarily function by recognizing viral nucleic acids and transmitting signals to downstream adapter molecules. RBPs are indispensable cellular constituents that play a pivotal role in the binding, regulation, and processing of RNA molecules, including their involvement in the intracellular replication processes of viruses. In this review, we summarize the roles and mechanisms of action of RBPs on various types of viral PRRs (Table S1). In this process of antiviral immune response, RBPs play a pivotal role in both virus recognition and the activation of PRRs (Fig. 3). Firstly, RBPs serve as RNA/DNA sensors, directly recognizing viral nucleic acids, such as RIG-I, ZNFX1, and ZBP1. RIG-I serves as the primary cytoplasmic RNA sensor, while ZNFX1 is a mitochondrial-localized dsRNA sensor, transmitting signals to MAVS to initiate antiviral responses. Secondly, RBPs can function as co-receptors for PRRs, enhancing the antiviral response induced by RNA/DNA viruses, such as Mex3B, DDX6 and ZCCHC3. Zinc-Finger Protein ZCCHC3 plays a dual role in the immune response to viral infections. It acts as an essential co-receptor for RLRs, facilitating the binding of RIG-I and MDA5 to viral RNA. Concurrently, ZCCHC3 augments the cGAS sensor’s ability to recognize dsDNA, thereby promoting cGAS-mediated immune signaling upon viral infection. Thirdly, RBPs can also serve as crucial sensors within the cell nucleus, recognizing viral nucleic acids or structural proteins, and initiating innate immune responses to combat viral intrusions, such as nuclear-localized hnRNPA2B1 and NONO. After recognizing viral DNA entering the cell nucleus, hnRNPA2B1 forms a homodimer and undergoes demethylation mediated by the demethylase JMJD6. Subsequently, hnRNPA2B1 translocated from the nucleus to the cytoplasm, where it activates the TBK1-IRF3 pathway and initiates the production of IFN-I. NONO serves as a crucial HIV capsid sensor within the nucleus, facilitating DNA sensing by cGAS and subsequent downstream antiviral signal transduction.
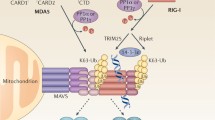
RBPs play a pivotal role in both virus recognition and the activation of PRRs. Some RBPs serve as RNA/DNA sensors (A), such as RIG-I and ZNFX1, or as a co-receptor for RIG-I and cGAS (B-C), such as Mex3B, DDX6 and ZCCHC3. RBPs can also serve as crucial sensors for viral structural proteins, such as NONO (D). On the other hand, many RBPs regulate the activation of PRRs by modulating their ubiquitination modifications (E-G), such as TRIM56 and RNF185, or regulating oligomerization for signal transduction (H), such as G3BP1.
Lastly, RBPs regulate the activation of PRRs by modulating their ubiquitination modifications or aggregation. Many RBPs possess E3 ubiquitin ligase activity, thereby promoting or inhibiting the activation of PRRs through various types of ubiquitination modifications, such as TRIM56 and RNF185. TRIM56 directly binds to cGAS, promoting its monoubiquitination, while RNF185 catalyzes K27-linked polyubiquitination of cGAS. TRIM14 indirectly enhances cGAS stability by inhibiting K48-linked polyubiquitylation through recruitment of the deubiquitinase USP14. On the other hand, RBPs can also modulate the activation of PRRs through alternative post-translational modification, such as RNF111. In addition, RBPs also influence the PRRs by oligomerization or liquid-liquid phase separation, to enhance the activation and signal transduction of PRRs, such as G3BP1. G3BP1 promotes the formation of larger cGAS complexes, facilitating the binding of cGAS to viral DNA. When cGAS binds to dsDNA, it triggers a robust phase transition into liquid-like droplets. RBPs are likely to be involved in this process, but further research is needed to identify the specific roles of RBPs. Additionally, it’s worth noting that RBPs play a pivotal role not only in the activation of PRRs but also in the regulation of downstream signaling molecules and the transcription of ISGs. These numerous RBPs, through intricate and diverse mechanisms, influence the host’s ability to recognize invading viruses and the strength of the antiviral immune response.
Data availability
No datasets were generated or analysed during the current study.
References
Lunde BM, Moore C, Varani G. RNA-binding proteins: modular design for efficient function. Nat Rev Mol Cell Biol. 2007;8:479–90.
Bao X, Guo X, Yin M, Tariq M, Lai Y, Kanwal S, Zhou J, Li N, Lv Y, Pulido-Quetglas C, et al. Capturing the interactome of newly transcribed RNA. Nat Methods. 2018;15:213–20.
Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, Youngs N, Penfold-Brown D, Drew K, Milek M, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell. 2012;46:674–90.
Rice GI, Kasher PR, Forte GM, Mannion NM, Greenwood SM, Szynkiewicz M, Dickerson JE, Bhaskar SS, Zampini M, Briggs TA, et al. Mutations in ADAR1 cause Aicardi-Goutieres syndrome associated with a type I interferon signature. Nat Genet. 2012;44:1243–8.
Kafasla P, Skliris A, Kontoyiannis DL. Post-transcriptional coordination of immunological responses by RNA-binding proteins. Nat Immunol. 2014;15:492–502.
Garcia-Moreno M, Noerenberg M, Ni S, Jarvelin AI, González-Almela E, Lenz CE, Bach-Pages M, Cox V, Avolio R, Davis T, et al. System-wide profiling of RNA-Binding proteins uncovers key regulators of virus infection. Mol Cell. 2019;74:196–.
Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801.
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–5.
Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511.
Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011;23:10–20.
Gurtler C, Bowie AG. Innate immune detection of microbial nucleic acids. Trends Microbiol. 2013;21:413–20.
Grandvaux N, Servant MJ, tenOever B, Sen GC, Balachandran S, Barber GN, Lin R, Hiscott J. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J Virol. 2002;76:5532–9.
Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–64.
Xu LG, Wang YY, Han KJ, Li LY, Zhai Z, Shu HB. VISA is an adapter protein required for virus-triggered IFN-beta signaling. Mol Cell. 2005;19:727–40.
Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–8.
Meylan E, Curran J, Hofmann K, Moradpour D, Binder M, Bartenschlager R, Tschopp J. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–72.
Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82.
Hou F, Sun L, Zheng H, Skaug B, Jiang QX, Chen ZJ. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell. 2011;146:448–61.
Yoneyama M, Fujita T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol Rev. 2009;227:54–65.
Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91.
Wu J, Sun L, Chen X, Du F, Shi H, Chen C, Chen ZJ. Cyclic GMP-AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. Science. 2013;339:826–30.
Zhong B, Yang Y, Li S, Wang YY, Li Y, Diao F, Lei C, He X, Zhang L, Tien P, Shu HB. The adaptor protein MITA links virus-sensing receptors to IRF3 transcription factor activation. Immunity. 2008;29:538–50.
Sun W, Li Y, Chen L, Chen H, You F, Zhou X, Zhou Y, Zhai Z, Chen D, Jiang Z. ERIS, an endoplasmic reticulum IFN stimulator, activates innate immune signaling through dimerization. Proc Natl Acad Sci U S A. 2009;106:8653–8.
Jin L, Hill KK, Filak H, Mogan J, Knowles H, Zhang B, Perraud AL, Cambier JC, Lenz LL. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. J Immunol. 2011;187:2595–601.
Shang G, Zhang C, Chen ZJ, Bai XC, Zhang X. Cryo-EM structures of STING reveal its mechanism of activation by cyclic GMP-AMP. Nature. 2019;567:389–93.
Zhang C, Shang G, Gui X, Zhang X, Bai XC, Chen ZJ. Structural basis of STING binding with and phosphorylation by TBK1. Nature. 2019;567:394–8.
Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 2001;8:761–5.
Kuriakose T, Man SM, Malireddi RK, Karki R, Kesavardhana S, Place DE, Neale G, Vogel P, Kanneganti TD. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci Immunol 2016; 1.
Maelfait J, Liverpool L, Bridgeman A, Ragan KB, Upton JW, Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36:2529–43.
Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nat Immunol. 2010;11:373–84.
Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–5.
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature. 2001;413:732–8.
Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–7.
Ma Y, Wang X, Luo W, Xiao J, Song X, Wang Y, Shuai H, Ren Z, Wang Y. Roles of emerging RNA-Binding activity of cGAS in Innate Antiviral Response. Front Immunol. 2021;12:741599.
Nunez RD, Budt M, Saenger S, Paki K, Arnold U, Sadewasser A, Wolff T. The RNA helicase DDX6 associates with RIG-I to augment induction of Antiviral Signaling. Int J Mol Sci 2018;19.
Miyashita M, Oshiumi H, Matsumoto M, Seya T. DDX60, a DEXD/H box helicase, is a novel antiviral factor promoting RIG-I-like receptor-mediated signaling. Mol Cell Biol. 2011;31:3802–19.
Stavrou S, Aguilera AN, Blouch K, Ross SR. DDX41 Recognizes RNA/DNA Retroviral Reverse Transcripts and Is Critical for In Vivo Control of Murine Leukemia Virus Infection. mBio 2018, 9.
Wang Y, Yuan S, Jia X, Ge Y, Ling T, Nie M, Lan X, Chen S, Xu A. Mitochondria-localised ZNFX1 functions as a dsRNA sensor to initiate antiviral responses through MAVS. Nat Cell Biol. 2019;21:1346–56.
Yang Y, Wang SY, Huang ZF, Zou HM, Yan BR, Luo WW, Wang YY. The RNA-binding protein Mex3B is a coreceptor of toll-like receptor 3 in innate antiviral response. Cell Res. 2016;26:288–303.
Zhong X, Feng L, Xu WH, Wu X, Ding YD, Zhou Y, Lei CQ, Shu HB. The zinc-finger protein ZFYVE1 modulates TLR3-mediated signaling by facilitating TLR3 ligand binding. Cell Mol Immunol. 2020;17:741–52.
Kok KH, Lui PY, Ng MH, Siu KL, Au SW, Jin DY. The double-stranded RNA-binding protein PACT functions as a cellular activator of RIG-I to facilitate innate antiviral response. Cell Host Microbe. 2011;9:299–309.
Miyamoto M, Komuro A. PACT is required for MDA5-mediated immunoresponses triggered by Cardiovirus infection via interaction with LGP2. Biochem Biophys Res Commun. 2017;494:227–33.
Zhang Z, Kim T, Bao M, Facchinetti V, Jung SY, Ghaffari AA, Qin J, Cheng G, Liu YJ. DDX1, DDX21, and DHX36 helicases form a complex with the adaptor molecule TRIF to sense dsRNA in dendritic cells. Immunity. 2011;34:866–78.
Meier-Stephenson V, Mrozowich T, Pham M, Patel TR. DEAD-box helicases: the Yin and Yang roles in viral infections. Biotechnol Genet Eng Rev. 2018;34:3–32.
Ye C, Yu Z, Xiong Y, Wang Y, Ruan Y, Guo Y, Chen M, Luan S, Zhang E, Liu H. STAU1 binds to IBDV genomic double-stranded RNA and promotes viral replication via attenuation of MDA5-dependent beta interferon induction. FASEB J. 2019;33:286–300.
Liao CY, Lei CQ, Shu HB. PCBP1 modulates the innate immune response by facilitating the binding of cGAS to DNA. Cell Mol Immunol. 2021;18:2334–43.
Lian H, Wei J, Zang R, Ye W, Yang Q, Zhang XN, Chen YD, Fu YZ, Hu MM, Lei CQ, et al. ZCCHC3 is a co-sensor of cGAS for dsDNA recognition in innate immune response. Nat Commun. 2018;9:3349.
Lian H, Zang R, Wei J, Ye W, Hu MM, Chen YD, Zhang XN, Guo Y, Lei CQ, Yang Q, et al. The zinc-finger protein ZCCHC3 binds RNA and facilitates viral RNA sensing and activation of the RIG-I-like receptors. Immunity. 2018;49:438–e448435.
Biswas J, Patel VL, Bhaskar V, Chao JA, Singer RH, Eliscovich C. The structural basis for RNA selectivity by the IMP family of RNA-binding proteins. Nat Commun. 2019;10:4440.
Takaoka A, Wang Z, Choi MK, Yanai H, Negishi H, Ban T, Lu Y, Miyagishi M, Kodama T, Honda K, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–5.
Xia P, Wang S, Ye B, Du Y, Li C, Xiong Z, Qu Y, Fan Z. A circular RNA protects dormant hematopoietic stem cells from DNA sensor cGAS-Mediated exhaustion. Immunity. 2018;48:688–e701687.
Wang L, Wen MY, Cao XT. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:656–.
Zhou Y, Wu W, Xie L, Wang D, Ke Q, Hou Z, Wu X, Fang Y, Chen H, Xiao S, Fang L. Cellular RNA helicase DDX1 is involved in transmissible gastroenteritis Virus nsp14-Induced Interferon-Beta production. Front Immunol. 2017;8:940.
Xue Q, Liu H, Zeng Q, Zheng H, Xue Q, Cai X. The DEAD-Box RNA helicase DDX1 interacts with the viral protein 3D and inhibits Foot-and-Mouth Disease Virus Replication. Virol Sin. 2019;34:610–7.
Grutter MG, Luban J. TRIM5 structure, HIV-1 capsid recognition, and innate immune signaling. Curr Opin Virol. 2012;2:142–50.
Lahaye X, Gentili M, Silvin A, Conrad C, Picard L, Jouve M, Zueva E, Maurin M, Nadalin F, Knott GJ, et al. NONO detects the Nuclear HIV Capsid to promote cGAS-Mediated Innate Immune activation. Cell. 2018;175:488–e501422.
Choudhury NR, Heikel G, Trubitsyna M, Kubik P, Nowak JS, Webb S, Granneman S, Spanos C, Rappsilber J, Castello A, Michlewski G. RNA-binding activity of TRIM25 is mediated by its PRY/SPRY domain and is required for ubiquitination. BMC Biol. 2017;15:105.
Williams FP, Haubrich K, Perez-Borrajero C, Hennig J. Emerging RNA-binding roles in the TRIM family of ubiquitin ligases. Biol Chem. 2019;400:1443–64.
Yan J, Li Q, Mao AP, Hu MM, Shu HB. TRIM4 modulates type I interferon induction and cellular antiviral response by targeting RIG-I for K63-linked ubiquitination. J Mol Cell Biol. 2014;6:154–63.
van Gent M, Sparrer KMJ, Gack MU. TRIM proteins and their roles in antiviral host defenses. Annu Rev Virol. 2018;5:385–405.
Gack MU, Shin YC, Joo CH, Urano T, Liang C, Sun L, Takeuchi O, Akira S, Chen Z, Inoue S, Jung JU. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature. 2007;446:916–20.
Li MM, Lau Z, Cheung P, Aguilar EG, Schneider WM, Bozzacco L, Molina H, Buehler E, Takaoka A, Rice CM, et al. TRIM25 enhances the antiviral action of zinc-finger antiviral protein (ZAP). PLoS Pathog. 2017;13:e1006145.
Meyerson NR, Zhou L, Guo YR, Zhao C, Tao YJ, Krug RM, Sawyer SL. Nuclear TRIM25 specifically targets Influenza Virus Ribonucleoproteins to Block the Onset of RNA chain elongation. Cell Host Microbe. 2017;22:627–e638627.
Choudhury NR, Trus I, Heikel G, Wolczyk M, Szymanski J, Bolembach A, Pinto RMD, Smith N, Trubitsyna M, Gaunt E, et al. TRIM25 inhibits influenza a virus infection, destabilizes viral mRNA, but is redundant for activating the RIG-I pathway. Nucleic Acids Res. 2022;50:7097–114.
Lang XT, Tang TT, Jin TC, Ding C, Zhou RB, Jiang W. TRIM65-catalized ubiquitination is essential for MDA5-mediated antiviral innate immunity. J Exp Med. 2017;214:459–73.
Hu MM, Liao CY, Yang Q, Xie XQ, Shu HB. Innate immunity to RNA virus is regulated by temporal and reversible sumoylation of RIG-I and MDA5. J Exp Med. 2017;214:973–89.
Zhao CY, Jia MT, Song H, Yu ZX, Wang WW, Li Q, Zhang LM, Zhao W, Cao XT. The E3 ubiquitin ligase TRIM40 attenuates antiviral Immune responses by targeting MDA5 and RIG-I. Cell Rep. 2017;21:1613–23.
Narayan K, Waggoner L, Pham ST, Hendricks GL, Waggoner SN, Conlon J, Wang JP, Fitzgerald KA, Kang J. TRIM13 is a negative Regulator of MDA5-Mediated type I Interferon Production. J Virol. 2014;88:10748–57.
Miyazato P, Matsuo M, Tan BJY, Tokunaga M, Katsuya H, Islam S, Ito J, Murakawa Y, Satou Y. HTLV-1 contains a high CG dinucleotide content and is susceptible to the host antiviral protein ZAP. Retrovirology. 2019;16:38.
Cassandri M, Smirnov A, Novelli F, Pitolli C, Agostini M, Malewicz M, Melino G, Raschella G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017;3:17071.
Oshiumi H, Matsumoto M, Hatakeyama S, Seya T. Riplet/RNF135, a RING finger protein, ubiquitinates RIG-I to promote interferon-beta induction during the early phase of viral infection. J Biol Chem. 2009;284:807–17.
Wang W, Jiang M, Liu S, Zhang S, Liu W, Ma Y, Zhang L, Zhang J, Cao X. RNF122 suppresses antiviral type I interferon production by targeting RIG-I CARDs to mediate RIG-I degradation. Proc Natl Acad Sci U S A. 2016;113:9581–6.
Arimoto K, Takahashi H, Hishiki T, Konishi H, Fujita T, Shimotohno K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc Natl Acad Sci U S A. 2007;104:7500–5.
Jiang X, Xiao Y, Hou W, Yu J, He TS, Xu LG. The RNA-binding protein ZFP36 strengthens innate antiviral signaling by targeting RIG-I for K63-linked ubiquitination. J Cell Physiol 2023.
Maeda M, Sawa H, Tobiume M, Tokunaga K, Hasegawa H, Ichinohe T, Sata T, Moriyama M, Hall WW, Kurata T, Takahashi H. Tristetraprolin inhibits HIV-1 production by binding to genomic RNA. Microbes Infect. 2006;8:2647–56.
Lai WS, Carballo E, Strum JR, Kennington EA, Phillips RS, Blackshear PJ. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol Cell Biol. 1999;19:4311–23.
Fu M, Blackshear PJ. RNA-binding proteins in immune regulation: a focus on CCCH zinc finger proteins. Nat Rev Immunol. 2017;17:130–43.
Seo GJ, Kim C, Shin WJ, Sklan EH, Eoh H, Jung JU. TRIM56-mediated monoubiquitination of cGAS for cytosolic DNA sensing. Nat Commun. 2018;9:613.
Liu ZS, Zhang ZY, Cai H, Zhao M, Mao J, Dai J, Xia T, Zhang XM, Li T. RINCK-mediated monoubiquitination of cGAS promotes antiviral innate immune responses. Cell Biosci. 2018;8:35.
Chen M, Meng Q, Qin Y, Liang P, Tan P, He L, Zhou Y, Chen Y, Huang J, Wang RF, Cui J. TRIM14 inhibits cGAS degradation mediated by selective autophagy receptor p62 to promote Innate Immune responses. Mol Cell. 2016;64:105–19.
Wang Q, Huang L, Hong Z, Lv Z, Mao Z, Tang Y, Kong X, Li S, Cui Y, Liu H, et al. The E3 ubiquitin ligase RNF185 facilitates the cGAS-mediated innate immune response. PLoS Pathog. 2017;13:e1006264.
Li C, Zhang L, Qian D, Cheng M, Hu H, Hong Z, Cui Y, Yu H, Wang Q, Zhu J, et al. RNF111-facilitated neddylation potentiates cGAS-mediated antiviral innate immune response. PLoS Pathog. 2021;17:e1009401.
Hu MM, Yang Q, Xie XQ, Liao CY, Lin H, Liu TT, Yin L, Shu HB. Sumoylation promotes the Stability of the DNA sensor cGAS and the adaptor STING to regulate the kinetics of response to DNA virus. Immunity. 2016;45:555–69.
Du M, Chen ZJ. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–9.
Gu H, Yang J, Zhang J, Song Y, Zhang Y, Xu P, Zhu Y, Wang L, Zhang P, Li L, et al. PCBP2 maintains antiviral signaling homeostasis by regulating cGAS enzymatic activity via antagonizing its condensation. Nat Commun. 2022;13:1564.
Yang W, Ru Y, Ren J, Bai J, Wei J, Fu S, Liu X, Li D, Zheng H. G3BP1 inhibits RNA virus replication by positively regulating RIG-I-mediated cellular antiviral response. Cell Death Dis. 2019;10:946.
Kim SS, Sze L, Liu C, Lam KP. The stress granule protein G3BP1 binds viral dsRNA and RIG-I to enhance interferon-beta response. J Biol Chem. 2019;294:6430–8.
Liu ZS, Cai H, Xue W, Wang M, Xia T, Li WJ, Xing JQ, Zhao M, Huang YJ, Chen S, et al. G3BP1 promotes DNA binding and activation of cGAS. Nat Immunol. 2019;20:18–28.
Song X, Liu S, Wang W, Ma Z, Cao X, Jiang M. E3 ubiquitin ligase RNF170 inhibits innate immune responses by targeting and degrading TLR3 in murine cells. Cell Mol Immunol. 2020;17:865–74.
Li WW, Nie Y, Yang Y, Ran Y, Luo WW, Xiong MG, Wang SY, Xu ZS, Wang YY. Ubiquitination of TLR3 by TRIM3 signals its ESCRT-mediated trafficking to the endolysosomes for innate antiviral response. Proc Natl Acad Sci U S A. 2020;117:23707–16.
Shen Y, Li NL, Wang J, Liu B, Lester S, Li K. TRIM56 is an essential component of the TLR3 antiviral signaling pathway. J Biol Chem. 2012;287:36404–13.
Chuang TH, Ulevitch RJ. Triad3A, an E3 ubiquitin-protein ligase regulating toll-like receptors. Nat Immunol. 2004;5:495–502.
Cai C, Tang YD, Zhai J, Zheng C. The RING finger protein family in health and disease. Signal Transduct Target Ther. 2022;7:300.
Funding
This work was supported by Grants from the National Natural Science Foundation of China (Grant Nos. 82201952), Natural Science Foundation of Jiangxi Province (20232BAB216036), Doctoral startup fund of Gannan Medical University (QD202126), Science and Technology Plan Project of Jiangxi Provincial Health Commission (202311176).
Author information
Authors and Affiliations
Contributions
JL and JY wrote the main manuscript; AS and SL prepared figures; TSH and ZL designed the study and revised the manuscript. All authors read and approved the submission of this manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
No animal experiments were involved in this article.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, J., Yu, J., Shen, A. et al. The RNA-binding proteins regulate innate antiviral immune signaling by modulating pattern recognition receptors. Virol J 21, 225 (2024). https://doi.org/10.1186/s12985-024-02503-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12985-024-02503-x