Abstract
Gamma-aminobutyric acid (GABA), the most important inhibitory neurotransmitter in the human brain, has long been considered essential in human behavior in general and learning in particular. GABA concentration can be quantified using magnetic resonance spectroscopy (MRS). Using this technique, numerous studies have reported associations between baseline GABA levels and various human behaviors. However, regional GABA concentration is not fixed and may exhibit rapid modulation as a function of environmental factors. Hence, quantification of GABA levels at several time points during the performance of tasks can provide insights into the dynamics of GABA levels in distinct brain regions. This review reports on findings from studies using repeated measures (n = 41) examining the dynamic modulation of GABA levels in humans in response to various interventions in the perceptual, motor, and cognitive domains to explore associations between GABA modulation and human behavior. GABA levels in a specific brain area may increase or decrease during task performance or as a function of learning, depending on its precise involvement in the process under investigation. Here, we summarize the available evidence and derive two overarching hypotheses regarding the role of GABA modulation in performance and learning. Firstly, training-induced increases in GABA levels appear to be associated with an improved ability to differentiate minor perceptual differences during perceptual learning. This observation gives rise to the ‘GABA increase for better neural distinctiveness hypothesis’. Secondly, converging evidence suggests that reducing GABA levels may play a beneficial role in effectively filtering perceptual noise, enhancing motor learning, and improving performance in visuomotor tasks. Additionally, some studies suggest that the reduction of GABA levels is related to better working memory and successful reinforcement learning. These observations inspire the ‘GABA decrease to boost learning hypothesis’, which states that decreasing neural inhibition through a reduction of GABA in dedicated brain areas facilitates human learning. Additionally, modulation of GABA levels is also observed after short-term physical exercise. Future work should elucidate which specific circumstances induce robust GABA modulation to enhance neuroplasticity and boost performance.
Similar content being viewed by others
Introduction
Information processing in the brain occurs through an interplay between excitatory and inhibitory processes. Gamma-aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the human brain that reduces the excitability of nearby neurons. More specifically, when GABA is released in the synaptic cleft, it inhibits the activity of the post-synaptic neurons by hyperpolarizing their membrane potential and reducing their likelihood of firing. On the contrary, lower synaptic GABA concentration increases the excitability of the post-synaptic neurons, making them more prone to activation by other neurotransmitters, such as glutamate (Glu). By reducing the excitability of the post-synaptic neurons and modulating the synaptic activity, GABA plays a crucial role in orchestrating and refining the activity of neuronal networks. This points to GABA as an important modulator of neural communication to support behavior and flexible adaptation to environmental changes.
Regulation of the GABAergic activity is considered a prerequisite for inducing long-term potentiation (LTP)-like cortical plasticity, which constitutes the neural basis of learning [1, 2]. Learning is defined as sustained behavioral modifications that result from experience or dedicated practice. Numerous studies using animal models have shown that the administration of drugs that facilitate or hinder GABAergic transmission can modulate learning outcomes [3] and the formation of memories [4, 5]. These findings further highlight the critical role of the GABAergic system in learning.
The advent of 1H magnetic resonance spectroscopy (1H MRS) has made it possible to reliably measure GABA levels in the human brain in vivo. So far, numerous studies have reported associations between MRS-assessed baseline GABA concentrations and different types of behavior. A recent review of these studies resulted in the proposition of three preliminary hypotheses that highlight the potential role of baseline GABA levels in perceptual distinctiveness, interference suppression, and cognitive flexibility [6]. First, the GABA-distinctiveness hypothesis states that the inhibition induced by higher levels of baseline GABA in the perceptual processing brain regions leads to higher perceptual sensitivity and an increased ability to discriminate perceptual features. Second, the GABA-interference suppression hypothesis suggests that higher baseline GABA levels may prevent irrelevant stimuli or preponderant responses from interfering during the execution of goal-oriented tasks. Third, the GABA-flexibility hypothesis proposes that lower levels of baseline GABA reduce the brake on neural activity, leading to higher excitability and behavioral flexibility. These hypotheses suggest a crucial role for baseline GABA levels in human performance and learning.
Despite the previous focus on static GABA, it is important to keep in mind that GABA levels are dynamic and adaptive to the internal and external demands faced by organisms. As such, static resting-state GABA levels, measured at a single time point, may not provide a comprehensive picture of the dynamics of inhibitory processes and their role in behavioral performance [6]. Conversely, MRS studies involving repeated GABA measurements provide complementary insights into the relationship between GABAergic dynamics and changes in brain activity and behavior, such as during skill learning or following brain stimulation. Study designs using repeated MRS acquisitions enable the comparison of neurometabolites obtained from specific brain areas at rest and during or after the execution of a particular task within the same individual, providing insights into the interplay between excitatory and inhibitory modulations required for behavioral performance. During the past decades, a growing number of MRS studies have looked into the modulations of GABA levels resulting from task performance and learning. A recent systematic review and meta-analysis has reported on functional MRS (fMRS) studies of Glu, Glx (Glutamate + Glutamine), and GABA in response to external stimuli such as pain, visual stimulation, and motor tasks. Our approach differs from this review because we primarily focus on assessment of dynamic modulation of GABA levels in humans following various types of interventions and their association with behavioral performance [7]. Even though the focus of this review is on GABA modulations, it is relevant to also summarize intervention-related Glu modulations, as they reflect excitatory neuromodulation, thereby exerting an influence on the excitatory/inhibitory (E/I) balance necessary for successful task performance. It is noteworthy that while the Glu peak is reliably detectable in the spectrum acquired using specific MRS sequences, it overlaps with the glutamine (Gln) peak in several conventional MRS sequences. In the latter cases, the obtained measurement is referred to as the concentration of the Glx compound (Glu + Gln).
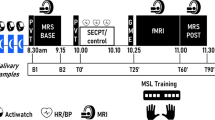
The procedure for the search and selection of studies was conducted according to the PRISMA guidelines [8]. The selection included relevant studies published before June 16, 2023, extracted from the PubMed, Web of Science, and Embase databases. We focused on interventional studies that acquired repeated MRS-assessed GABA measurements to investigate the effect of sensory stimulation, behavioral training, or other interventions promoting learning. Any further interventions, such as pharmacological and brain stimulation, were excluded from this review. More detailed information about inclusion criteria, the search strategy, and the selection of publications can be found in the Supplementary Material. The systematic search yielded a sample of 33 studies. Additionally, eight records were identified through other sources, such as references, leading to a final sample of 41 studies. Figure 1 illustrates the PRISMA chart of this search. A summary of the singular features of each study can be found in Table 1. Even though these studies commonly investigated dynamic changes in GABA resulting from behavioral intervention, it is noteworthy that the reported methods greatly vary in intervention type, intervention length, the time interval between the intervention and MRS acquisitions, behavioral measurements, the sample of participants, and target brain regions. Figure 2 illustrates the variations in the timing of MRS data acquisitions in the studies reporting dynamic changes in GABA. Hence, because of the heterogeneity in the included studies, a qualitative–narrative review was considered more suitable than a systematic–quantitative one. Furthermore, the selected studies were classified according to the behavioral protocols employed for intervention. One limitation inherent in research is the prevalence of publication bias towards null findings. In our investigation, we stumbled upon references revealing null findings concerning GABA, which were not indexed by PubMed. It's likely that numerous studies with non-significant GABA findings either remained unpublished or did not report such results, implying a potential bias in our review.
PRISMA flowchart. Titles and abstracts of 962 full-length articles, found by the search were screened; 851 studies were excluded based on the exclusion criteria (see supplementary materials) to yield 111 studies for full-text screening. Subsequently, the same investigators scrutinized the full texts of relevant articles (n = 111) against the proposed inclusion and exclusion criteria, which resulted in further exclusion of 78 articles. This resulted in 33 articles included in the qualitative synthesis. Another eight publications were manually added when screening reference lists of retrieved articles
GABA modulation in response to perceptual stimulation and plasticity
Perceptual stimulation
Perception can be understood as the processing of sensory information to create mental representations of our environment. The link between neurometabolic changes (particularly GABA) and perceptual processing has triggered considerable attention because it is considered a useful framework to understand how excitatory and inhibitory systems modulate information processing in the brain. So far, numerous studies have investigated neurometabolic alterations during visual and tactile stimulation and plasticity, which are discussed next. However, no study has yet explored neurometabolic alterations in response to auditory stimulations.
Visual stimulation
The encoding, processing, and transmission of visual inputs are of great importance for daily life. Thus far, several studies have investigated neurometabolic activity in the visual cortex following different types of visual stimulation, among which the flickering checkerboard is the most commonly used. This type of stimulation has been widely implemented in electroencephalography (EEG) and functional magnetic resonance imaging (fMRI) studies as a reliable paradigm to induce evoked potentials and blood oxygen level-dependent (BOLD) responses, respectively. However, the observed neurochemical modulations following various visual stimuli have not always been consistent across the different paradigms.
The perception of visual stimuli has been reported to induce detectable changes in the levels of MRS-assessed GABA. For example, in one study, participants were exposed to a visual stimulation protocol, starting with closed eyes in darkness, followed by opened eyes in darkness, and finally, watching a flickering checkerboard. The GABA-to-creatine levels (GABA/Cr) and Glx/Cr levels in the occipital cortex (OCC) during these three states were found to change differently. Specifically, GABA/Cr levels decreased from the eyes-closed to eyes-open states but did not change with the visual checkerboard stimuli, whereas the Glx/Cr levels did not change significantly from the eyes-closed to eyes-open state but increased with the visual checkerboard stimuli [9]. These results indicate that modulations in the excitation/inhibition balance are visual-state dependent. Additionally, Koush et al. [10] found a significant reduction of OCC GABA/NAA (N-acetylaspartate) levels and an increase of Glx/NAA levels when participants were exposed to a flickering checkerboard as compared to a cross-fixation condition. Furthermore, they found that a greater reduction of GABA/NAA and a greater increase of Glx/NAA were correlated with a higher task-related BOLD activation during the visual stimulation. In another study, Lin et al. [11] used contrast-defined wedges moving towards or away from a fixation cross as a visual stimulation paradigm, which is considered to induce less neural adaptation through time as compared with a checkerboard. They also reported an increase in Glu levels (with water as an internal reference) during visual stimulation. However, changes in the GABA levels did not reach significance, and both GABA and Glu levels returned to the resting state levels after removing the visual stimuli. Altogether, results obtained from the aforementioned studies [9,10,11] suggest that an increase in the cortical excitability induced by increased Glu and/or reduced GABA levels may play an important role in the activation of the primary visual cortex when exposed to visual stimulation. Additionally, an increase in Glu levels along with checkerboard stimulation was observed consistently at ultra-high field strengths with event-related study designs [12,13,14]. Even though the latter studies did not reveal significant GABA modulations, the obtained findings generally support the notion of increases in excitation in the primary visual cortex when exposed to a visual stimulus.
Not only can the neurometabolite levels be modulated in response to the perception of different visual stimuli, but they can also change in response to the perception of different features of the same stimuli. Ip et al. [15] introduced a variation of the checkerboard paradigm by presenting a flashing checkerboard in four different blocks with a distinct degree of contrast in each one. Results showed an increase of Glu under the highest contrast and a significant effect of image contrast on Glu modulation. However, GABA levels in the visual cortex remained constant across the different contrast conditions and in reference to the baseline. These findings suggest that while adjusting features of the perceived images (such as contrast) modulates Glu levels, this might not be sufficient to induce detectable MRS-assessed GABA changes in the primary visual cortex. In another study, Boillat et al. [16] observed that when the perceived images elicited different BOLD responses (positive or negative), these changes in brain activity were accompanied by different dynamics of both GABA and Glx. More specifically, in the group that was exposed to an image inducing a positive BOLD response, the Glx levels were increased, whereas, in the group exposed to an image inducing a negative BOLD response, both Glx and GABA levels were decreased.
Additionally, one study has addressed GABA modulations during visual stimulation presented in only one hemifield, such that the unstimulated hemisphere could act as a control region. Mekle et al., [17] measured concentrations of GABA in the right OCC in two visual stimulation conditions. In the “voxel activation” condition, half of a rotating checkerboard in the form of a torus was presented in the left visual field, while in the “control activation” condition, the mirror-symmetric stimulus was presented in the right visual field. Results showed a significant GABA decrease of 5% in the right OCC in the “voxel activation” condition as compared with the “control activation” condition [17], suggesting reduced neuronal inhibition during the activation of primary visual cortex.
Tactile stimulation
Thus far, a limited number of studies have addressed whether tactile stimulation induces neurometabolic changes in the human brain and whether such neurometabolic fluctuations vary as a function of the frequency of tactile stimulation. As the first to investigate this hypothesis, Heba et al. [18] measured GABA levels in the bilateral sensorimotor (SM1) cortex at baseline and after 45 min of high-frequency repetitive tactile stimulation, and they observed no significant changes in GABA levels. Hence, tactile stimulation could not induce measurable changes in the SM1 GABA levels [18], suggesting that not all types of perceptual stimulation are effective modulators of GABA levels.
Aiming to investigate perceptual adaptation under different stimulation frequencies, Lea-Carnall et al. [19] applied tactile stimulation with two different stimulation frequencies in two separate sessions of 46 min. In one session, the stimulation frequency was set to match the average endogenous frequency of the sensorimotor cortex (at-resonance), whereas in the other session, it was set above this frequency (above-resonance). MRS data were acquired in the early (first 12 min) and late (last 12 min) phases of the stimulation to assess adaptive changes as a result of the stimulation. Results showed that the above-resonance stimulation induced tactile discrimination impairments, as indexed by higher mis-location error in a forced-choice tactile discrimination task and significant decreases in the S1 GABA/NAA levels. However, the adaptive behavioral effect was not significantly correlated with the amount of GABA modulation at the individual level. Furthermore, the results of the fMRI data from the latter study revealed that the above-resonance stimulation led the digit regions in the motor cortex to come closer to each other while the functional connectivity among them increased [19]. The findings were interpreted as evidence for GABA modulation to play a role in allowing the brain to adapt and reorganize its digit mapping in response to tactile input, especially when the sensorial stimulation fell outside the endogenous range.
Summary
Altogether, current evidence seems to point to an increase in excitation and a reduction of inhibition as a result of visual or tactile stimulation. Firstly, using a regular visual stimulation paradigm (such as a flickering checkerboard), reductions in GABA levels were observed in a few reported studies [9, 10, 17], but not in some others [11,12,13,14,15]. Additionally, increases in Glx or Glu levels were observed consistently along with dynamic visual stimulation [9,10,11,12,13,14,15]. Therefore, the available evidence seems to support the idea that reduction of inhibition, as indexed by increased Glu levels and/or reduced GABA levels, may play an important role in visual processing in the primary visual cortex. Secondly, a reduction of GABA levels was also observed in tactile stimulation [19]. It is important to note that the interventions in this study seem to have an evident element related to adaptation-induced neuroplasticity.
Overall, whereas consistent increases in OCC Glx or Glu levels have been observed along with dynamic visual stimulation, modulation of OCC GABA in response to the perception of images has only been observed in a limited number of studies and it appears to be more strongly induced by changes in the eye status, for example, transitioning from closed to open eyes. Therefore, we speculate that in contrast to Glu (or Glx), GABA modulation in response to visual inputs might be more difficult to induce or it may exhibit a greater and quicker tendency to return to the baseline level as a result of perceptual habituation. For example, when the visual stimuli are very simple, there is a greater likelihood of visual habituation effects. An electrocorticographic study has shown that continuously being exposed to a simple visual stimulus may lead to a decrease in the magnitude of the response in the visual cortex over time [20]. In contrast, it is possible to defy the perceptual habituation effect by presenting the stimuli in a less ordinary fashion, for example, presenting them in only one hemifield. Thus, there may be a higher chance of inducing GABA modulation in the latter condition.
Perceptual plasticity
Perceptual learning and adaptation are forms of plasticity whereby neural representations of the world change in response to repeated exposure to a specific stimulus or task, leading to improved performance. Perceptual learning and adaptation can occur in various sensory domains, such as vision, hearing, and touch. Although it has been observed that baseline GABA levels in the sensory cortex relate to perceptual discrimination ability [6, 21], less is known about the modulations of GABA during or after perceptual training, such as perceptual discrimination or noise suppression training. A few pioneering studies focusing on perceptual learning have documented the associations between GABA modulations in brain regions involved in perception.
Visual learning and adaptation after monocular deprivation
Visual learning
In a study by Frangou et al. [22], two types of visual perceptual training were carried out to assess learning ability with regard to visual signal extraction and fine feature discrimination. The signal-in-noise task (SN) presented patterns embedded in noisy dots, whereas the feature difference task (FD) presented patterns with delicate morphological characteristics (radial or concentric). For both tasks, observers were required to judge whether the presented dotted images had either radial or concentric patterns. The behavioral training consisted of 7–8 runs, with each run comprising 36 trials for each training stimulus. Participants were provided with feedback on their average performance, specifically the percentage of the correct responses, every 10–15 trials. GABA levels in the posterior occipitotemporal (OCT) cortex and posterior-parietal cortex (PPC) were measured before and after the training (~ 40 min) using a 3T MR scanner. On a group level, no significant differences were observed between GABA levels before and after the behavioral training. However, on an individual level, the training-induced performance improvements in these two tasks consistently showed a correlation with OCT GABA changes but no significant correlation with PPC GABA changes. More specifically, a larger reduction in the right OCT GABA levels was associated with a faster learning rate in the SN task (extracting signals by suppressing noisy background), whereas a larger increase in the right OCT GABA levels was related to a better final learning outcome in the FD task (extracting signals by discriminating fine feature differences) [22]. These results imply that building refined visual representations through learning is associated with significant increases in OCT GABA levels (FD task). Potentially, these GABA increases induce increased neural inhibition. Conversely, sufficient suppression of visual noise through learning is associated with greater release from inhibition, possibly induced by the reduction of OCT GABA levels (SN task). To further scrutinize the dynamic modulation of neurotransmitters during these SN and FD tasks, Frangou et al. conducted another study in which they identified OCT and PPC GABA and Glu levels at baseline and three consecutive times during training using a 7T MR scanner [23]. 400 trials of stimuli were presented during each MRS measurement and trial-by-trial feedback was provided. On a group level, results showed that the OCT GABA levels changed in opposite directions during training of the two tasks, i.e., decreased in SN but increased in FD. However, the PPC GABA levels increased with training in both tasks. Moreover, this training-induced modulation was only observed for GABA and was not detectable for Glu. Compatible with their previous results [22], the larger improvement of individual perceptual sensitivity, measured as the perceptual change in accuracy between the best performance block and the first block, divided by accuracy in the first block, was associated with a larger decrease in the OCT GABA levels in the SN task while it was associated with a larger increase in the OCT GABA levels in the FD task. Furthermore, this correlation was only observed for the OCT GABA levels and not for either the OCT Glu levels or the PPC GABA levels [23]. The results of the aforementioned studies [22, 23] highlight the specificity of neurometabolites, brain regions, and tasks in the associations between behavioral learning and the modulation of neurometabolites.
Based on the promising role of OCT GABA levels in predicting perceptual learning after single-session training [22, 23], Ziminski et al. [24] further investigated whether GABA modulation related to perceptual improvement following a multisession training protocol. In this study, the SN task was trained on three consecutive days, with 800 trials of concentric and 800 trials of radial patterns on each day. Trial-by-trial feedback was provided for all trials during training. Moreover, GABA and Glu levels in the OCT and PPC were measured before and after the multisession behavioral training, during which participants completed the behavioral tests (200 trials) without feedback. Consistent with the role of decreased GABA levels in a single session of perceptual learning [22, 23], a greater decrease in OCT GABA from pre- to post-measurement related to greater behavioral progress as measured by the accuracy difference between the post-test and pre-test. Furthermore, this brain-behavior association was specific to OCT GABA levels and did not generalize to PPC neurometabolites and OCT Glu levels [24].
Visual overlearning
Besides perceptual learning, neurometabolic modulations during perceptual overlearning have also been investigated. Overlearning refers to extra practice of an already mastered skill. Although overlearning may seem inefficient because performance gains are not always detected [25], many skills show improvement over months and even years of practice, as demonstrated by athletes, musicians, or factory employees. Shibata et al. [26] used an orientation detection learning task to study the modulation of the excitation/inhibition balance in two groups of participants: a non-overlearning group that was trained on the task for ~ 20 min and an overlearning group that was trained on the task for ~ 40 min. Additionally, they also investigated the association between the changes of neurometabolites and the anterograde interference of learning, which refers to the phenomenon whereby prior learning disrupts subsequent learning. For this purpose, the authors compared the Glu/GABA ratio from pre- and post-training MRS measurements in groups undergoing different learning stages, i.e., non-overlearning and overlearning stages. Participants were asked to select a stimulus that contained a Gabor patch out of two interleaved masked stimuli, and the task difficulty was modulated by changing the signal-to-noise ratios in the Gabor patch. Neurochemically, the OCC Glu/GABA ratio measured 30 min after the end of training was increased in the non-overlearning group and decreased in the overlearning group. However, at 3.5 h after the end of training, it returned close to baseline levels in both groups. When looking at each neurometabolite separately, they observed trends toward an increase in GABA levels in the overlearning group and an increase in Glx levels in the non-overlearning group 30 min after the training. These marginally significant results (0.05 < p-value < 0.1) might suggest that an excitation/inhibition ratio could be a more sensible measure than GABA or Glu alone for showing the plasticity of the visual cortex. Interestingly, in a subsequent control experiment, the modulation of the OCC Glu/GABA ratio was only observed in the non-overlearning and overlearning groups and not in the non-learning group. Behavioral results revealed that overlearning of a certain orientation detection made it much more resilient, such that it even interfered with subsequent learning of a new orientation. In addition, the degree of OCC Glu/GABA ratio reduction from baseline to after overlearning was positively associated with a greater magnitude of stronger anterograde interference that was observed in a second grating orientation being learned in a subsequent session [26].
Using the same perceptual learning task, Frank et al. [27] further compared GABA modulation in both children and adults while continuous measurements of GABA levels were taken in the early visual cortex throughout three phases: pre-training (9 min), during training (12 min), and post-training the task (18 min). Behaviorally, perceptual learning progress was not significantly different between the children and adult groups. However, the neurochemical data analysis showed that children exhibited a rapid increase of GABA during visual training, and it persisted until the end of training, whereas GABA levels in adults remained unchanged. Inspired by the previous study investigating overlearning and stabilization [26], the authors predicted that this boost of GABA relates to the fast stabilization of learning in children. To investigate this, behavioral experiments were conducted wherein both children and adults underwent training on two different orientations, with a 10- (Experiment 1) and 60-min (Experiment 2) intermission. Confirming the prediction, significant perceptual learning was observed in both trained orientations in children, suggesting no retrograde interference in children. However, perceptual learning was only observed in the second trained orientation but not in the first trained orientation in adults, suggesting that retrograde interference occurred in adults. In general, these findings provide support for the notion that children might exhibit rapid stabilization of learned content by means of a prompt increase in GABA levels within the brain [27].
Visual learning reactivation
Bang et al. [28] studied GABA modulation during the reactivation of an already learned and consolidated Gabor orientation. In this paradigm, participants learned a Gabor orientation on the first day and did a short test on the second day to reactivate the neural networks of this task. Meanwhile, on the second day, the OCC Glu/GABA ratios were measured before the pre-test, immediately after the post-test, and 3.5 h later. Their results showed that compared to the pre-test measurement, the OCC Glu/GABA ratio increased during the reactivation and decreased to baseline level at the 3.5 h post-test [28]. Consistent with the results of the study by Shibata et al. [26], the modulation of GABA and Glu alone was not significant, again suggesting that the excitation/inhibition ratio is a more sensitive measure for plasticity induced by visual perceptual learning.
Visual adaptation after monocular deprivation
Another study used monocular deprivation interventions (i.e., covering one eye with an eyepatch). Lunghi et al. [29] measured the changes in binocular rivalry performance and the MRS-assessed GABA levels in the OCC region and posterior cingulate cortex (PCC, as a control region) before and after a 150-min monocular deprivation of the dominant eye. They investigated whether monocular deprivation could induce changes in visual perception (quantified by measuring binocular rivalry performance) and neuroplasticity (quantified by measuring MRS-assessed neurometabolite levels). Binocular rivalry occurs when two dissimilar images are displayed to each eye simultaneously, causing the perception to switch between them. Their findings revealed a significant reduction of the OCC GABA levels, but not the PCC GABA levels, following monocular deprivation. Furthermore, a higher reduction in the OCC GABA levels correlated with a greater increment in the predominance of the deprived eye, as assessed by a deprivation index, which was transformed from the mean perceptive duration of each eye [29]. Therefore, this report not only showed modulation of GABA levels during a visual intervention but also linked the changes in GABA levels to binocular rivalry performance. This opens perspectives for using interventions to modulate neurotransmitter levels in the visual cortex to achieve behavioral improvement.
Summary
Overall, in the orientation detection learning tasks mentioned [26, 28], the critical component to success appeared to be learning to better suppress visual interference and filtering background noise from the target signal (the grating orientation). The increased OCC Glu/GABA ratio during learning and reactivation showed that optimizing the ability to cope with interference may be accomplished by a release from inhibition, consistent with the findings by Frangou et al. and Ziminski et al. [22,23,24], which linked a larger magnitude of GABA reduction to better learning in visual noise suppression.
Audio-motor mapping learning
Van Vugt et al. [30] investigated the role of GABA modulation in the discrimination of audio perception and the ability to map motor responses with perceived sounds. In this audio-motor mapping learning task, participants used their right hand to make a center-out movement, which corresponded to a feedback sound with a specific frequency. In each trial, participants were required to make a movement with an angle they thought was corresponding to the target sound. After each movement, the correct feedback sound corresponding to their movement was delivered. Participants learned to map their movement to a target sound with a specific frequency, and the reaching error was the behavioral metric. Additionally, the MRS-assessed left SM1 GABA levels were measured before, during, and after behavioral training. The results showed that the left SM1 GABA levels increased along with training, and larger increases in GABA levels were associated with greater behavioral learning [30]. Notably, the key to improvement in this audio-motor mapping task was learning to distinguish between variations in frequency, memorize the sound-movement combination, and finally generate the precise movement. As such, the link between the increment in SM1 GABA levels and better learning outcomes may be due to the beneficial role of GABA modulation in the formation of distinctive audio-motor representations.
Summary
Although higher resting-state (baseline) GABA levels in perceptual brain areas were suggested to mediate both better distinctiveness and interference suppression function in our previous review [6], enhancing perception to discriminate fine differences and to filter noise through learning appears to be achieved through differential adjustment of GABA levels, i.e., an increase for distinguishing features and a decrease for interference suppression.
Specifically, when the critical component to success was learning to better suppress visual interference and filter background noise from the target signal (the grating orientation), the increased OCC Glu/GABA ratio during learning and reactivation suggested that optimizing the ability to cope with interference may be accomplished by a decrease in inhibition [22,23,24, 26, 28], which linked the bigger magnitude of GABA reduction with better learning in visual noise suppression. In contrast, when building distinctive representations is a crucial component in learning, dynamic increases in GABA levels are associated with a maximal learning outcome [22, 23, 30]. Surprisingly, the accumulation of GABA has also been observed in the SM1 during an audio-motor mapping learning task, which again suggests that this role may not necessarily be restricted to the perceptual processing areas.
Additionally, the decreased OCC Glu/GABA ratio, mainly driven by GABA increase, during overlearning indicates that an increase of inhibition may play a role in the process of the learning stabilization along with overlearning, where the brain becomes specialized in a specific task and can shield the interference from other tasks [26]. This rapid increase in GABA levels during training may have comparable effects in facilitating the swift consolidation of acquired knowledge in children [27].
GABA modulation in response to physical exercise and motor learning
Physical exercise
Physical exercise has been associated with the prevention of cognitive decline [31]. Importantly, recent studies have shown the beneficial effects of cardiovascular exercise on boosting neuroplasticity as mediated by, for example, increases in brain-derived neurotrophic factor (BDNF) [32, 33]. Additionally, several MRS studies have looked into neurometabolic changes in the human brain as a result of physical exercise. Regarding physical exercise specifically targeting the upper limb, Chen et al. [34] used a motor training task that included a 5-min rest arranged before and after a 10-min hand-clenching exercise, during which MRS measures were continuously obtained. They found a pronounced decrease in SM1 GABA levels and an increase in SM1 Glx levels. Andrushko's study [35] measured the levels of GABA and Glx in the left primary motor cortex (M1), right M1, left supplementary motor area (SMA), and right SMA before and after a nine-minute session of right handgrip contraction at both 5% and 50% maximum voluntary contraction (MVC). Additionally, the study recorded MVC as well as the reaction times of a response task of both hands before and after the hand contraction training. The results showed that the 50% MVC contractions of the right hand resulted in better performance of the contralateral hand, i.e., decreased reaction times in the left hand. In contrast to Chen’s study, their results showed no significant difference in GABA levels before and after the right-hand contraction at the group level. However, at the individual level, a greater decrease in GABA levels in the left M1, left SMA, and right SMA was linked to larger improvements in reaction times of the left hand [35]. Hence, these findings imply that disinhibition of motor areas may facilitate contralateral hand movements.
Regarding physical exercises that predominantly involve gross motor activity and cardiovascular endurance, Coxon et al. [36] investigated modulations in neurometabolite levels after a 20-min high-intensity interval exercise performed on a stationary bicycle ergometer and observed a significant increase in the SM1 GABA levels but not in SM1 Glu levels or dorsolateral prefrontal cortex (DLPFC) neurometabolic levels [36]. These findings support the notion that physical exercise affects regional GABA levels. In another study, Maddock et al. [37] conducted a series of MRS experiments to investigate modulations in GABA and Glu after a course of vigorous cycling (up to 20 min). They acquired MRS data from the primary visual cortex and anterior cingulate cortex (ACC) before vigorous cycling and two or three times after a 1-min cool-down period following the exercise. The GABA/Cr levels in the primary visual cortex were found to be significantly higher after vigorous cycling on the first post-exercise measurement and then decreased on the second post-exercise measurement, no longer being significantly different from baseline. Additionally, increased Glu/Cr levels in the primary visual cortex and ACC were also observed after 20 min of cycling [37]. Even though the latter findings support the idea that GABA neurometabolism induced by physical exercise or other physical interventions is not limited to SM1, it remains to be investigated to what extent the GABA changes induced by physical exercise are regionally specific or cover broader brain territory.
In addition to cycling training, yoga exercise has also been implemented as a form of physical intervention. In a long-term (12 weeks) yoga training paradigm, the authors did not observe significant changes in thalamic GABA levels [38]. It is possible that modulations of GABA are not detectable after a long-term period of yoga training, or it may occur in other brain regions than the thalamus.
Overall, the aforementioned scarce evidence shows a GABA decrease along with hand clenching [34], as well as an association between a larger GABA decrease and a larger improvement in reaction times [35]. However, GABA increases seem to be rather consistently induced by short-term physical exercise involving full-body exercise or cardiovascular endurance [36, 37]. This modulation of GABA seems to occur in certain brain regions and may be modulated by different exercise intensities, structures, and durations. Consequently, these findings collectively appear to indicate that GABA metabolism plays a significant role in physical exercise.
Motor learning
Motor learning broadly refers to the process of improving the accuracy and/or speed of previously unfamiliar motor behavior through experience or dedicated practice [39, 40]. In principle, the neural basis of expert motor performance refers to the formation of neural connections that can satisfy the motor requirements in a stable manner [41]. However, performing a new task efficiently may not only require building of new motor activation patterns but also suppression of pre-existing default coordination patterns (such as in-phase inter-hand coordination) that intrude into learning the new task [42,43,44]. Modulation of the GABAergic system has been proposed to play a crucial role in the neural rewiring and reorganization processes underlying motor learning [45].
Visuomotor coordination learning
Visuomotor coordination refers to the ability to integrate visual information with performance of precise and coordinated movements.
The first study conducted in humans exploring changes in the MRS-assessed SM1 GABA levels during motor learning was conducted by Floyer-Lea et al. [46]. This seminal study made use of a 30-min repetitive force-tracking task, utilizing a pressure sensor positioned between the thumb and fingers of the right hand. Participants were required to continuously modulate the pressure between the thumb and fingers to match a target force on the screen. Therefore, inter-finger coordination is important for performing this task. GABA levels in the left SM1 were continuously measured during the performance of the task and decreased steadily during the task, yet partially recovered 20 min after completion of the training. Interestingly, these GABA modulations were not observed in a group merely executing random force-tracking movements without a learning component and in a resting (no movement) group. Furthermore, GABA modulations were not observed in the ipsilateral sensorimotor cortex during the same motor learning task.
Juggling is considered a visuomotor coordination task because individuals must visually track the trajectory of objects (such as juggling balls) and simultaneously coordinate their hand and arm movements to catch and throw these objects in a rhythmic sequence. Sampaio-Baptista investigated the effect of long-term training on juggling on the SM1 GABA levels [47]. As such, in a 6-week juggling practice protocol, participants were assigned to either a high-intensity group (30 min daily) or low-intensity group (15 min daily). While the groups ended up with comparable levels of behavioral performance, the SM1 GABA levels decreased from baseline to after 6 weeks of training in the low-intensity practice group, whereas no significant modulations in SM1 GABA were observed in the high-intensity training group. These peculiar findings appear to suggest that different intensities of practice schedules may be associated with differential neurochemical dynamics across a larger time epoch despite similar final behavioral outcomes. The authors speculated that GABA might have changed during the training period in the higher-intensity group but recovered after the end of training. More research about long-term effects of GABA is certainly warranted.
Another interesting avenue of research is to assess how GABA modulation relates to different learning protocols that have the potential to maximize skill acquisition and consolidation. Chalavi et al. [48] used a bimanual visuomotor task and investigated the acquisition of a set of three subtasks under a blocked or a random practice schedule across three days of training in a sample of young and older adult group [48]. Participants were instructed to closely track a white dot on the screen by rotating two dials simultaneously with their wrists/fingers. Participants in the blocked practice group only learned one of the subtasks on each training day, while participants in the random practice group were exposed to a randomized presentation of all three subtasks across each practice day, leading to a richer but also more demanding training context. Notably, the MRS-assessed GABA levels in the SM1 and OCC voxels were obtained before and after task training during the first and last training days. At the behavioral level, blocked practice led to a better performance during the training phase, while random practice led to a better performance during the retention phase (representing the ultimate test of learning). At the neural level, neither learning condition significantly modulated the SM1 GABA levels. In contrast, it was observed that the OCC GABA levels increased in the blocked, but decreased in the random condition as a result of within-day practice, and this effect was mainly driven by the older group. The authors argued that the decreased levels of GABA in the OCC region may be due to the more challenging learning condition in the random as compared to the blocked practice conditions, as the participants needed to frequently switch task sets and re-plan hand movements in the former condition. On the other hand, increased levels of GABA in the OCC region may have supported the building of specific neural representations, as required for the execution of this visuomotor task under blocked practice [48]. Due to the lower difficulty of the bimanual training during blocked practice, participants might have already performed the task in a stable manner. Consequently, the increased levels of GABA in the OCC region may contribute to the stabilization of acquired information.
Another study assessed GABA levels before, during, and after a unimanual/bimanual action selection task in a sample of young and older adults [49]. Participants were instructed to place their fingers on a device with force sensors and lift specific finger(s) of the left and/or right hand as cued on the screen. In both young and older adults, GABA levels within left SM1 were found to decrease during the task and returned to baseline levels afterwards. Even though investigating the learning effect was not the focus of this study, some degree of implicit learning seems to have occurred as the behavioral performance improved during the course of the experiment. Interestingly, a greater decrease in GABA was related to better bimanual performance as assessed by movement accuracy, in older adults but not in young adults [49].
Motor sequence learning
Motor sequence learning refers to the process by which individuals acquire and refine the ability to perform a sequence of movements or actions with increasing proficiency over time. It is often studied through tasks such as finger tapping and serial reaction time task (SRTT).
Kolasinski et al. [50] used a visually cued explicit SRTT in which participants pressed the target button using a specific finger of the right hand as fast and as accurately as possible following the appearance of the cue. Participants were randomly assigned to a learning group, a movement group, or a rest group. For the learning group, the sequence consisting of 16 cues was repeated, whereas for the movement group, the same task was presented without a learnable sequence (pseudorandom sequences), and the rest group only watched a video during the scan session. During the entire experiment of ~ 30 min, the left SM1 MRS data were continuously acquired at six time points. Over time, the SM1 GABA levels were observed to decrease continuously in the learning group, such that the GABA levels obtained during the last measurement were significantly lower than those obtained during the first measurement. However, no significant changes in GABA levels were observed in the movement group and the rest group. However, using the same button-pressing SRTT and a cross-over design, Bell and colleagues did not observe any significant modulation of GABA or Glx levels in either the motor learning or the movement control condition [51]. The authors speculated that using a lower magnitude field (3T) MRI scanner, a relatively bigger voxel size, and different analysis software may account for the discrepancies between their findings and those of Kolasinski et al. [50] (using 7T).
While findings of the aforementioned studies suggest modulations (i.e. decreases) in the SM1 GABA levels induced by motor learning, other studies did not observe these modulations but found a relationship between the individual modulation of GABA and performance improvement. For instance, King et al. [52] tested dynamic GABA modulations in older adults using a motor sequence learning task in which participants were required to tap their fingers following a repeated sequence. While no significant differences were found between the SM1 GABA levels measured at the pre-measurement and post-measurement epochs, at the individual level, a greater reduction of GABA levels following motor training was related to a greater learning magnitude. When interpreting findings of GABA modulations in aging samples, it is important to consider that baseline GABA levels decline with increasing age [53,54,55], hence the modulatory capacity of GABA may be decreased. Along the same line, in the latter study, a larger learning-related reduction of GABA was also associated with higher baseline GABA levels and lower age [52]. These results indicated that the participants with lower age exhibited a greater decrease in GABA and greater learning gains. Consequently, the authors tentatively concluded that a minimum amount of resting-state GABA seems necessary such that enough GABA modulation in relation to learning can take place.
In another study, Maruyama et al. [56] used a sequential finger-tapping learning task in which participants repeated the same movement sequence with their left hand, and concentrations of neurometabolites in the right M1 were collected before, during, and after training of the task. They observed motor learning-induced increases in Glu/Cr but no significant change in GABA/Cr. Additionally, they found that a greater decrease in the right M1 GABA/Glu correlated with a faster average reaction time. It is noteworthy that the MRS data during task performance were acquired 13 min after the initiation of task training (i.e., during training). Therefore, GABA level may already have reached a steady-state level at this advanced stage of training.
Visuomotor adaptation learning
In another visuomotor task, participants were instructed to make ballistic movements to move a cursor through one of eight radial targets using their right hand in two sessions on two separate days [57]. During each training session, participants performed a visuomotor task with either a rotation component (adaptation condition) or no rotation component (control condition), and measurements of magnetic resonance spectroscopic imaging (MRSI) covering bilateral cerebellum were taken four times (9 min each) at baseline, early task, late task, and after training. At the group level, adaptation-driven GABA, as measured by the GABA difference between the adaptation and control session at each time point, in the left cerebellar nuclei and the right cerebellar nuclei diverged. Specifically, adaptation-driven GABA levels in the left cerebellar nuclei increased significantly, while changes in the right cerebellar nuclei were not significant. Moreover, at the individual level, a greater decrease of GABA in the right cerebellar nuclei in the early learning phase was related to better motor adaptation [57].
Summary
Overall, relatively consistent results have been reported for the modulation of GABA during the learning of motor skills. On a group level, training-induced reductions of GABA levels were observed in the context of various motor learning paradigms, including force tracking [46], serial reaction time [50], bimanual tracking under random practice conditions [48], and juggling [47] tasks. Additionally, the modulation of GABA was only observed in motor learning conditions rather than non-learning practice conditions [46, 50]. Furthermore, several studies have reported that a more pronounced decrease in GABA levels at the individual level is associated with larger behavioral improvements [51, 52, 57]. These findings support the hypothesis that repeated practice of a specific task, rather than movement per se or rest, leads to a reduction in the SM1 GABA levels. A few studies did not observe GABA modulation along with motor learning at a group level, and this may be related to the timing of the MRS measurement, among other accounts. For example, in the study by Maruyama et al. [56], the MRS measurement was only acquired after 13 min following initiation of the 30-min motor training. As such, the critical window for GABA modulation may have been missed. Moreover, when the post-MRS measurement was acquired 15 min after completion of practice, GABA modulation may have already returned to the baseline level [52]. This prompts questions about the critical temporal window for the detection of motor learning-related GABA modulation in relation to task complexity. The discrepancy observed among the findings of the existing literature also raises the question regarding the critical brain regions for assessing GABA modulation. For example, decreases in GABA levels following short-term training were observed in other regions than the SM1 (i.e., in the OCC) and this may relate to the specific nature of the task and its sensory requirements [48].
GABA modulation in response to cognitive task performance and learning
Thus far, the relationship between cognitive task performance and dynamic modulation of GABA levels has been investigated in relation to working memory, associative learning, and inhibition and self-regulation, as discussed next.
Attention and working memory
Attention and working memory, which are two important executive functions, are both crucial for task performance and learning new information. Although it is apparent that these processes are closely intertwined, they each possess their unique characteristics. Attention allows us to select and also take in useful information, while working memory helps store this information instantly and retrieve it when it is still active. The DLPFC is regarded as a crucial node in executive functions, as it is involved in attentional control and updating of information [58, 59].
Regarding neurometabolite modulations during different levels of attentional load, Frank et al. [60] tested whether GABA and Glx levels in the parieto-insular vestibular cortex vary with attentional load. Visually tracking 1 or 2 targets out of 8 objectives was considered a low cognitive load and tracking 3 or 4 targets out of 8 objectives was considered a high cognitive load. The results revealed that Glx levels decreased while GABA levels remained stable from low to high visual attentional load [60], indicating that manipulating visual attentional load is not enough to induce MRS-detectable GABA changes.
Regarding neurometabolite modulations in the working memory task, Vijayakumari et al. [61] used the letter N-back task to assess working memory performance while the DLPFC GABA and Glu levels were obtained at baseline, during, and after task performance. In the N-back task, participants respond when the current stimulus is a specific letter (0-back) or when it is identical to the one preceding it (1-back), depending on the task condition. Their findings revealed no significant change in DLPFC GABA levels across different time points. In contrast, there was a significant increase in the Glu levels during the task and a decrease after the task [61].
In another study, Michels et al. [62] investigated the baseline and dynamics of GABA levels in the DLPFC during the Sternberg working memory training task. Five or seven letters were displayed on the screen for 2 s, and participants were required to retain these stimuli in memory for 5 s, after which they were asked to determine whether a single presented item was part of the previous stimulus set. The task was executed during four MRS blocks (10 min each) with a preceding resting MRS block. During the first working memory block, the DLPFC GABA showed a significant increase relative to baseline, followed by a significant decrease in the subsequent task runs, whereas no changes were observed in the DLPFC Glx levels. Moreover, the GABA reductions after the initial training block co-occurred with a faster reaction time and higher accuracy as a result of training. The authors proposed that an increased GABA release and an inhibitory-excitatory rebalance became evident following initial practice. The discrepancy in results between these two working memory studies may be due to differences in the required cognitive load or attention. It appears that cognitive load was significantly lower in the first (0-back and 1-back) as compared to the second experiment (5 and 7 letters). Additionally, the deployment of attention seems to be of greater importance in the 0-back and 1-back tasks, while working memory plays a more important role in the retention of multiple letters in memory. It is possible that GABA modulation is more likely detected when cognitive processing demands are higher, but this hypothesis requires further investigation. Additionally, it is important to note that in the first study, neurometabolites were measured using the PRESS sequence, which is a non-edited sequence with lower reliability for measuring GABA levels.
In summary, even though GABA modulations have been observed in working memory tasks with relatively higher cognitive load, it is currently not possible to draw solid conclusions from the aforementioned scarce and incompatible evidence. In terms of attention, it appears that manipulating visual attentional load may not suffice to trigger MRS-detectable GABA changes within attention-related brain regions.
Associative learning
Associative learning refers to the type of learning whereby an individual associates two or more stimuli with each other and/or forms connections between stimuli or behaviors. Reinforcement learning is a type of associative learning, in which behaviors are strengthened or weakened based on their consequences. One study made use of a probability discrimination reinforcement learning task in which participants were faced with two-alternative forced-choice auditory stimuli corresponding to a different probability of monetary loss or gain in three successive experimental conditions, namely uncertainty (high cognitive load: 50/50 loss/gain probability), discrimination (lower cognitive load: 80/20 loss/gain probability), and control (null condition: 00/00 loss/gain probability) [63]. Given that dorsal anterior cingulate cortex (dACC) is crucial for reward- and error-guided learning [64, 65], levels of neurochemicals in dACC were determined at baseline, during each condition and post-learning. During the uncertainty condition, as compared to the discrimination condition, the dACC GABA levels increased, and Glx/GABA decreased. In addition, no difference was observed between the initial and final resting-state dACC GABA. Glx levels did not show significant changes during the whole study. It was argued that the observed GABA modulation potentially reflects the recruitment of inhibitory networks during practice under high cognitive load (high uncertainty) conditions. These results appear to suggest that GABA modulations are more easily induced in more, as compared to less, challenging task conditions, which is in line with the previously reported evidence from the working memory task.
Furthermore, changes in GABA levels have also been investigated following a 21-day period of facial associative learning [66]. Fifty-two out of 200 facial pairs were randomly chosen and studied each day, and retrieval performance after practice was measured on each day. GABA and Glx levels in the hippocampal, insular, and thalamic regions were measured on the first and last days of training. The results revealed no significant changes in GABA or Glx levels in the three tested regions from the pre- to post-training epochs. However, considering that the period of 21 days is relatively long, it can be speculated that this study design may have missed the modulation of neurometabolites that may have occurred between the first and final days of training.
Koolschijn et al. [67] implemented a novel imaging sequence, which allowed them to alternatively measure near-whole brain fMRI together with fMRS in the primary visual cortex to further investigate the changes in both brain activity and neurometabolites levels during the recall of memory. The task was learned through a mixed protocol involving associative and reinforcement learning. Their protocol consisted of 3 training days: on the first day, participants associated sounds with visual patterns; on the second day, different visual patterns were conditioned to a monetary reward or with a neutral stimulus; on the third day, participants were asked to predict whether the presented sound would lead to a reward. The task-related fMRI and fMRS data were obtained on the third day. During this test, an increase in the OCC Glu/GABA ratio (driven by a decrease in GABA levels) occurred when participants made the right inferences relative to erroneous answers. Moreover, the BOLD signal response in the hippocampus predicted the Glu/GABA increase and GABA decrease during correct associative inferences. Accordingly, the evidence supports the authors’ hypothesis that the hippocampus may orchestrate the E/I dynamics distributed across the neocortex in order to recall memories [67].
Inhibition and self-regulation
To investigate response conflict monitoring, Kühn et al. [68] used a Color/Word Stroop task and measured levels of ACC neurometabolites at baseline, during, and after the 13-min task. During the task, names of some colors were presented on the screen, where half of them were congruent (e.g., the word ‘red’ printed in red color) and the other half were incongruent (e.g., the word ‘red’ printed in green color). During the latter condition, participants were required to inhibit the prepotent word reading tendency in order to indicate the printed color of the presented word. The ACC was regarded as the targeted function-related brain area as it is involved in detecting and monitoring response conflict [69]. The results showed that the ACC GABA and Glu levels increased during the performance of the manual Stroop task and decreased after the completion of the task. Furthermore, a greater increase in GABA levels during the task performance was associated with less increase in BOLD activation in the congruent and incongruent conditions [68], which suggests an increased inhibitory function of ACC GABA levels when coping with conflicting information.
GABA modulations have also been studied following long-term self-regulation training. Specifically, Namgung et al. [70] tested the effect of a 10-day relaxation training program on GABA levels in young women with subclinical levels of stress. The training consisted of 30 min of cognitive relaxation training (cognitive restructuring for automatic thinking and progressive muscle relaxation) and 10 min of breathing-relaxation training. GABA levels were measured in the medial prefrontal cortex before and after the 10-day training. A significant decrease in the levels of GABA was observed after the training, although this change was not related to the levels of stress reported post-intervention.
Summary
The limited evidence in the field of short-term and long-term cognitive task learning is greatly diverse, making it difficult to draw solid conclusions at the current time. The preliminary evidence appears to suggest that task-induced reduction of GABA levels is related to better working memory [62] and to more successful reinforcement learning [67]. However, modulation of GABA levels is not observed after long-term learning [66]. Furthermore, modulation of GABA in the higher cognitive brain areas (such as ACC and DLPFC) is more easily induced when the behavioral task poses a higher cognitive load or demand. Additionally, an increment of GABA levels in the ACC during conflicting [68] or uncertain situations [63] has been observed. As ACC is involved in conflict resolution and reinforcement-based learning, enhanced GABA may help maintain inhibition or prevent excessive excitation, allowing the individual to cope better with the interference along with decision-making.
Discussion
This review summarizes research on the dynamics of MRS-assessed GABA (and, when available, Glu or Glx) in humans following various types of intervention and their associations with or implications for behavioral performance. Figure 3 summarizes the interventions that have been reported to induce GABA modulations in the human brain.
GABA modulation in human learning
The regulation of GABAergic activity is widely considered a crucial process in facilitating plasticity and learning. In recent work reviewing evidence for the role of ‘baseline GABA’ in behavior, we proposed the GABA-distinctiveness hypothesis, which implies that maintaining appropriate neural suppression in perceptual processing regions via higher baseline GABA levels is associated with more distinctive perceptual performance [6]. Consistent with this hypothesis, the present review identified converging evidence for a beneficial role for the task-induced GABA increase in effectively discerning subtle perceptual differences and possibly building distinct perceptual neural representations in the context of perceptual feature difference learning tasks (section "Perceptual plasticity"). We refer to this as the GABA increase for better neural distinctiveness hypothesis.
Taken together, we tentatively speculate that higher GABA levels at baseline and the increase of GABA levels during task performance may improve the distinctiveness of neural representations. In this context, representations mainly refer to the way that internal or external information is encoded and stored in the brain. This can be expressed at various levels, i.e., from the firing patterns of individual neurons to the activation of complex brain networks.
From the perspective of the cellular level, the distinctiveness of neural representations might be achieved through intricate adjustments and refinements in the functioning and connectivity of neurons, which is known as neuronal fine-tuning. For example, animal studies showed that the tuning curves of neurons are improved as a result of the administration of GABA and GABA agonists. More specifically, in a study by Leventhal et al. [71], it was observed that neuronal tuning became increasingly selective in old monkeys after the administration of GABA and its agonists, resembling the tuning functions observed in younger counterparts [71]. These GABAergic functional changes ultimately resulted in enhanced visual function. This evidence appears to support the GABA increase for better neural distinctiveness hypothesis.
From the perspective of brain activity using fMRI techniques, a higher distinctiveness of neural representations can become expressed by lower levels of overlap among representations as a function of different task conditions. For example, a number of studies have shown that the distributed patterns of brain activation elicited by visual stimuli [72, 73] or motor tasks [74] are less selective in older than in young adults. Although the role of GABA was not tested in the abovementioned studies, it can be speculated that the age-related decline in baseline GABA levels is (at least) partially responsible for the reduced selectiveness of patterns of brain activity in older adults. To directly test the GABA increase for better neural distinctiveness hypothesis, future studies could integrate MRS and fMRI techniques. This integration would allow us to examine the potential correlation between increased GABA levels and reduced overlap in brain activation patterns for different stimuli or task conditions as behavioral skills develop. Additionally, randomized controlled trials combining MRS techniques and pharmacological interventions may offer a promising approach to establishing causal relationships between neuronal GABA levels and human behavior.
However, in contrast to the aforementioned studies supporting a beneficial effect for GABA increases in neural distinctiveness, findings of other studies suggested beneficial effects of training-induced GABA reduction, not only in enhancing perceptual noise filtering capacity (section "Perceptual plasticity" ) but also in (visuo)motor task improvement (section "Motor learning" ). Additionally, limited evidence provides first hints that the reduction of GABA levels is related to better working memory and successful (reinforcement) learning, again suggesting a beneficial role for GABA reduction in learning (section "Attention and working memory" and section "Associative learning" ). Therefore, we coin this as the GABA decrease to boost learning hypothesis, which implies that decreasing neural inhibition through a reduction of GABA boosts human learning.
Regarding the underlying mechanisms, the reduction of GABA levels may decrease the overall inhibition among neuronal ensembles, thereby promoting neural communication and reorganization as underlying mechanisms of human learning. It has been established that the temporary activation of the N-methyl-D-aspartate (NMDA) receptor system is essential for the induction of LTP [75], which serves as the neural basis for learning. Notably, the activation of the NMDA receptor system could be modulated by GABA-mediated synaptic inhibition. This modulation occurs through the effect of GABAB autoreceptors on the presynaptic membrane. By exerting an effect on these receptors, the presynaptic cell inhibits its own GABA release, depolarizing the postsynaptic membrane and facilitating adequate activation of NMDA receptors, permitting the induction of LTP [76]. Given the relationship observed between MRS-assessed GABA levels and human behavior, one might speculate that when a decrease in GABA release from the pre-synaptic neuron is induced by human behavior, the synaptic GABA concentration diminishes, consequently resulting in reduced MRS-assessed GABA levels. This mechanism may account for the consistent GABA reduction observed through MRS in numerous learning conditions. Nevertheless, there may also be other mechanisms coming into play.
GABA modulation according to the different phases of learning
MRS studies in the field of learning have primarily addressed the early stage in the process of encoding, storage, and retrieval of information that takes place during task practice. However, learning also encompasses a phase in which temporary, fragile memories are transformed into a more stable, long-lasting form, known as memory consolidation. Preliminary findings indicate that modulation direction of GABA may vary according to the specific phase (early or late) of task learning. For example, the OCC Glu/GABA ratio exhibited an increase following the learning phase of a visual learning task, whereas it demonstrated a decrease after the overlearning phase [26]. More specifically, within the domain of perceptual learning, only two studies have delved into the role of GABA in learning stabilization or consolidation. The preliminary findings point to a beneficial effect of increased GABA levels in aiding the fast online (not sleep-dependent) consolidation of acquired information and guarding against subsequent interference. Previous studies that made use of zolpidem, a GABAA agonist, to modulate sleep patterns during a daytime nap and overnight sleep showed an improvement in hippocampal-dependent episodic memories after this pharmacological intervention [77, 78], suggesting that increased GABA activity during sleep is related to memory consolidation. Currently, the limited studies on MRS and learning suggest that higher inhibition induced by increased GABA levels during learning supports consolidation and guards against interference. Further research is certainly necessary to validate and substantiate these findings.
Although GABA modulation has been observed in various learning conditions, it is noteworthy that such changes are not unique to learning. GABA modulation has been observed in other conditions as well, as discussed below.
GABA modulation in other human behaviors besides learning
In addition to the study of learning processes, GABA modulation has been observed in some conditions with an implicit learning component as well as in various non-learning conditions, such as exposure to perceptual stimulation, physical exercise, and performance of cognitive tasks.
In the context of perceptual stimulation, a form of adaptive plasticity in perceptual processing areas can be induced. Specifically, the release from inhibition caused by the modulation of Glx and/or GABA co-occurs with most of the stimulation paradigms (3.1 perceptual stimulation). This is in line with results from animal studies, which have shown that stimulus-specific adaptation in the perceptual cortex is exhibited by both excitatory neurons and inhibitory interneurons [79, 80]. Specifically, the neuron’s response to a frequently occurring perceptual stimulus differs from its reaction to a rare stimulus, and this stimulus-specific neuroplasticity is mediated by the excitatory and inhibitory system. At the cellular level, the substantial facilitation caused by an increase in excitation and/or reduction of inhibition is thought to enhance overall cortical responsiveness to perceptual task stimuli [81]. Hence, the release from inhibition (facilitation) in perceptual brain regions induced by visual stimuli may be beneficial for general perceptual information processing.
In the context of physical exercise, increases in GABA have been observed following intensive short-term cardiovascular endurance activity, such as cycling, but GABA modulation has also been connected with yoga. Conversely, decreases in GABA have also been observed after, for example, repeated hand movement practice (section "Physical exercise" ). This suggests that the brain’s adaptive plasticity may vary in response to different types of physical activity intervention. Lastly, it remains to be studied whether modulations of GABA following an exercise intervention benefit subsequent behavioral performance and learning of motor or other tasks. For example, can exercise-induced GABA modulation create the optimal conditions for increased neuronal interactions associated with learning new skills (section "Perceptual plasticity" and section "Motor learning" )?
In the context of cognition, increases in GABA levels have been observed in tasks with high cognitive load levels, such as conflict resolution (section "Inhibition and self-regulation" ). Nevertheless, given the notable variations in cognitive task subtypes, it is premature to draw firm conclusions at this stage.
GABA modulation varies according to the function of brain areas
Converging evidence suggests that GABA modulation exhibits brain regional specificity. On the one hand, this refers to a dynamic GABA modulation in those brain areas that are involved in the performance of a given task, while no strong modulation occurs in brain areas that are less relevant for that task. For instance, given that the SM1 brain region serves as the primary brain region for motor control, it is expected that cycling training results in modulations of GABA levels in the SM1 region and not in the cognitive processing-related brain regions [36]. On the other hand, different directions of GABA modulation in the task-involved brain areas may take place. For example, during the signal-in-noise (SN) task requiring participants to discern the presented patterns embedded in noisy dots, GABA levels increased in the PPC, whereas they decreased in the OCT [23]. Future research should address whether broader changes across the entire brain (global changes) can also be induced by interventions or whether they primarily affect specific brain regions (local changes). Furthermore, under which circumstances can local versus global changes in neurochemical modulation be accomplished?
The effect of perceptual stimulus complexity, task difficulty and cognitive demand on GABA modulation
Using fMRI, a number of studies have shown that the scope of brain activity in the multiple demand system varies depending on the difficulty of the behavioral task [82,83,84]. Nevertheless, there remains a gap in research specifically designed to directly investigate the modulation of MRS-assessed GABA in response to variations in behavioral task difficulty.
In the perceptual domain, it has been shown that presentation of an experimental stimulus, such as a checkerboard or wedges, as compared to a simple fixation cross, is more likely to induce GABA modulation [9,10,11]. Additionally, presentation of abnormal perceptual stimulation, such as monocular deprivation [29], seems to better induce GABA modulation as compared to often used experimental stimuli, such as a checkerboard.
The motor tasks addressed in this review included visuomotor coordination learning, motor sequence learning, visuomotor adaptation learning, and various types of physical exercise, etc. Thus, comparing the difficulty of motor tasks across studies becomes challenging. However, it was reported that training of a visually-guided bimanual coordination task resulted in decreased OCC GABA levels in the more challenging (random practice) as compared with the less challenging (blocked practice) motor learning condition [48].
In the cognitive domain, across studies, it appears that GABA modulation occurs in working memory tasks with relatively higher cognitive load rather than those with lower cognitive load [61, 62]. However, attempts to induce GABA modulation by manipulating visual attentional load in a visual tracking study were unsuccessful [60].
Overall, the current state of the literature does not provide sufficient evidence to draw definitive conclusions on the relationship between task complexity or difficulty and GABA modulation. Future studies may look into GABA modulations of more generic (pre-frontal) brain regions, such as DLPFC, that become activated when increased cognitive effort is required, especially during the initial phase of various types of learning.
Factors influencing the accuracy of MRS-assessed GABA
Quantifying GABA levels accurately is essential and a prerequisite for exploring their associations with behavior. Thus, it is crucial to assess whether proper MRS measurement techniques have been used in the reported studies. Although a detailed comparison of the outputs of different MRS techniques falls outside the main scope of this review, here we list several factors that affect MRS measurement accuracy, including MR field strength, MR sequence type and the methodology employed to eliminate the macromolecule contamination. Additionally, we provide suggestions for improving the overall accuracy of GABA measurement in practice.
MR field strength
Higher MR field strength leads to better signal-to-noise ratios, which contributes to higher MRS measurement accuracy. Therefore, studies conducted at ultra-high MR field strengths (≥ 7T) are likely to obtain more sensitive and reliable results than those conducted under 3T or 1.5T. In this review, 63.4% of the included articles utilized a 3T magnetic field strength, 31.7% employed a 7T field strength (31.7%) and 4.9% employed a 4T field strength.
MRS sequence type
GABA signal overlaps with signals from other molecules in the brain. Therefore, accurate quantification of GABA requires the employment of an edited sequence that can isolate GABA signals from those of overlapping molecules. The edited MRS sequences exploit known J-coupling relationships to distinguish signals originating from low-concentration metabolites, such as GABA, from stronger overlapping signals [85].
Detailed distribution of the type of MR sequences used in the reviewed studies is reported in Table 2. It is noteworthy that over half of the included studies (56%) utilized an edited sequence. Furthermore, the most commonly employed edited sequence was MEGA-PRESS, accounting for 78.3% of the reported studies utilizing the edited sequence. Less than half of the included studies (44%) used a non-edited sequence, which delivers broader spectral information from multiple metabolites rather than focusing specifically on GABA. The choice of non-edited sequences varies across studies, with no clear preference for any specific sequence.
Macromolecule contamination
Although spectral editing allows the detection and quantification of GABA, the edited signal contains a significant contribution from the macromolecular signal when using conventional editing approaches. It is important to note that failure to account for macromolecule contamination may lead to inaccurate estimation of metabolite levels and misinterpretation of their relationship with human behavior. While macromolecule contamination is an acknowledged problem, there is currently no clear consensus on the best approach to tackle it. Methods used in the correction of macromolecules in the reviewed studies are reported in Table 2. Among the 41 studies analyzed, only 16 employed methods to eliminate the contamination of macromolecules, of which six studies employed an inversion-recovery sequence to acquire a macromolecule spectrum during data collection; two studies used a symmetric-suppression editing, and eight studies used a set of macromolecule basis functions during fitting. A detailed discussion of these three techniques and their caveats has been reported by Mullins et al. [86]. Even though over half of the included studies did not account for contaminating macromolecule signals, it is assumed that this is not a major confounder for studies focused on GABA changes (using repeated measures) rather than those reporting a one-time measurement. This is because macromolecule signals are believed to be relatively stable within healthy participants during multiple measurements in fMRS studies.
MRS measurement time points
The precise time course of neurochemical modulations is still unclear, and therefore, no definite consensus exists on the temporal resolution that is necessary to detect neurometabolic fluctuations with optimal sensitivity and reliability. Stated differently: what is the critical window for GABA modulation to occur in the context of task performance and learning? It appears likely that GABA modulation occurs within milliseconds, but changes over minutes, hours, days, or weeks can also possibly take place. This prompts questions about setting up the appropriate experimental paradigms for capturing the window of change that scientists wish to investigate while taking into account the current MRS imaging constraints (relatively long measuring time: 8–10 min for each brain area).
Figure 2 provides an overview of the MRS timing designs in various studies, summarized as pre-post, pre-during, pre-during-post, and continuous measurement during task performance. It is important to mention that even with similar MRS timing designs, the exact measurement times may differ across studies. For example, most of the studies conducted the post measurement immediately after the end of training, but a few studies acquired MRS data with some time delay following the end of training. The time gap between task completion and MRS measurement may impact results because GABA levels could return to baseline over time. Moreover, adaptation and/or habituation processes may occur rapidly such that the concentrations of neurometabolites recover to the resting state level before or during the post-MRS measurement. In addition, MRS measures assessed during task performance can show different modulation directions depending on the different phases of task performance/learning. Accordingly, scientists should give careful consideration to the design of behavioral tasks when planning the timing of the MRS acquisition and provide highly detailed, accurate, and replicable descriptions of their experimental setup when reporting their findings.
Recent progress in event-related fMRS techniques allows continuous collection of MRS data at a time resolution of seconds during the presentation of intermixed experimental conditions in a sequence of trials [87]. As compared to measuring the GABA modulation via repeated measures, this event-related method appears very promising to detect the dynamics of neurometabolites with a comparably high temporal resolution. However, the other side of the temporal spectrum is also of interest and addresses questions about the sustainability of training-induced neurochemical changes. At least some evidence suggests that neurochemical modulation occurs across a time window of weeks of training, even though it is not yet clear how neurochemical concentrations evolve after training has been completed [47]. It is important to gain deeper insights into the temporal characteristics of task-related neurochemical fluctuations in order to decide upon the appropriate MRS measurement protocol.
Calculating GABA modulation
Throughout the reviewed studies, substantial inconsistencies exist with regard to the methods employed to assess the modulation of GABA at a group level. Here, we use ‘scan 1’ to refer to the first MRI scan across these studies, which is often measured during a resting state before the task or intervention starts. We use ‘scan 2’ to refer to the subsequent MRI scans, which are often measured during or after the performance or training of a task. Initially, most studies (around 60%) addressed the “absolute” GABA changes, which are calculated by the difference between ‘scan 2’ and ‘scan 1’ (scan 2-scan 1). In contrast, around 36% of the studies took the baseline GABA levels into consideration to normalize or standardize the GABA modulation, i.e., GABA modulation was obtained by calculating the difference between ‘scan 2’ and ‘scan 1’, divided by ‘scan 1’ [(scan 2- scan 1)/scan 1]. Furthermore, only a limited number of studies (about 4%) reported their results using both calculation methods. There is currently no gold standard for which method to use. However, it is important to acknowledge that results obtained through different calculation procedures are not always consistent.
Individual differences approach
Even though task- or training-induced changes in GABA and/or Glx at the group level have been demonstrated, it is fruitful to complement this with an individual differences approach to explore whether changes in neurometabolites exhibit different directions across individuals. The same experimental manipulation may cause increases, decreases, or no changes in neurometabolites in different individuals. This prompts questions about the potential interactions between baseline levels of neurometabolites and the directions of modulation as well as their range of modulation. For example, does a higher baseline level of GABA in learners imply that they have a larger window for change available to reduce GABA during task practice? Will the larger modulation further promote inter-neuronal interactions and increase neural plasticity? Preliminary evidence provides some hints that this might be the case. A motor sequence learning study in older adults revealed that higher baseline GABA levels were associated with a larger training-induced reduction of GABA, even though the age of the participants may potentially have mediated this effect. Moreover, in the latter study, a greater reduction of GABA levels following motor training was related to a greater learning magnitude [52]. However, it is noteworthy that the dynamics of neural circuits are highly intricate and non-linear [88, 89], and hence the brain-behavior associations might also follow a non-linear and more complex relationship. Therefore, further research is required to confirm such findings and to explore whether causal interventions to boost baseline GABA promote task-induced GABA change. This may have important implications for those individuals confronted with decreased GABA as a result of normal aging [52,53,54] or in pathological conditions associated with acute or chronic GABA depletion [90, 91].
GABA, Glu and E/I balance
In the present review, GABA levels were the primary focus because recent studies have suggested that GABA-induced disinhibition is tightly related to the learning process [50]. However, it is crucial to bear in mind that the majority of GABA is synthesized directly from Glu [92]. Consequently, there may be an association between GABA and Glu levels during the resting state. Additionally, the modulation of GABA levels may coincide with changes in Glu levels, influencing E/I balance. Moreover, the optimal MRS sequences for GABA and Glu may differ. Therefore, measuring both metabolites simultaneously using a sequence targeted at either GABA or Glu may reduce the accuracy of the other.
Concerning the associations between baseline MRS-assessed GABA and Glu (or Glx) levels, previous research has yielded inconsistent findings [93,94,95]. More specifically, while Rideaux discovered evidence against a positive correlation in both the visual and motor cortices, Steel et al. showed a positive link between MRS-assessed GABA + and Glx levels in the posterior cingulate gyrus. These discrepancies could be explained by different functions of the brain areas investigated. However, the latest work using a large sample size attempting to resolve this inconsistency showed that there is a regionally non-specific common ratio between MRS-assessed GABA and Glu levels [96]. Overall, these results suggest the existence of a inter-individual common ratio between baseline GABA and Glu levels. Concerning the associations between the modulation of MRS-assessed GABA and Glu (or Glx), to the best of our knowledge, there is only one study addressing this question and reporting a positive correlation between both [56].
Here, we summarized and reported the associations between the modulation of MRS-assessed GABA and various human behaviors. Since this is still a relatively new area of research and the current literature focusing on GABA does not always report the E/I balance, we cannot provide conclusive evidence on the effect of GABA modulation on Glu modulation and how the E/I balance changes along with behavior.
We recommend that future studies investigating the associations between human behavior and E/I balance also report the results on independent neuro-metabolites, such as GABA and Glu. This would enable us to discern which metabolites drive the observed correlations between E/I balance and behaviors.
Conclusion
Neurochemical modulation is an emerging area of research with important implications for behavioral neuroscience. GABA modulation has been observed in both learning and non-learning conditions. The current review has led us to propose two preliminary hypotheses regarding GABA modulation in the brain in the context of learning across a broad range of tasks: the ‘GABA increase for better neural distinctiveness’ hypothesis and the ‘GABA decrease to boost learning hypothesis’. These preliminary hypotheses may encourage scientists from various disciplines to further investigate neurochemical dynamics in order to establish a solid body of knowledge for the field of (cognitive) neuroscience. From the behavioral side, further refinement of task protocols is required to capture the dynamics of learning, memory, and retention and to select appropriate brain regions of interest. Moreover, the temporal resolution of neurometabolite modulation in the human system needs to be considered across the time scale from milliseconds to weeks or months. From the technical side, it will be of utmost importance to further optimize current MRS sequences and software procedures in order to capture the temporal dynamics of neurochemical modulation and to explore larger brain territory with increased (sub)regional resolution. We are only at the initial stage of this exciting field of neurochemical modulation in the human system.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- ACC:
-
Anterior cingulate cortex
- BOLD:
-
Blood oxygen level-dependent
- BTT:
-
Bimanual tracking task
- Cr:
-
Creatine
- dACC:
-
Dorsal anterior cingulate cortex
- DLPFC:
-
Dorsolateral prefrontal cortex
- EEG:
-
Electroencephalography
- E/I:
-
Excitatory/inhibitory
- FD:
-
Feature difference task
- fMRI:
-
Functional magnetic resonance imaging
- GABA:
-
Gamma-aminobutyric acid
- Glx:
-
Glutamate + glutamine
- Glu:
-
Glutamate
- MRS:
-
Magnetic resonance spectroscopy
- MRSI:
-
Magnetic resonance spectroscopic imaging
- NAA:
-
N-acetylaspartic acid
- LTP:
-
Long-term potentiation
- OCC:
-
Occipital cortex
- OCT:
-
Occipitotemporal cortex
- PCC:
-
Posterior cingulate cortex
- PPC:
-
Posterior-parietal cortex
- SM1:
-
Primary sensorimotor cortex
- SN:
-
Signal-in-noise task
- SRTT:
-
Serial reaction time task
9. References
Hebb DO. The organization of behavior; a neuropsychological theory. Hoboken: Wiley; 1949.
Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290(5491):533–6. https://doi.org/10.1126/science.290.5491.533.
Ishikawa K, Saito S. Effect of intraventricular gamma-aminobutyric acid (GABA) on discrimination learning in rats. Psychopharmacology. 1978;56(2):127–32. https://doi.org/10.1007/BF00431837.
Brioni JD, McGaugh JL. Post-training administration of GABAergic antagonists enhances retention of aversively motivated tasks. Psychopharmacology. 1988;96(4):505–10. https://doi.org/10.1007/BF02180032.
Makkar SR, Zhang SQ, Cranney J. Behavioral and neural analysis of GABA in the acquisition, consolidation, reconsolidation, and extinction of fear memory. Neuropsychopharmacology. 2010;35(8):1625–52. https://doi.org/10.1038/npp.2010.53.
Li H, Heise K-F, Chalavi S, Puts NAJ, Edden RAE, Swinnen SP. The role of MRS-assessed GABA in human behavioral performance. Prog Neurobiol. 2022;212: 102247. https://doi.org/10.1016/j.pneurobio.2022.102247.
Pasanta D, He JL, Ford T, Oeltzschner G, Lythgoe DJ, Puts NA. Functional MRS studies of GABA and glutamate/Glx-A systematic review and meta-analysis. Neurosci Biobehav Rev. 2023;144: 104940. https://doi.org/10.1016/j.neubiorev.2022.104940.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339: b2535. https://doi.org/10.1136/bmj.b2535.
Kurcyus K, Annac E, Hanning NM, Harris AD, Oeltzschner G, Edden R, Riedl V. Opposite dynamics of GABA and glutamate levels in the occipital cortex during visual processing. J Neurosci. 2018;38(46):9967–76. https://doi.org/10.1523/JNEUROSCI.1214-18.2018.
Koush Y, de Graaf RA, Kupers R, Dricot L, Ptito M, Behar KL, Rothman DL, Hyder F. Metabolic underpinnings of activated and deactivated cortical areas in human brain. J Cereb Blood Flow Metab. 2021;41(5):986–1000. https://doi.org/10.1177/0271678X21989186.
Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab. 2012;32(8):1484–95. https://doi.org/10.1038/jcbfm.2012.33.
Bednařík P, Tkáč I, Giove F, DiNuzzo M, Deelchand DK, Emir UE, Eberly LE, Mangia S. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab. 2015;35(4):601–10. https://doi.org/10.1038/jcbfm.2014.233.
Mangia S, Tkáč I, Gruetter R, Van de Moortele P-F, Maraviglia B, Uğurbil K. Sustained neuronal activation raises oxidative metabolism to a new steady-state level: evidence from 1 H NMR spectroscopy in the human visual cortex. J Cereb Blood Flow Metab. 2007;27(5):1055–63. https://doi.org/10.1038/sj.jcbfm.9600401.
Schaller B, Mekle R, Xin L, Kunz N, Gruetter R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J Neurosci Res. 2013;91(8):1076–83. https://doi.org/10.1002/jnr.23194.
Ip IB, Emir UE, Parker AJ, Campbell J, Bridge H. Comparison of neurochemical and BOLD signal contrast response functions in the human visual cortex. J Neurosci. 2019;39(40):7968–75. https://doi.org/10.1523/JNEUROSCI.3021-18.2019.
Boillat Y, Xin L, Van der Zwaag W, Gruetter R. Metabolite concentration changes associated with positive and negative BOLD responses in the human visual cortex: a functional MRS study at 7 Tesla. J Cereb Blood Flow Metab. 2020;40(3):488–500. https://doi.org/10.1177/0271678X19831022.
Mekle R, Kühn S, Pfeiffer H, Aydin S, Schubert F, Ittermann B. Detection of metabolite changes in response to a varying visual stimulation paradigm using short-TE 1 H MRS at 7 T. NMR Biomed. 2017. https://doi.org/10.1002/nbm.3672.
Heba S, Puts NAJ, Kalisch T, Glaubitz B, Haag LM, Lenz M, Dinse HR, Edden RAE, Tegenthoff M, Schmidt-Wilcke T. Local GABA concentration predicts perceptual improvements after repetitive sensory stimulation in humans. Cereb Cortex. 2016;26(3):1295–301. https://doi.org/10.1093/cercor/bhv296.
Lea-Carnall CA, Williams SR, Sanaei-Nezhad F, Trujillo-Barreto NJ, Montemurro MA, El-Deredy W, Parkes LM. GABA modulates frequency-dependent plasticity in humans. iScience. 2020;23(11): 101657. https://doi.org/10.1016/j.isci.2020.101657.
Groen IIA, Piantoni G, Montenegro S, Flinker A, Devore S, Devinsky O, Doyle W, Dugan P, Friedman D, Ramsey NF, Petridou N, Winawer J. Temporal dynamics of neural responses in human visual cortex. J Neurosci. 2022;42(40):7562–80. https://doi.org/10.1523/JNEUROSCI.1812-21.2022.
Hammett ST, Cook E, Hassan O, Hughes C-A, Rooslien H, Tizkar R, Larsson J. GABA, noise and gain in human visual cortex. Neurosci Lett. 2020;736: 135294. https://doi.org/10.1016/j.neulet.2020.135294.
Frangou P, Correia M, Kourtzi Z. GABA, not BOLD, reveals dissociable learning-dependent plasticity mechanisms in the human brain. Elife. 2018. https://doi.org/10.7554/eLife.35854.
Frangou P, Emir UE, Karlaftis VM, Nettekoven C, Hinson EL, Larcombe S, Bridge H, Stagg CJ, Kourtzi Z. Learning to optimize perceptual decisions through suppressive interactions in the human brain. Nat Commun. 2019;10(1):474. https://doi.org/10.1038/s41467-019-08313-y.
Ziminski JJ, Frangou P, Karlaftis VM, Emir U, Kourtzi Z. Microstructural and neurochemical plasticity mechanisms interact to enhance human perceptual decision-making. PLOS Biol. 2023;21(3): e3002029. https://doi.org/10.1371/journal.pbio.3002029.
Pashler H, Rohrer D, Cepeda NJ, Carpenter SK. Enhancing learning and retarding forgetting: choices and consequences. Psychon Bull Rev. 2007;14(2):187–93. https://doi.org/10.3758/bf03194050.
Shibata K, Sasaki Y, Bang JW, Walsh EG, Machizawa MG, Tamaki M, Chang L-H, Watanabe T. Overlearning hyperstabilizes a skill by rapidly making neurochemical processing inhibitory-dominant. Nat Neurosci. 2017;20(3):470–5. https://doi.org/10.1038/nn.4490.
Frank SM, Becker M, Qi A, Geiger P, Frank UI, Rosedahl LA, Malloni WM, Sasaki Y, Greenlee MW, Watanabe T. Efficient learning in children with rapid GABA boosting during and after training. Curr Biol. 2022;32(23):5022-5030.e7. https://doi.org/10.1016/j.cub.2022.10.021.
Bang JW, Shibata K, Frank SM, Walsh EG, Greenlee MW, Watanabe T, Sasaki Y. Consolidation and reconsolidation share behavioural and neurochemical mechanisms. Nat Hum Behav. 2018;2:507–13. https://doi.org/10.1038/s41562-018-0366-8.
Lunghi C, Emir UE, Morrone MC, Bridge H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr Biol. 2015;25(11):1496–501. https://doi.org/10.1016/j.cub.2015.04.021.
van Vugt FT, Near J, Hennessy TJ, Doyon J, Ostry DJ. Early stages of sensorimotor map acquisition: neurochemical signature in primary motor cortex and its relation to functional connectivity. J Neurophysiol. 2020;124(6):1615–24. https://doi.org/10.1152/jn.00285.2020.
Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis. 2017;55(1):1–18. https://doi.org/10.3233/JAD-160665.
de Sousa Fernandes MS, Ordônio TF, Santos GCJ, Santos LER, Calazans CT, Gomes DA, Santos TM. Effects of physical exercise on neuroplasticity and brain function: a systematic review in human and animal studies. Neural Plast. 2020. https://doi.org/10.1155/2020/8856621.
El-Sayes J, Harasym D, Turco CV, Locke MB, Nelson AJ. Exercise-induced neuroplasticity: a mechanistic model and prospects for promoting plasticity. Neuroscientist. 2019;25(1):65–85. https://doi.org/10.1177/1073858418771538.
Chen C, Sigurdsson HP, Pépés SE, Auer DP, Morris PG, Morgan PS, Gowland PA, Jackson SR. Activation induced changes in GABA: functional MRS at 7T with MEGA-sLASER. Neuroimage. 2017;156:207–13. https://doi.org/10.1016/j.neuroimage.2017.05.044.
Andrushko JW, Levenstein JM, Zich C, Edmond EC, Campbell J, Clarke WT, Emir U, Farthing JP, Stagg CJ. Repeated unilateral handgrip contractions alter functional connectivity and improve contralateral limb response times. Sci Rep. 2023;3(1):6437. https://doi.org/10.1038/s41598-023-33106-1.
Coxon JP, Cash RFH, Hendrikse JJ, Rogasch NC, Stavrinos E, Suo C, Yücel M. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol. 2018;596(4):691–702. https://doi.org/10.1113/JP274660.
Maddock RJ, Casazza GA, Fernandez DH, Maddock MI. Acute modulation of cortical glutamate and GABA content by physical activity. J Neurosci. 2016;36(8):2449–57. https://doi.org/10.1523/JNEUROSCI.3455-15.2016.
Streeter CC, Whitfield TH, Owen L, Rein T, Karri SK, Yakhkind A, Perlmutter R, Prescot A, Renshaw PF, Ciraulo DA, Jensen JE. Effects of yoga versus walking on mood, anxiety, and brain GABA levels: a randomized controlled MRS study. J Altern Complement Med. 2010;16(11):1145–52. https://doi.org/10.1089/acm.2010.0007.
Schmidt RA, Lee TD, Winstein CJ, Wulf G, Zelaznik HN. 2019. Motor control and learning: A behavioral emphasis (Sixth edition). Human Kinetics.
Shmuelof L, Krakauer JW. Recent insights into perceptual and motor skill learning. Front Hum Neurosci. 2014;8:683. https://doi.org/10.3389/fnhum.2014.00683.
Diedrichsen J, Kornysheva K. Motor skill learning between selection and execution. Trends Cogn Sci. 2015;19(4):227–33. https://doi.org/10.1016/j.tics.2015.02.003.
Fontaine RJ, Lee TD, Swinnen SP. Learning a new bimanual coordination pattern: reciprocal influences of intrinsic and to-be-learned patterns. Can J Exp Psychol. 1997;51(1):1–9. https://doi.org/10.1037/1196-1961.51.1.1.
Swinnen SP. Age-related deficits in motor learning and differences in feedback progressing during the production of bimanual coordination pattern. Cogn Neuropsychol. 1998;15(5):439–66. https://doi.org/10.1080/026432998381104.
Swinnen SP, Walter CB, Lee TD, Serrien DJ. Acquiring bimanual skills: contrasting forms of information feedback for interlimb decoupling. J Exp Psychol Learn Mem Cogn. 1993;19(6):1328–44. https://doi.org/10.1037/0278-7393.19.6.1328.
Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124(Pt 6):1171–81. https://doi.org/10.1093/brain/124.6.1171.
Floyer-Lea A, Wylezinska M, Kincses T, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95(3):1639–44. https://doi.org/10.1152/jn.00346.2005.
Sampaio-Baptista C, Filippini N, Stagg CJ, Near J, Scholz J, Johansen-Berg H. Changes in functional connectivity and GABA levels with long-term motor learning. Neuroimage. 2015;106:15–20. https://doi.org/10.1016/j.neuroimage.2014.11.032.
Chalavi S, Pauwels L, Heise K-F, Zivari Adab H, Maes C, Puts NAJ, Edden RAE, Swinnen SP. The neurochemical basis of the contextual interference effect. Neurobiol Aging. 2018;66:85–96. https://doi.org/10.1016/j.neurobiolaging.2018.02.014.
Maes C, Cuypers K, Peeters R, Sunaert S, Edden RAE, Gooijers J, Swinnen SP. Task-related modulation of sensorimotor GABA+ levels in association with brain activity and motor performance: a multimodal MRS-fMRI study in young and older adults. J Neurosci. 2022;42(6):1119–30. https://doi.org/10.1523/JNEUROSCI.1154-21.2021.
Kolasinski J, Hinson EL, Divanbeighi Zand AP, Rizov A, Emir UE, Stagg CJ. The dynamics of cortical GABA in human motor learning. J Physiol. 2019;597(1):271–82. https://doi.org/10.1113/JP276626.
Bell TK, Craven AR, Hugdahl K, Noeske R, Harris AD. Functional changes in GABA and glutamate during motor learning. eNeuro. 2023;10(2): ENEURO.0356-20.2023. https://doi.org/10.1523/ENEURO.0356-20.2023.
King BR, Rumpf J-J, Verbaanderd E, Heise KF, Dolfen N, Sunaert S, Doyon J, Classen J, Mantini D, Puts NAJ, Edden RAE, Albouy G, Swinnen SP. Baseline sensorimotor GABA levels shape neuroplastic processes induced by motor learning in older adults. Hum Brain Mapp. 2020;41(13):3680–95. https://doi.org/10.1002/hbm.25041.
Gao F, Edden RAE, Li M, Puts NAJ, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, Wang X, Barker PB. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. https://doi.org/10.1016/j.neuroimage.2013.04.012.
Hermans L, Leunissen I, Pauwels L, Cuypers K, Peeters R, Puts NAJ, Edden RAE, Swinnen SP. Brain GABA levels are associated with inhibitory control deficits in older adults. J Neurosci. 2018;38(36):7844–51. https://doi.org/10.1523/JNEUROSCI.0760-18.2018.
Maes C, Cuypers K, Heise K-F, Edden RAE, Gooijers J, Swinnen SP. GABA levels are differentially associated with bimanual motor performance in older as compared to young adults. Neuroimage. 2021;231: 117871. https://doi.org/10.1016/j.neuroimage.2021.117871.
Maruyama S, Fukunaga M, Sugawara SK, Hamano YH, Yamamoto T, Sadato N. Cognitive control affects motor learning through local variations in GABA within the primary motor cortex. Sci Rep. 2021;11(1):18566. https://doi.org/10.1038/s41598-021-97974-1.
Nettekoven C, Mitchell L, Clarke WT, Emir U, Campbell J, Johansen-Berg H, Jenkinson N, Stagg CJ. Cerebellar GABA change during visuomotor adaptation relates to adaptation performance and cerebellar network connectivity: a magnetic resonance spectroscopic imaging study. J Neurosci. 2022;42(41):7721–32. https://doi.org/10.1523/JNEUROSCI.0096-22.2022.
Kluen LM, Dandolo LC, Jocham G, Schwabe L. Dorsolateral prefrontal cortex enables updating of established memories. Cereb Cortex. 2019;29(10):4154–68. https://doi.org/10.1093/cercor/bhy298.
Rodríguez-Nieto G, Seer C, Sidlauskaite J, Vleugels L, Van Roy A, Hardwick R, Swinnen S. Inhibition, shifting and updating: inter and intra-domain commonalities and differences from an executive functions activation likelihood estimation meta-analysis. Neuroimage. 2022;264: 119665. https://doi.org/10.1016/j.neuroimage.2022.119665.
Frank SM, Forster L, Pawellek M, Malloni WM, Ahn S, Tse PU, Greenlee MW. Visual attention modulates glutamate-glutamine levels in vestibular cortex: evidence from magnetic resonance spectroscopy. J Neurosci. 2021;41(9):1970–81. https://doi.org/10.1523/JNEUROSCI.2018-20.2020.
Vijayakumari AA, Thomas B, Menon RN, Kesavadas C. Task-based metabolic changes in the left dorsolateral prefrontal region during the letter N-back working memory task using proton magnetic resonance spectroscopy. NeuroReport. 2018;29(2):147–52. https://doi.org/10.1097/WNR.0000000000000943.
Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, Lüchinger R, Brandeis D, O’Gorman RL. Frontal GABA levels change during working memory. PLoS ONE. 2012;7(4): e31933. https://doi.org/10.1371/journal.pone.0031933.
Bezalel V, Paz R, Tal A. Inhibitory and excitatory mechanisms in the human cingulate-cortex support reinforcement learning: a functional Proton Magnetic Resonance Spectroscopy study. Neuroimage. 2019;184:25–35. https://doi.org/10.1016/j.neuroimage.2018.09.016.
Heilbronner SR, Hayden BY. Dorsal anterior cingulate cortex: a bottom-up view. Annu Rev Neurosci. 2016;39(1):149–70. https://doi.org/10.1146/annurev-neuro-070815-013952.
Kolling N, Wittmann MK, Behrens TEJ, Boorman ED, Mars RB, Rushworth MFS. Value, search, persistence and model updating in anterior cingulate cortex. Nat Neurosci. 2016;19(10):1280–5. https://doi.org/10.1038/nn.4382.
Spurny B, Seiger R, Moser P, Vanicek T, Reed MB, Heckova E, Michenthaler P, Basaran A, Gryglewski G, Klöbl M, Trattnig S, Kasper S, Bogner W, Lanzenberger R. Hippocampal GABA levels correlate with retrieval performance in an associative learning paradigm. Neuroimage. 2020;204: 116244. https://doi.org/10.1016/j.neuroimage.2019.116244.
Koolschijn RS, Shpektor A, Clarke WT, Ip IB, Dupret D, Emir UE, Barron HC. Memory recall involves a transient break in excitatory-inhibitory balance. Elife. 2021;10: e70071. https://doi.org/10.7554/eLife.70071.
Kühn S, Schubert F, Mekle R, Wenger E, Ittermann B, Lindenberger U, Gallinat J. Neurotransmitter changes during interference task in anterior cingulate cortex: evidence from fMRI-guided functional MRS at 3 T. Brain Struct Funct. 2016;221(5):2541–51. https://doi.org/10.1007/s00429-015-1057-0.
Fan J, Kolster R, Ghajar J, Suh M, Knight R, Sarkar R, McCandliss B. Response anticipation and response conflict: an event-related potential and functional magnetic resonance imaging study. J Neurosci. 2007;27(9):2272–82. https://doi.org/10.1523/JNEUROSCI.3470-06.2007.
Namgun E, Kim J, Jeong H, Ma J, Hong G, Kang I, Kim J, Joo Y, Kim RY, Lyoo IK. Changes in prefrontal gamma-aminobutyric acid and perfusion after the computerized relaxation training in women with psychological distress: a preliminary report. Front Psychol. 2021;12: 569113. https://doi.org/10.3389/fpsyg.2021.56911.
Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Scienc. 2003;300(5620):812–5. https://doi.org/10.1126/science.1082874.
Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV. Age-related changes in cortical blood flow activation during visual processing of faces and location. J Neurosci. 1994;14(3 Pt 2):1450–62. https://doi.org/10.1523/JNEUROSCI.14-03-01450.1994.
Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR. Aging reduces neural specialization in ventral visual cortex. Proc Natl Acad Sciof the U S A. 2004;101(35):13091–5. https://doi.org/10.1073/pnas.0405148101.
Carp J, Park J, Hebrank A, Park DC, Polk TA. Age-related neural dedifferentiation in the motor system. PLoS ONE. 2011;6(12): e29411. https://doi.org/10.1371/journal.pone.0029411.
Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. https://doi.org/10.1113/jphysiol.1983.sp014478.
Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349(6310):609–11. https://doi.org/10.1038/349609a0.
Mednick SC, McDevitt EA, Walsh JK, Wamsley E, Paulus M, Kanady JC, Drummond SPA. The critical role of sleep spindles in hippocampal-dependent memory: a pharmacology study. J Neurosci. 2013;33(10):4494–504. https://doi.org/10.1523/JNEUROSCI.3127-12.2013.
Zhang J, Yetton B, Whitehurst LN, Naji M, Mednick SC. The effect of zolpidem on memory consolidation over a night of sleep. Sleep. 2020;43(11): zsaa084. https://doi.org/10.1093/sleep/zsaa084.
Chen I-W, Helmchen F, Lütcke H. Specific early and late oddball-evoked responses in excitatory and inhibitory neurons of mouse auditory cortex. J Neurosci. 2015;35(36):12560–73. https://doi.org/10.1523/JNEUROSCI.2240-15.201.
Natan RG, Briguglio JJ, Mwilambwe-Tshilobo L, Jones SI, Aizenberg M, Goldberg EM, Geffen MN. Complementary control of sensory adaptation by two types of cortical interneurons. Elife. 2015;4: e09868. https://doi.org/10.7554/eLife.09868.
Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nat Neurosci. 2003;6(11):1216–23.
Badre D, D’Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. J Cogn Neurosci. 2007;19(12):2082–99. https://doi.org/10.1162/jocn.2007.19.12.2082.
Badre D, D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat Rev Neurosci. 2009;10(9):659–69. https://doi.org/10.1038/nrn2667.
Crittenden BM, Duncan J. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb Cortex. 2014;24(2):532–40. https://doi.org/10.1093/cercor/bhs333.
Choi I-Y, Andronesi OC, Barker P, Bogner W, Edden RAE, Kaiser LG, Lee P, Marjańska M, Terpstra M, de Graaf RA. Spectral editing in 1 H magnetic resonance spectroscopy: experts’ consensus recommendations. NMR Biomed. 2021;34: e4411.
Mullins PG, McGonigle DJ, O’Gorman RL, Puts NAJ, Vidyasagar R, Evans CJ, Cardiff Symposium on MRS of GABA, Edden RAE. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. https://doi.org/10.1016/j.neuroimage.2012.12.004.
Koolschijn RS, Clarke WT, Ip IB, Emir UE, Barron HC. Event-related functional magnetic resonance spectroscopy. Neuroimage. 2023;276: 120194. https://doi.org/10.1016/j.neuroimage.2023.120194.
Bhatia A, Moza S, Bhalla US. Precise excitation-inhibition balance controls gain and timing in the hippocampus. Elife. 2019;8: e43415. https://doi.org/10.7554/eLife.43415.
Liang J, Yang Z, Zhou C. Excitation-inhibition balance, neural criticality, and activities in neuronal circuits. Neuroscientist. 2024. https://doi.org/10.1177/10738584231221766.
Blicher JU, Near J, Næss-Schmidt E, Stagg CJ, Johansen-Berg H, Nielsen JF, Østergaard L, Ho Y-CL. GABA levels are decreased after stroke and GABA changes during rehabilitation correlate with motor improvement. Neurorehabil Neural Repair. 2015;29(3):278–86. https://doi.org/10.1177/1545968314543652.
Cuypers K, Verstraelen S, Maes C, Hermans L, Hehl M, Heise K-F, Chalavi S, Mikkelsen M, Edden R, Levin O, Sunaert S, Meesen R, Mantini D, Swinnen SP. Task-related measures of short-interval intracortical inhibition and GABA levels in healthy young and older adults: a multimodal TMS-MRS study. Neuroimage. 2020;208: 116470. https://doi.org/10.1016/j.neuroimage.2019.116470.
Martin DL, Rimvall K. Regulation of gamma-aminobutyric acid synthesis in the brain. J Neurochem. 1993;60(2):395–407. https://doi.org/10.1111/j.1471-4159.1993.tb03165.x.
Rideaux R. Temporal dynamics of GABA and Glx in the visual cortex. eNeuro. 2020. https://doi.org/10.1523/ENEURO.0082-20.2020.
Rideaux R. No balance between glutamate+glutamine and GABA+ in visual or motor cortices of the human brain: a magnetic resonance spectroscopy study. Neuroimage. 2021;237: 118191. https://doi.org/10.1016/j.neuroimage.2021.118191.
Steel A, Mikkelsen M, Edden RAE, Robertson CE. Regional balance between glutamate+glutamine and GABA+ in the resting human brain. Neuroimage. 2020;220: 117112. https://doi.org/10.1016/j.neuroimage.2020.117112.
Rideaux R, Ehrhardt SE, Wards Y, Filmer HL, Jin J, Deelchand DK, Marjańska M, Mattingley JB, Dux PE. On the relationship between GABA+ and glutamate across the brain. Neuroimage. 2022. https://doi.org/10.1016/j.neuroimage.2022.119273.
Acknowledgements
The authors declare no competing financial interests.
Funding
This work is supported by the Research Foundation Flanders (FWO) (G089818N, G039821N), the Excellence of Science grant (EOS, 30446199, MEMODYN) and the KU Leuven Research Fund (C16/15/070), awarded to SPS and co-workers. HL is supported by a doctoral fellowship from the China Scholarship Council (201906170063). SC is supported by a postdoctoral fellowship from FWO (K174216N). MM received salary support from NIH grant K99 EB028828. RAEE received salary support from NIH grants R01 EB016089, R01 EB023963, and P41 EB031771.
Author information
Authors and Affiliations
Contributions
HL performed the study design; screened and selected the papers; analyzed and interpreted the data and wrote the paper. GRN wrote and revised the paper. SC wrote and revised the paper. CS revised the paper. MM revised the paper. RAEE revised the paper. SPS performed the study design; formulated and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent for publication of the manuscript in its present form.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, H., Rodríguez-Nieto, G., Chalavi, S. et al. MRS-assessed brain GABA modulation in response to task performance and learning. Behav Brain Funct 20, 22 (2024). https://doi.org/10.1186/s12993-024-00248-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12993-024-00248-9