Abstract
Background
Breast cancer is the most widespread cancer in women and young women worldwide. Moving towards customised radiotherapy, balancing the use of the available technology with the best treatment modality may not be an easy task in the daily routine. This study aims to evaluate the effectiveness of introducing IQ-feasibility into clinical practice to support the decision of free-breathing (FB) versus breath-hold (BH) left-sided breast irradiations, in order to optimise the technology available and the effectiveness of the treatment.
Methods
Thirty-five patients who received 3D radiotherapy treatment of the left breast in deep-inspiration BH were included in this retrospective study. Computed tomography scans in FB and BH were acquired for each patient; targets contoured in both imaging datasets by an experienced radiation oncologist, and organs at risk delineated using automatic segmentation software were exported to PlanIQ™ (Sun Nuclear Corp.) to generate feasibility dose volume histogram (FDVHs). The dosimetric parameter of BH versus FB FDVH, and BH clinical dataset versus BH FDVH were compared.
Results
A total of 30 patients out of 35 patients analysed, presented for the BH treatments a significant reduction (p < 0.05) in the heart mean dose (\({{\text{D}}}_{{\text{m}}}\)), volume receiving 5 Gy (\({{\text{V}}}_{5{\text{Gy}}}\)) and 20 Gy (\({{\text{V}}}_{20{\text{Gy}}}\)), of 35.7%, 54.5%, and 2.1%, respectively; for the left lung, a lower reduction was registered and significant only for \({{\text{V}}}_{5{\text{Gy}}}\) (21.4%, p = 0.046). For the remaining five patients, the FDVH cut-off points of heart and lung were superimposable with differences of less than 1%. Heart and left lung dosimetric parameters of the BH clinical plans are located in the difficult zone of the FDVH and differ significantly (p < 0.05) from the corresponding parameters of the FDVH curves delimiting this buffer area between the impossible and feasible zones, respectively.
Conclusion
The use of PlanIQTM as a decision-support tool for the FB versus BH treatment delivery modality allows customisation of the treatment technique using the most appropriate technology for each patient enabling accurate management of available technologies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Breast cancer is the most widespread cancer in women and young women (< 45 years) worldwide [1, 2]. Adjuvant radiotherapy is part of the standard of care, and the focus on undue doses to organs at risk makes breast treatment one of the most studied because of the important sequelae that can occur even years later. A primary concern is unwanted pulmonary and cardiac irradiation, which may result in late injury [3, 4]. Increased risk of fatal cardiac events, pneumonitis, as well as of a second primary cancer of the breast has been largely reported [5,6,7,8]. Considering the incidence of this pathology even at a younger age and the increase in life expectancy [1], it is paramount to limit as much as possible long-term complications reducing pulmonary and cardiac volumes irradiated without compromising the success of the target irradiation. The treatment is delivered in free breathing or deep inspiration breath hold (DIBH) depending on the availability and indications of the centre. The topic of comparing the two techniques regarding their effectiveness, although widely discussed, remains to date very relevant [9, 10].
During DIBH the chest wall expands along with the inferior displacement of the diaphragm, and for left breast treatments, the heart is displaced medially posteriorly and inferiorly away from the target [11]. DIBH allows to minimize the irradiation of nearby organs at risk while maintaining an adequate target dose coverage [12,13,14,15] and has therefore become part of clinical practice in many institutions [16,17,18,19]. Although it appears clear in the scientific community that there may be an advantage for cardiac dosimetry in performing breath-hold irradiation [20, 21], there are still conflicting opinions and research regarding the advantage of lung dosimetry [22, 23]. The interinstitutional study by Nelms et al. [24] demonstrated that considerable variation in the quality of treatment plans may be attributed to the planner’s general skills. Many studies have focused on a priori estimation of the best possible sparing of organs at risk (OARs) before proceeding to plan optimization, to reduce variability in plan quality [25, 26]. Rocket et al.[27] found that 75% of left-side breast treatments can benefit from breath hold (BH) irradiation suggesting its use as routine clinical practice. On the other hand, some particularly overloaded departments might benefit from a preliminary assessment of the real advantage of proceeding in BH, a practice that nevertheless remains more challenging both in its preparation and execution, and involves the use of specific technology.
Feasibility dose volume histogram (FDVH) is a tool that was introduced in the PlanIQ software (Sun Nuclear Corp., Melbourne, FL) able to estimate for each patient, the lowest possible dose volume histogram (DVH) for OARs, given the full coverage of the target volume with the prescribed dose [28, 29]. This retrospective study aims to evaluate the usefulness of introducing IQ-feasibility into clinical practice to support case by case the decision of free-breathing versus breath-hold left-sided breast irradiation.
Methods
Patient selection
This retrospective study included 35 patients with early-stage left breast cancer consecutively admitted to our hospital in 2020. Patients were referred by physicians for whole-breast radiotherapy in BH. The study was approved by the institutional Ethics Committee (approval number: SCCHEC-02-2021-026). Informed consent was obtained from all subjects and/or their legal guardian(s); data were anonymized before use and patient details were de-identified.
Clinical workflow
The process followed by patients undergoing radiotherapy on the left breast is standardised in our department and has been previously described [30,31,32]; patient immobilization (supine) was achieved using WingStep (IT-V, Innsbruck, Austria) breast board. Patients were imaged in free breathing (FB) and BH consecutively, with a 16-slice Brilliance Big Bore computed tomography (CT) scanner (Philips Medical Systems, Cleveland, OH) using 3-mm slice thickness; a copper wire was placed along with the palpated breast tissue during the simulation as a support for the target delineation. A surface-guided radiotherapy (SGRT) system was used at simulation CT and throughout the treatment fractions for patient monitoring. CT scans were exported to a commercial platform MIM Version 7.0.5 (MIM Software Inc., Cleveland, Ohio, USA) used for contour segmentation. Target volume and organs at risk were outlined manually on the BH imaging dataset by an experienced radiation oncologist of the breast department following the breast cancer atlas for radiotherapy consensus definitions [33, 34]. Clinical target volume (CTV) included all mammary tissues of the whole breast after lumpectomy as visualized on the CT scans. The planning target volume (PTV) was generated as an isotropic expansion of the CTV with a margin of 3 mm in all directions; the first 5 mm within the outer contour of the body were excluded from both the CTV and the PTV. The heart and left lung contoured on the BH imaging dataset used for the treatment plan clinically delivered will be indicated as \({{\text{Heart}}}_{{\text{man}}}\) and \({{\text{Lung}}}_{{\text{man}}}\), respectively. CT images and the delineated structures were imported into Pinnacle 3™ Version 9.10 (Philips Medical Systems, Eindhoven, the Netherlands) treatment planning system. All plans were created for the Elekta Infinity (Elekta, Stockholm, Sweden) LINAC with a photon beam energy of 6MV and calculated with the full collapsed cone convolution algorithm and a 3-mm dose grid. For all patients the tangential field-in-field technique (TFiF) was used, consisting of two opposing tangential fields and additional fields manually created with the multileaf collimator (MLC) to homogenise the target volume; three to five sub-segments per beam were used, while the tangential field gantry angles ranged between 300° and 315° for the medial beam, and 120° and 135° for the lateral beam. Patients received hypofractionated radiotherapy consisting of a prescription dose (\({{\text{D}}}_{{\text{p}}}\)) of 42.56 Gy delivered in 16 fractions [35]. The plan was optimised to achieve a mean dose (\({{\text{D}}}_{{\text{m}}}\)) to the PTV equal to the \({{\text{D}}}_{{\text{p}}}\) with a dose reaching 95% of the target volume (\({{\text{D}}}_{95}\)) no lower than 95% of the \({{\text{D}}}_{{\text{p}}}\); hotspots were not to exceed 107% of \({{\text{D}}}_{{\text{p}}}\) although they were considered acceptable if the dose received by 2 cm3 of the target (\({{\text{D}}}_{2{\text{cc}}}\)) remained below the 110% isodose line [30].
The dose to the OARs was kept as low as possible without compromising target coverage; constraints normally used in clinical practice were adopted [36] trying to keep the mean dose of the heart under 2 Gy [37], and less than 15% of the left lung receiving more than 20 Gy.
FB and BH patient’s dataset preparation for FDVH assessment
For each patient, the PTV contoured in the clinical workflow was used to generate the BH FDVH, while for the FB FDVH, the PTV was delineated by the same radiation oncologist who identified it in the corresponding BH imaging. Considering that organ delineation remains operator-dependent, to avoid uncertainties when comparing FB and BH FDVHs, commercial automatic segmentation software (AiPlan, Lianxin Company, Beijing, China) [38] was used to contour the heart (\({{\text{Heart}}}_{{\text{auto}}}\)) and left lung (\({{\text{Lung}}}_{{\text{auto}}}\)) on FB and BH CT scans; the contours were successively reviewed and validated by an experienced radiation oncologist. The consistency of automatic segmentation versus manual contouring was assessed on the BH dataset for which manual contours were available for the heart and lungs. Quantitative metrics such as the Dice Similarity Coefficient (DSC), the mean absolute surface-to-surface distance (MASD), and the Hausdorff distance (HD) were used to compare \({{\text{Heart}}}_{{\text{man}}}\) and \({{\text{Heart}}}_{{\text{auto}}}\), and \({{\text{Lung}}}_{{\text{man}}}\) and \({{\text{Lung}}}_{{\text{auto}}}\). DSC provides a measure of overlap between automatic and manual delineations, with 0 indicating no overlap and 1 indicating perfect overlap. MASD and HD are indicative of deviations between the delineations on the surface, with the HD being more sensitive to local surface deviations.
FB and BH treatment plan quality assessment
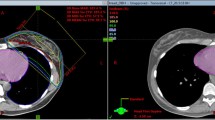
The FDVH module of PlanIQ™ (Sun Nuclear Corp., Melbourne, FL) is marketed as software for the analysis of treatment plan quality metrics. FDVH is based on a falloff of the ideal dose from the prescribed dose at the target boundary, allowing the quantitative determination of the best possible OAR FDVH that can be generated based on the benchmark dose, and making FDVH curves more easily achievable. FDVH can simulate the dose prescribed to a clinical site in four zones of dose decay modes, starting from the boundary of the target volume. Based on the geometric relationship between the organs at risk and the target volume, the exposure of the organ at risk and the corresponding dose received can be quantified in each zone [26]. The four zones are defined as “impossible”, “difficult”, “feasible”, and “easy” and for clear identification, they are bounded by red, orange, and blue FDVH curves, respectively, as depicted in Fig. 1 for a representative patient treated in BH.
In this study, the FDVH module was used to compare the dosimetric difference resulting from a FB versus BH treatment plan approach of left-sided breast cancer patients. For each patient, FB and BH CT datasets and patient structures were exported to PlanIQ™ to generate the corresponding OAR FDVHs; heart and left lung \({{\text{D}}}_{{\text{m}}}\), \({{\text{V}}}_{5{\text{Gy}}}\) and \({{\text{V}}}_{20{\text{Gy}}}\) (percentage volume receiving 5 and 20 Gy, respectively) were evaluated assuming the entire PTV was receiving 40.43 Gy equal to 95% of the \({{\text{D}}}_{{\text{p}}}\). BH and FB OARs dosimetry was compared for the red and orange FDVH curves bounding the impossible and difficult zone, respectively, deemed more challenging by PlanIQ™. Statistical analysis of the data was performed by using SPSS software (IBM, Armont, USA) version 19.0, used to test the results of the radiation treatment plans of the BH and FB groups by paired t-test; a difference of p < 0.05 was considered statistically significant.
Results
Accuracy of the datasets used
Automatic segmentation of the whole heart and left lung volumes are consistent with contours performed manually in the clinical practice, with minor deviations not statistically significant. In Table 1, the results of DSC, MASD, HD and p-value are reported. Results are given as the mean ± standard deviation.
BH Clinical DVH versus BH FDVH
The results dosimetric parameters of the heart and left lung of the clinically delivered plans and the corresponding dosimetric parameters evaluated by the red and orange FDVH curves at the boundary between the impossible and feasible areas are shown in Table 2.
Clinical treatment plans in BH meet the target dose coverage and dose sparing requirements for OARs; the dosimetric parameters of the heart and left lung of the clinical plans are in the FDVH difficult zone, while they differ significantly from the corresponding parameters of the red and orange FDVH curves (p < 0.05), respectively, which delimit this area, a buffer between the impossible and feasible zones.
Comparison of BH and FB contours datasets
In Table 3, PTV and OAR volumes corresponding to the FB and BH are reported. In BH, PTV and heart median volumes did not present a significant difference (p = 0.064 and p = 0.106, respectively); the volume of the left lung increased significantly in BH (p = 0.001) with a median value of 75.1%, range (25.59—139.70) %.
BH FDVH versus FB FDVH
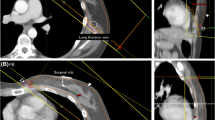
The target dose distribution of FB and BH FDVHs meets clinical requirements and does not present a statistical difference (p > 0.05) in the target \({{\text{D}}}_{95}\), \({{\text{D}}}_{2{\text{cc}}}\). For 30 patients out of 35 patients the heart and left lung BH FDVHs dosimetric parameters, are reduced compared with the corresponding FB FDVHs; the results obtained are shown in Tables 4 and 5 for the red and orange FDVHs curves, respectively. The largest differences in the FDVH were found for heart \({{\text{V}}}_{5{\text{Gy}}}\) and \({{\text{D}}}_{{\text{m}}}\) with mean percentage reductions of 54.5% and 35.7%, respectively, in the red curves, and 16.7%, and 16.0%, respectively, in the orange curve; similarly, for the left lung \({{\text{V}}}_{5{\text{Gy}}}\) and \({{\text{D}}}_{{\text{m}}}\) a mean percentage reduction of 21.4% and 15.1%, respectively, for the red curve, and 13.7%, and 7.1%, respectively, for the orange FDVH. Left lung \({{\text{D}}}_{{\text{m}}}\) and \({{\text{V}}}_{20{\text{Gy}}}\) did not present significant differences in the boundaries for the red and orange boundary curves. The comparison of FB and BH heart and left lung FDVH curves obtained for a representative patient are shown in Fig. 2.
Timing of the procedure
The time required to automatically segment the heart and left lung is about 5 min, mainly due to the time needed to import the CT scan into the automatic segmentation software and the subsequent export of CT images and structures to PlanIQ™. The average time needed for the radiation oncologist to verify/adjust the target on the FB CT dataset is around 10 min. The time to generate the FDVH is 2 min for each structure. The total average time of the procedure setting to compare the FB and BH FDVH for the heart and left lung is 20 min with a range of 18 to 22 min.
Discussion
Radiotherapy is rapidly evolving towards precise targeting, precise planning and precise treatment [39, 40]. Accurate treatment depends on the dose distribution received by the tumor tissue, which is determined by many factors including the delivery technique chosen. In recent years, automatic planning optimization solutions have received much attention to address the high cost and low efficiency of the reverse planning process [18,19,20].
FDVH is a tool to estimate the best possible sparing dose of OARs a priori before starting the plan optimization [41]. The algorithm does not require a database of prior plans but rather derives the FDVH from nearly first principles, assuming that the targets are uniformly covered with the prescription doses. It is easily parametrized based on a short list of model geometrical datasets. The method is agnostic to the planning technique and beam arrangement, requiring only the regions of interest, the energy, and optionally the CT dataset as inputs.
In this study, FDVH was used as an independent method to compare in large clinical left-sided breast datasets the dosimetry of the heart and left lung for FB and BH treatments. The dosimetric parameters provided by the FDVH in the FB datasets and the BH datasets were compared and analysed to explore and quantify differences in the OARs dosimetry. The time needed for the whole procedure is about 20 min per patient. The results obtained showed that the dose distribution of the FB and BH FDVH met the clinical prescription requirements, and there was no statistical difference in the target area dosimetric parameters. OARs dose can be reduced with BH datasets, particularly heart \({{\text{V}}}_{5{\text{Gy}}}\) and \({{\text{D}}}_{{\text{m}}}\). The results obtained are not systematically valid for all patients; a percentage of patients, which in the case of our study stands at 14.2% have no advantage in following the breath-hold irradiation procedure. The method is well-suited for approximating the best-possible OAR DVH curve; however, because it enforces 100% of the target coverage whereas a real-world plan often sacrifices target coverage near OARS there can be deviations of the clinical DVH versus FDVH. In particular, the geometry of the OAR in relation to the target is the major driver of the achievable OAR FDVH [42, 43]. Its simplicity is nevertheless a cause of the algorithm’s limitation; because it is designed to minimize the OARs DVH as much as possible, based on the geometry and distance between the OAR and the target volume.
Tools capable of providing predictions of what is dosimetrically achievable (and ideally optimal) are greatly needed in radiation treatment planning not only to reduce plan variability and ensure quality but also as a tool to support the radiation oncologist in the decision process. The use of artificial intelligence (AI) in setting up predictive models for BH treatment decisions is to date an investigated option [44]. Vendrame et al. [45], after generating FB and BH treatment plans, considered the decrease of the maximum dose to the left anterior descending artery as a parameter to select patients eligible for BH or not; the predictive model set up used AI to analyse different phases of the respiratory cycle.
This work showed with an independent method that left-sided-breast treatments performed in BH and FB enable target dose distribution that met the clinical requirements without statistical differences; the dosimetric advantages that can arise in the heart and left lung dosimetry for BH delivery exist but not indiscriminately for all cases; for even a small percentage of patients, the use of the breath-hold technique does not lead to additional benefits. A personalised assessment is important to decide on the appropriate type of treatment and to optimise the use of the available technology, avoiding more demanding treatments for cases that will not have any benefits.
Conclusion
The use of PlanIQTM as a decision-support tool for the FB versus BH treatment modality may allow the customisation of the treatment technique using the most appropriate delivery techniques for each patient while enabling an accurate use of available technologies.
Availability of data and materials
The data are fully available and will be included within the article and in its additional files.
Abbreviations
- BH:
-
Breath-hold
- OARs:
-
Organs at risk
- FDVH:
-
Feasibility dose volume histogram
- DVH:
-
Dose-volume histogram
- CTV:
-
Clinical target volume
- CT:
-
Computed tomography
- PTV:
-
Planning target volume
- \({{\text{D}}}_{{\text{p}}}\) :
-
Prescription dose
- \({{\text{D}}}_{{\text{m}}}\) :
-
Mean dose
- FB:
-
Free breathing
- \({{\text{Lung}}}_{{\text{auto}}}\) :
-
Automatic Lung contour
- \({{\text{Heart}}}_{{\text{auto}}}\) :
-
Automatic heart contour
- \({{\text{Lung}}}_{{\text{man}}}\) :
-
Manual contour of the Lung
- \({{\text{Heart}}}_{{\text{man}}}\) :
-
Manual contour of the heart
- MASD:
-
Mean absolute surface-to-surface distance
- DSC:
-
Dice similarity coefficient
- HD:
-
Hausdorff distance
References
Anastasiadi Z, Lianos GD, Ignatiadou E, Harissis HV, Mitsis M. Breast cancer in young women: an overview. Updates Surg. 2017;69(3):313–7.
Lima SM, Kehm RD, Terry MB. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. e-ClinicalMedicine Part Lancet Discov Sci. 2021;38:100985.
Darby S, Mcgale P, Correa C, Taylor T, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–16.
Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41.
Boero IJ, Paravati AJ, Triplett DP, Hwang L, Murphy JD. Modern radiation therapy and cardiac outcomes in breast cancer. Int J Radiat Oncol Biol Phys. 2016;94(4):700–8.
Darby SC, Ewertz M, Mcgale P, Bennet AM, Blomgoldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987–98.
Cuzick J, Stewart H, Rutqvist LE, Houghton J, Host H. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol. 1994;12(3):447–53.
Stovall M, Smith SA, Langholz MB. Dose to the contralateral breast from radiotherapy and risk of second primary breast cancer in the WECARE study. Int J Radiat Oncol Biol Phys. 2008;74:1021–30.
Lu Y, Yang D, Zhang X, Teng Y, Yuan W, Zhang Y, et al. Comparison of deep inspiration breath hold versus free breathing in radiotherapy for left sided breast cancer. Front Oncol. 2022;12: 845037.
Shagun M, Ashutosh M, Punita L, Resham S, Mrinalani V, Sellepolyam K, et al. Cardiac dose reduction using deep inspiratory breath hold (DIBH) in radiation treatment of left sided breast cancer patients with breast conservation surgery and modified radical mastectomy. J Med Imaging Radiat Sci. 2021;52(1):57–67.
Lu H-M, Cash E, Chen MH, Chin L, Manning WJ, Harris J, et al. Reduction of cardiac volume in left-breast treatment fields by respiratory maneuvers: a CT study. Int J Radiat Oncol Biol Phys. 2000;47(4):895–904.
Swanson T, Grills IS, Ye H, Entwistle A, Teahan M, Letts N, et al. Six-year experience routinely using moderate deep inspiration breath-hold for the reduction of cardiac dose in left-sided breast irradiation for patients with early-stage or locally advanced breast cancer. Am J Clin Oncol. 2013;36(1):24–30.
Latty D, Stuart KE, Wang W, Ahern V. Review of deep inspiration breath-hold techniques for the treatment of breast cancer. J Med Radiat Sci. 2015;62(1):74–81.
Remouchamps VM, Vicini FA, Sharpe MB, Kestin LL, Martinez AA, Wong JW. Significant reductions in heart and lung doses using deep inspiration breath hold with active breathing control and intensity-modulated radiation therapy for patients treated with locoregional breast irradiation. Int J Radiat Oncol Biol Phys. 2003;55(2):392–406.
Pham TT, Ward R, Latty D, Owen C, Gebski V, Chojnowski J, et al. Left-sided breast cancer loco-regional radiotherapy with deep inspiration breath-hold: Does volumetric-modulated arc radiotherapy reduce heart dose further compared with tangential intensity-modulated radiotherapy? J Med Imaging Radiat Oncol. 2016;60(4):545–53.
Mika J, Laaksomaa M, Jani P, Mikko H, Helmi L, Turkka L, et al. Effect of multiple breath hold reproducibility on treatment localization and dosimetric accuracy in radiotherapy of left-sided breast cancer with voluntary deep inspiration breath hold technique. Med Dosim. 2017;42:177–84.
Vikström J, Hjelstuen MHB, Mjaaland I, Dybvik KI. Cardiac and pulmonary dose reduction for tangentially irradiated breast cancer, utilizing deep inspiration breath-hold with audio-visual guidance, without compromising target coverage. Acta Oncol. 2011;50(1):42–50.
Xiao A, Crosby J, Malin M, Malin H, Kang H, Washington M, et al. Single-institution report of setup margins of voluntary deep-inspiration breath-hold (DIBH) whole breast radiotherapy implemented with real-time surface imaging. J Appl Clin Med Phys. 2018;19(4):205–13.
Nissen HD, Appelt AL. Improved heart, lung and target dose with deep inspiration breath hold in a large clinical series of breast cancer patients. Radiother Oncol. 2013;106(1):28–32.
Bartlett FR, Donovan EM, McNair HA, Corsini LA, Colgan RM, Evans PM, et al. The UK HeartSpare Study (Stage II): multicentre evaluation of a voluntary breath-hold technique in patients receiving breast radiotherapy. Clin Oncol (R Coll Radiol). 2017;29(3):e51–6.
Yeung R, Conroy L, Long K, Walrath D, Li H, Smith W, et al. Cardiac dose reduction with deep inspiration breath hold for left-sided breast cancer radiotherapy patients with and without regional nodal irradiation. Radiat Oncol. 2015;10:200.
Walston S, Quick AM, Kuhn K, Rong Y. Dosimetric considerations in respiratory-gated deep inspiration breath-hold for left breast irradiation. Technol Cancer Res Treat. 2016;16(1):22.
Oechsner M, Düsberg M, Borm KJ, Combs SE, Duma MN. Deep inspiration breath-hold for left-sided breast irradiation: analysis of dose-mass histograms and the impact of lung expansion. Radiat Oncol. 2019;14(1):1.
Nelms BE, Robinson G, Markham J, Velasco K, Boyd S, Narayan S, et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2(4):296–305.
Ahmed S, Nelms B, Gintz D, Caudell J, Zhang G, Moros EG, et al. A method for a priori estimation of best feasible DVH for organs-at-risk: Validation for head and neck VMAT planning. Med Phys. 2017;44(10):5486–97.
Perumal B, Sundaresan HE, Ranganathan V, Ramar N, Meher SR. Evaluation of plan quality improvements in PlanIQ-guided Autoplanning. Rep Pract Oncol Radiother. 2019;24:1.
Nathalie R, Julie ID, Kyla H, John AW, Brian N, Betro TS, et al. Deep inspiration breath-hold technique in left-sided breast cancer radiation therapy: evaluating cardiac contact distance as a predictor of cardiac exposure for patient selection. Pract Radiat Oncol. 2015;5(3):e127–34.
Sasaki M, Nakaguuchi Y, Kamomae T, Tsuzuki A, Kobuchi S, Kuwahara K, Ueda S, Endo Y, Ikushima H. Analysis of prostate intensityand volumetric—dulated arc radiation therapy planning quality with PlanIQ™. J Appl Clin Med Phys. 2021;2021:1.
Xia W, Han F, Chen J, Miao J, Dai J. Personalized setting of plan parameters using feasibility dose volume histogram for auto-planning in Pinnacle system. J Appl Clin Med Phys. 2020;21(7):119–27.
Xin X, Li J, Zhao Y, Wang P, Tang B, Yao X, et al. Retrospective study on left-sided breast radiotherapy: dosimetric results and correlation with physical factors for free breathing and breath hold irradiation techniques. Technol Cancer Res Treat. 2021;20:15330338211062428.
Zhao Y, Diao P, Zhang D, Wu J, Xin X, Fontanarosa D, et al. Impact of positioning errors on the dosimetry of breath-hold-based volumetric arc modulated and tangential field-in-field left-sided breast treatments. Front Oncol. 2020;10:1.
Liao X, Wu F, Wu J, Peng Q, Yao X, Kang S, et al. Impact of positioning errors in the dosimetry of VMAT left-sided post mastectomy irradiation. Radiat Oncol. 2020;15(1):103.
Harriet EG, Lauren M, Kirsty S, Najmun N, Ken T, Tim W, et al. Delineation of target volumes and organs at risk in adjuvant radiotherapy of early breast cancer: national guidelines and contouring atlas by the Danish Breast Cancer Cooperative Group. Acta Oncol. 2013;52(4):703–10.
Gentile MS, Usman AA, Neuschler EI, Sathiaseelan V, Hayes JP Jr, SW. Contouring guidelines for the axillary lymph nodes for the delivery of radiation therapy in breast cancer: evaluation of the RTOG breast cancer atlas. Int J Radiat Oncol Biol Phys. 2015;93(2):257–65.
Whelan TJ, Pignol J-P, Levine MN, et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. New Engl J Med. 2010;1:1.
Marks LB, Yorke ED, Jackson A, Haken RKT, Constine LS, Eisbruch A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S10.
National Cancer Center NCQCC. Guideline of target delineation and treatment planning of adjuvant radiotherapy for breast cancer. Chin J Radiat Oncol. 2022;31(10):863–78.
XiongFei L, Ke Y, Peng X, Churong L, Min L, Junxiang W, et al. Clinical dosimetry testing of the AiPlan radiotherapy treatment planning system. J Med Devices. 2022;37(01):27–9.
Clarke S, Goodworth J, Westhuyzen J, Chick B, Hoffmann M, Pacey J, et al. Software-based evaluation of a class solution for prostate IMRT planning. Rep Pract Oncol Radiother. 2017;22:441–69.
Huang L, Park K, Boike T, Lee P, Papiez L, Solberg T, et al. A study on the dosimetric accuracy of treatment planning for stereotactic body radiation therapy of lung cancer using average and maximum intensity projection images. Radiother Oncol. 2010;96:48–54.
Ahmed S, Nelms B, Gintz D, Caudell J, Zhang G, Moros EG, et al. A method for a priori estimation of best feasible DVH for organs-at-risk: validation for head and neck VMAT planning. Med Phys. 2017;44(10):1.
Petit SF, Wu B, Kazhdan M, Dekker A, Simari P, Kumar R, et al. Increased organ sparing using shape-based treatment plan optimization for intensity modulated radiation therapy of pancreatic adenocarcinoma. Radiother Oncol. 2011;102(1):38–44.
Shiraishi S, Moore KL. Knowledge-based prediction of three-dimensional dose distributions for external beam radiotherapy. Med Phys. 2016;43:1.
Avanzo M, Trianni A, Botta F, Talamonti C, Iori M. Artificial Intelligence and the Medical Physicist: Welcome to the Machine. Appl Sci. 2021;11(4):1691.
Vendrame A, Cappelletto C, Chiovati P, Vinante L, Parvej M, Caroli A, et al. Artificial intelligence-based patient selection for deep inspiration breath-hold breast radiotherapy from respiratory signals. Appl Sci. 2023;13(8):1.
Acknowledgements
None.
Funding
The authors did not receive any funding for this work.
Author information
Authors and Affiliations
Contributions
Study concept and design of the Research: LX, LCO, YX, KY; Acquisition of data and images dataset processing: XX, MJ, DP, KY; Analysis and interpretation of data: XL, YX, LJ; Manuscript Preparation: JL, YX, YK; Writing Manuscript LCO, LJ, Manuscript critical revision: LX, YK. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors declare that this retrospective study “Using PlanIQTM in the clinical workflow for breath-hold versus free-breathing left-sided breast irradiation” received the approval of the ethic Committee of Sichuan Cancer Hospital located in 55th Renmin South Road, 4th Section, 610041, Chengdu, China (Approval number SCCHEC-02-2021-026).
Consent for publication
Even if no individual patient data were reported, consensus has been received by every patient for the elaboration of its data and for future scientific publication.
Competing Interests
None of the authors have any competing interests (financial and non-financial) in the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yuan, K., Yao, X., Liao, X. et al. Comparing breath hold versus free breathing irradiation for left-sided breast radiotherapy by PlanIQ™. Radiat Oncol 18, 200 (2023). https://doi.org/10.1186/s13014-023-02386-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-023-02386-2