Abstract
Background
Fractionated stereotactic radiosurgery (fSRS) is an important treatment strategy for unresected brain metastases. We previously reported that a good volumetric response 6 months after fSRS can be the first step for local control. Few studies have reported the association between gross tumor volume (GTV) dose, volumetric response, and local control in patients treated with the same number of fractions. Therefore, in this study, we aimed to investigate the GTV dose and volumetric response 6 months after fSRS in five daily fractions and identify the predictive GTV dose for local failure (LF) for unresected brain metastasis.
Methods
This retrospective study included 115 patients with 241 unresected brain metastases treated using fSRS in five daily fractions at our hospital between January 2013 and April 2022. The median prescription dose was 35 Gy (range, 30–35 Gy) in five fractions. The median follow-up time after fSRS was 16 months (range, 7–66 months).
Results
GTV D80 > 42 Gy and GTV D98 > 39 Gy were prognostic factors for over 65% volume reduction (odds ratio, 3.68, p < 0.01; odds ratio, 4.68, p < 0.01, respectively). GTV D80 > 42 Gy was also a prognostic factor for LF (hazard ratio, 0.37; p = 0.01).
Conclusions
GTV D80 > 42 Gy in five fractions led to better volume reduction and local control. The goal of planning an inhomogeneous dose distribution for fSRS in brain metastases may be to increase the GTV D80 and GTV D98. Further studies on inhomogeneous dose distributions are required.
Similar content being viewed by others
Background
Stereotactic radiosurgery (SRS) and fractionated stereotactic radiosurgery (fSRS) are essential treatment strategies for brain metastases [1, 2]. The clinical application of fSRS is increasing with advancements in linear accelerator (Linac) and radiation technologies [3]. Various radiation doses and fractions have been used in fSRS for brain metastases [1, 4, 5] and different formulas have been used to calculate the biological effective dose for fSRS [4,5,6,7,8]. These inconsistencies result in different doses calculated for tumor control. The same number of fractions is preferable when discussing tumor control and radiotherapy doses. However, only a few reports discuss tumor control in the same number of treatment fractions using Linac fSRS for unresected brain metastases [3, 9,10,11,12,13,14].
As per the Response Assessment in Neuro-Oncology Brain Metastases (RANO-BM) guidelines, the assessment and reporting of volumetric measurements are important, and their inclusion as a study endpoint should be encouraged [15] as volumetric measurements have lower variability than linear measurements [16]. However, few studies use volumetric measurements owing to the associated complexity [8, 15, 17, 18].
We previously reported over 65% and over 90% volume reductions 6 months after fSRS and SRS predicted local control [19]. Few studies have reported the association between gross tumor volume (GTV) dose and volumetric response, and local control for fSRS with the same number of fractions [9]. Therefore, in this study, we aimed to identify the prognostic GTV dose for volumetric response—specifically, over 65% reduction (O65R) and over 90% reduction (O90R) in volume—of brain metastases 6 months after fSRS in five fractions, and identify the prognostic GTV dose for local failure (LF).
Methods
Patients
Overall, 115 patients with 241 unresected brain metastases treated by fSRS in five daily fractions at our institute between January 2013 and April 2022 were included in this study. Patient data were retrospectively collected from our electronic database. The inclusion criteria were unresected brain metastases treated by five daily fractions at our institute. The exclusion criteria were the following: brain metastases < 0.3 cc at baseline, no magnetic resonance imaging (MRI) 5.0–8.5 months after fSRS, whole-brain radiotherapy before MRI evaluation, and brain metastases with local tumor progression before MRI evaluation, performed approximately 6 months (median, 6.2 months; range, 5.0–8.3 months) after fSRS.
The patient characteristics and details of the brain metastases and fSRS are presented in Table 1. A total of 179 brain metastases treated with systemic therapy concurrently (within 1 month before or after fSRS); 33 treated with immunotherapy, including immune checkpoint inhibitors; and 88 treated with target therapy, including angiogenesis inhibitors, anti-human epidermal growth factor receptor type 2 antibodies, anti-epidermal growth factor receptor antibodies, tyrosine kinase inhibitors, serine/threonine kinase inhibitors, and cyclin-dependent kinase inhibitors.
Treatments
The fSRS treatment has been described previously [19,20,21]. All patients were immobilized using a thermoplastic mask, and planning computed tomography was performed using an iodine-based contrast agent. The GTV was delineated using T1-weighted gadolinium-enhanced MRI. An isotropic margin of 1 mm (range, 1–3 mm) was applied to the GTV to obtain the planning target volume (PTV).
The median prescription dose was 35 Gy (range, 30–35 Gy) in five daily fractions on weekdays. The dose was prescribed to cover 95% or 99% of the combined PTVs. The median isodose line (prescription dose/maximum dose for GTV) was 52% (range, 40–95%). An inhomogeneous dose distribution was allowed. The doses to the brain tissue were reduced to a minimum during the optimization process. Automated non-coplanar volumetric-modulated arc therapy (VMAT) (HyperArc; Varian Medical Systems, Palo Alto, CA), coplanar VMAT, or dynamic conformal arc therapy with C-arm Linac (Clinac 23Ex, Ture Beam STX, or Edge; Varian Medical Systems, Palo Alto, CA, USA) was used for fSRS.
Follow-ups included clinical examination and MRI. Brain MRI every 3 months was recommended. The interval was shortened when the tumor volume was increased or new symptoms developed. The median follow-up time after fSRS was 16 months (range, 7–66 months).
The evaluated MRIs were imported into the radiotherapy planning system. The tumor volume was delineated by a radiation oncologist, including the tumor and the fSRS effects. The tumor volume reduction rate from GTV was evaluated.
Definitions
LF was calculated from the time of MRI evaluation to the radiological observation of tumor progression in a treated lesion. Tumor progression was defined according to the RANO-BM guidelines [15]. The definition of tumor progression was applied to each brain metastasis. The differential diagnoses of tumor progression and brain necrosis have been described previously [19]. We used adverse radiation effect (ARE) to capture all cases of radiation necrosis. The definition of ARE is consistent with the prior report by Sneed et al. [22]. ARE was calculated from the first day of fSRS.
Statistical analyses
LF and ARE were estimated using the cumulative incidence function with death as a competing risk. Univariate and multivariate analyses of factors associated with LF and ARE were performed using the Fine–Gray model to determine hazard ratios (HRs). Univariate and multivariate analyses of the factors associated with O65R and O90R were performed using a logistic regression model to determine the odds ratios (ORs). Furthermore, GTV D98, D80, D60, D40, D20, and D2 were analyzed. Pearson’s correlation coefficient (r) was used to evaluate the correlation between the GTV parameters. Moreover, Spearman’s correlation coefficient (ρ) was used to evaluate the correlation between the clinical and GTV parameters. The Kendall rank correlation coefficient (τ) was used to evaluate the correlation between binary variables. When (r), (ρ), and (τ) were > 0.60, only one variable was used for the multivariate analysis. The Youden index was used to identify the optimal threshold value for volume reduction. The lowest Akaike information criterion (AICc) value was considered the most predictive. Evidence ratios (EVRs) were calculated, and models with an EVR < 2.7 were considered to have substantial support [23]. We assessed the variance inflation factor (VIF) testing for multicollinearity in the multivariate analysis of O65R and O90R. Statistical significance was set at a p-value of < 0.05. The statistical analyses were performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan).
Results
Volume reduction and LF
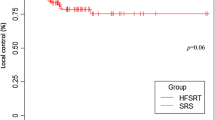
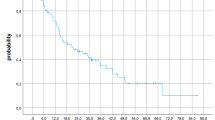
The LF at 0.5 and 1.5 years from the MRI evaluation (approximately 1 and 2 years from fSRS) for O65R was 1.80 and 7.80%, respectively (Fig. 1). The results of univariate and multivariate analyses of LF are presented in Table 2. The multivariate analysis revealed that O65R and O90R were prognostic factors for LF (HR, 0.35, p < 0.01 and HR, 0.41, p = 0.04, respectively) (Table 2).
Analysis of volume reduction
All correlations between the GTV parameters had (r) > 0.60. All correlations between the clinical and GTV parameters had (ρ) < 0.60 and (τ) < 0.60. Univariate analyses for O65R and O90R are presented in Supplementary Table 1.
Models that included GTV D80, age, and time for the 6-month MRI had the lowest AICc values for O65R (Supplementary Table 2). Overall, six models had EVR < 2.7 for O65R (Supplementary Table 2). Of the six models with EVR < 2.7 for O65R, five included GTV D80 and one included GTV D98 (Supplementary Table 2). Details of the multivariate analysis, including age, time for MRI evaluation, and GTV D80 or GTV D98, are presented in Supplementary Table 3; no collinearity was observed in each model. Both GTV D80 and GTV D98 were significant predictive factors for O65R (Supplementary Table 3).
Models including GTV D80, age, primary cancer, and time for MRI evaluation had the lowest AICc values for O90R (Supplementary Table 2). In addition to the lowest AICc model, the only model that included GTV D98 had EVR < 2.7 for O90R (Supplementary Table 2). Details of the multivariate analyses, including age, primary cancer, time for MRI evaluation, and GTV D80 or GTV D98, are presented in Supplementary Table 3; no collinearity was observed in any of the models (Supplementary Table 3). Both GTV D80 and GTV D98 were significant predictive factors for O90R (Supplementary Table 3).
We defined 42 Gy for GTV D80 and 39 Gy for GTV D98 as the thresholds for O65R and O90R. A strong correlation was observed between GTV D80 > 42 Gy and D98 > 39 Gy (τ = 0.88). The multivariate analysis revealed GTV D80 > 42 Gy and D98 > 39 Gy as predictive factors for O65R (OR, 3.68, p < 0.01 and OR, 4.68, p < 0.01, respectively; Table 3). Furthermore, GTV D80 > 42 Gy and D98 > 39 Gy were predictive factors for O90R (OR, 4.70, p < 0.01 and OR, 6.41, p < 0.01, respectively; Table 3).
Dose for GTV, LF, and ARE
GTV (cc) was a risk factor for LF (Table 2). GTV (cc) exhibited no correlation between GTV D80 greater or smaller than 42 Gy (τ = 0.21) and D98 greater or smaller than 39 Gy (τ = 0.22). For GTV > 1 cc, 87 brain metastases (69.6%) were GTV D80 > 42 Gy and 90 brain metastases (72.0%) were GTV D98 > 39 Gy.
D98 > 39 Gy was not a prognostic factor for LF in multivariate analysis (Table 2); however, D80 > 42 Gy was a significant prognostic factor for LF (HR, 0.37; p = 0.01; Table 2). The LF at 0.5 and 1.5 years from the MRI evaluation (approximately 1 and 2 years from fSRS) was 2.70 and 7.31%, respectively, for D80 > 42 Gy (Fig. 2).
The ARE at 1 and 2 years from fSRS was 6.01% and 13.14%, respectively. D80 > 42 Gy and D98 > 39 Gy were not a prognostic factor for ARE (Supplementary Tables 4 and Supplementary Figure).
Discussion
To our knowledge, this is the first study to report that GTV D80 > 42 Gy in five fractions is a significant prognostic factor for both tumor volume reduction and LF in Linac fSRS for brain metastases > 0.3 cc. Prescribed dose has been established as a predictive factor for LF [5, 24]. A higher prescribed dose is associated with an improved local control trend [4, 5, 24]. Moreover, a prescribed dose of > 30 Gy in five fractions was a predictive factor for LF [3, 11, 14]. However, different isodose and PTV margins result in different doses for the GTV even with the same prescribed dose [4]. Few studies have reported the GTV dose and LF [9]. This is the first study to discuss GTV dose and LF treated by fSRS in five fractions. Further studies incorporating GTV dose and LF are needed.
As previously recommended by the International Commission on Radiation Units and Measurements (ICRU), the dose in the PTV should be confined within 95–107% of the prescribed dose [25]; therefore, 80–90% isodose was used for patients undergoing Linac fSRS [3, 12, 24]. In this study, achieving a GTV D80 > 42 Gy when prescribed 35 Gy (the median dose) with 80% isodose was almost impossible, and an inhomogeneous distribution was needed. Inhomogeneous distribution has been reported to predict good local control in gamma knife SRS [26, 27] and may lead to better tumor volume reduction and LF.
Some problems are associated with LF in fSRS for brain metastases. First, many fSRS characteristics have underpowered significance because many patients die from extracranial disease. Second, tumor recurrence and brain necrosis are often difficult to diagnose. In cases with volume progression after fSRS, viable tumor tissue and brain necrotic change are often mixed on pathological evaluation [11, 28]. Misdiagnoses of tumor recurrence and brain necrosis, evaluated only using imaging, are unavoidable. In this study, O65R and O90R 6 months after fSRS were prognostic factors for LF. This was consistent with the results of our previous study [19]. O65R in brain metastases corresponds to a partial response according to the RANO-BM guidelines [15]. The median volume reduction rates were 44.2% at 3 months and 69.6% at 6 months after SRS and fSRS [17]. A partial response at 6 months after fSRS may be the first step toward long-term local control.
In this study, GTV D80 and GTV D98 were more predictive of volume reduction than GTV D2. Increasing the GTV D80 dose predicted good local control. Lucia et al. reported that an inhomogeneous dose distribution was a significant prognostic factor for local control compared to a homogeneous distribution with the same PTV dose; however, D2 was not a significant prognostic factor for local control [29]. These results may seem contradictory; however, GTV D80 and GTV D98 might be higher in inhomogeneous dose distributions, leading to good local control. Inhomogeneous dose distribution should be used to increase mainly GTV D80 and GTV D98.
GTV D80 was the most predictive for volume reduction in this study and our previous one [19]. The HyTEC group recommends that the maximum (D2) and peripheral (D98) doses for GTV should be included when reporting outcomes [4]. This study indicates that reporting only GTV D98 may be insufficient. A more inhomogeneous dose distribution can now be easily obtained with the advancement in Linac fSRS, including non-coplanar VMAT [30].
Our study has some limitations. First, it was retrospective. Second, the median tumor volume was 1.1 cc. However, we excluded brain metastases < 0.3 cc because volumetric measurements are unsuitable for very small brain metastases. The optimal GTV dose for very large brain metastases should be evaluated in future studies. Third, the effects of systemic therapy were not analyzed because this study included a variety of primary cancers. When reporting the same number of fractions in fSRS for brain metastases, the number of analyzed brain metastases is often small. The strength of our study is that this is one of the largest studies of its kind, with 241 brain metastases treated with five fractions and a relatively long follow-up period.
Conclusions
GTV D80 was the most predictive factor for volume reduction after fSRS. GTV D80 > 42 Gy in five fractions is required for good volume reduction and local control. The goal of planning an inhomogeneous dose distribution for fSRS in brain metastases is to increase the GTV D80 and GTV D98. An inhomogeneous dose distribution can now be easily obtained with the advancement of Linac fSRS, including non-coplanar VMAT. Further studies on inhomogeneous dose distributions are required.
Data availability
The data that support the findings of this study are available upon request from the corresponding author. The data are not publicly available because of privacy or ethical restrictions.
Abbreviations
- AICc:
-
Akaike information criterion
- ARE:
-
Adverse Radiation Effect
- EVRs:
-
Evidence ratios
- fSRS:
-
Fractionated stereotactic radiosurgery
- GTV:
-
Gross tumor volume
- HR:
-
Hazard ratios
- LF:
-
Local failure
- MRI:
-
Magnetic resonance imaging
- OR:
-
Odds ratios
- PTV:
-
Planning target volume
- VIF:
-
Variance inflation factor
References
Gondi V, Bauman G, Bradfield L, Burri SH, Cabrera AR, Cunningham DA, et al. Radiation therapy for brain metastases: an ASTRO clinical practice guideline. Pract Radiat Oncol. 2022;12:265–82. https://doi.org/10.1016/j.prro.2022.02.003.
Vogelbaum MA, Brown PD, Messersmith H, Brastianos PK, Burri S, Cahill D, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. Neuro Oncol. 2022;24:331–57. https://doi.org/10.1200/JCO.21.02314.
Myrehaug S, Hudson J, Soliman H, Ruschin M, Tseng CL, Detsky J, et al. Hypofractionated stereotactic radiation therapy for intact brain metastases in 5 daily fractions: Effect of dose on treatment response. Int J Radiat Oncol Biol Phys. 2022;112:342–50. https://doi.org/10.1016/j.ijrobp.2021.09.003.
Redmond KJ, Gui C, Benedict S, Milano MT, Grimm J, Vargo JA, et al. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2021;110:53–67. https://doi.org/10.1016/j.ijrobp.2020.10.034.
Wiggenraad R, Verbeek-de Kanter A, Kal HB, Taphoorn M, Vissers T, Struikmans H. Dose-effect relation in stereotactic radiotherapy for brain metastases. A systematic review. Radiother Oncol. 2011;98:292–7. https://doi.org/10.1016/j.radonc.2011.01.011.
Gago-Arias A, Neira S, Pombar M, Gómez-Caamaño A, Pardo-Montero J. Evaluation of indirect damage and damage saturation effects in dose–response curves of hypofractionated radiotherapy of early-stage NSCLC and brain metastases. Radiother Oncol. 2021;161:1–8. https://doi.org/10.1016/j.radonc.2021.05.012.
Guerrero M, Li XA. Extending the linear-quadratic model for large fraction doses pertinent to stereotactic radiotherapy. Phys Med Biol. 2004;49:4825–35. https://doi.org/10.1088/0031-9155/49/20/012.
Putz F, Weissmann T, Oft D, Schmidt MA, Roesch J, Siavooshhaghighi H, et al. FSRT vs. SRS in brain metastases—differences in local control and radiation necrosis—A volumetric study. Front Oncol. 2020;10:559193. https://doi.org/10.3389/fonc.2020.559193.
Dupic G, Brun L, Molnar I, Leyrat B, Chassin V, Moreau J, et al. Significant correlation between gross tumor volume (GTV) D98% and local control in multifraction stereotactic radiotherapy (MF-SRT) for unresected brain metastases. Radiother Oncol. 2021;154:260–8. https://doi.org/10.1016/j.radonc.2020.11.021.
Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81:18–24. https://doi.org/10.1016/j.radonc.2006.08.024.
Marcrom SR, McDonald AM, Thompson JW, Popple RA, Riley KO, Markert JM, et al. Fractionated stereotactic radiation therapy for intact brain metastases. Adv Radiat Oncol. 2017;2:564–71. https://doi.org/10.1016/j.adro.2017.07.006.
Ogura K, Mizowaki T, Ogura M, Sakanaka K, Arakawa Y, Miyamoto S, et al. Outcomes of hypofractionated stereotactic radiotherapy for metastatic brain tumors with high risk factors. J Neurooncol. 2012;109:425–32. https://doi.org/10.1007/s11060-012-0912-6.
Saitoh JI, Saito Y, Kazumoto T, Kudo S, Ichikawa A, Hayase N, et al. Therapeutic effect of linac-based stereotactic radiotherapy with a micro-multileaf collimator for the treatment of patients with brain metastases from lung cancer. Jpn J Clin Oncol. 2010;40:119–24. https://doi.org/10.1093/jjco/hyp128.
Zhou C, Xia Y, Huang P, Guan L, Shen X, Hao D, et al. Fractionated stereotactic radiation therapy using volumetric modulated arc therapy in patients with solitary brain metastases. BioMed Res Int. 2020;2020:6342057. https://doi.org/10.1155/2020/6342057.
Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015;16:e270–8. https://doi.org/10.1016/S1470-2045(15)70057-4.
Bauknecht HC, Romano VC, Rogalla P, Klingebiel R, Wolf C, Bornemann L, et al. Intra-and interobserver variability of linear and volumetric measurements of brain metastases using contrast-enhanced magnetic resonance imaging. Invest Radiol. 2010;45:49–56. https://doi.org/10.1097/RLI.0b013e3181c02ed5.
Oft D, Schmidt MA, Weissmann T, Roesch J, Mengling V, Masitho S, et al. Volumetric regression in brain metastases after stereotactic radiotherapy: Time course, predictors, and significance. Front Oncol. 2020;10:590980. https://doi.org/10.3389/fonc.2020.590980.
Sharpton SR, Oermann EK, Moore DT, Schreiber E, Hoffman R, Morris DE, et al. The volumetric response of brain metastases after stereotactic radiosurgery and its post-treatment implications. Neurosurgery. 2014;74:9–15. https://doi.org/10.1227/NEU.0000000000000190.
Kanayama N, Ikawa T, Ohira S, Hirata T, Morimoto M, Ogawa K, et al. Volumetric reduction of brain metastases after stereotactic radiotherapy: prognostic factors and effect on local control. Cancer Med. 2022;11:4806–15. https://doi.org/10.1002/cam4.4809.
Ikawa T, Kanayama N, Arita H, Ohira S, Takano K, Hirata T, et al. Linear accelerator-based stereotactic radiotherapy for brain metastases, including multiple and large lesions, carries a low incidence of acute toxicities: a retrospective analysis. Radiat Oncol. 2023;18:80. https://doi.org/10.1186/s13014-023-02262-z.
Ohira S, Ueda Y, Kanayama N, Isono M, Inui S, Komiyama R, et al. Impact of multileaf collimator width on dose distribution in HyperArc fractionated stereotactic irradiation for multiple (-) brain metastases. Anticancer Res. 2021;41:3153–9. https://doi.org/10.21873/anticanres.15101.
Sneed PK, Mendez J, Vemer-van den Hoek JG, Seymour ZA, Ma L, Molinaro AM, et al. Adverse radiation effect after stereotactic radiosurgery for brain metastases: incidence, time course, and risk factors. J Neurosurg. 2015;123:373–86. https://doi.org/10.3171/2014.10.JNS141610.
Burnham KP, Anderson DR. Multimodel inference: understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261–304. https://doi.org/10.1177/0049124104268644.
Remick JS, Kowalski E, Khairnar R, Sun K, Morse E, Cherng HR, et al. A multi-center analysis of single-fraction versus hypofractionated stereotactic radiosurgery for the treatment of brain metastasis. Radiat Oncol. 2020;15:1–11. https://doi.org/10.1186/s13014-020-01522-6.
The International Commission on Radiation Units and Measurements. NP.2-NP. J ICRU. 2010;10. https://doi.org/10.1093/jicru/ndq001.
Abraham C, Garsa A, Badiyan SN, Drzymala R, Yang D, DeWees T, et al. Internal dose escalation is associated with increased local control for non-small cell lung cancer (NSCLC) brain metastases treated with stereotactic radiosurgery (SRS). Adv Radiat Oncol. 2018;3:146–53. https://doi.org/10.1016/j.adro.2017.11.003.
Kennedy WR, DeWees TA, Acharya S, Mahmood M, Knutson NC, Goddu SM, et al. Internal dose escalation associated with increased local control for melanoma brain metastases treated with stereotactic radiosurgery. J Neurosurg. 2020;135:855–61. https://doi.org/10.3171/2020.7.JNS192210.
Ohtakara K, Tanahashi K, Kamomae T, Ito E, Suzuki K. Local control failure after five-fraction stereotactic radiosurgery alone for symptomatic brain metastasis from squamous cell lung carcinoma despite 43 gy to gross tumor margin with internal steep dose increase and tumor shrinkage during irradiation. Cureus. 2023;15:e38645. https://doi.org/10.7759/cureus.38645.
Lucia F, Key S, Dissaux G, Goasduff G, Lucia AS, Ollivier L, et al. Inhomogeneous tumor dose distribution provides better local control than homogeneous distribution in stereotactic radiotherapy for brain metastases. Radiother Oncol. 2019;130:132–8. https://doi.org/10.1016/j.radonc.2018.06.039.
Ohtakara K, Suzuki K. An extremely inhomogeneous gross tumor dose is suitable for volumetric modulated arc-based radiosurgery with a 5-mm leaf-width multileaf collimator for single brain metastasis. Cureus. 2023;15:e35467. https://doi.org/10.7759/cureus.3546.
Funding
This work was supported by JSPS KAKENHI (Grant No.: 21K15857). The study sponsor was not involved in the study design, collection, analysis, or interpretation of data.
Author information
Authors and Affiliations
Contributions
All authors contributed to the conceptualization. Data curation was performed by NK. Formal analysis, investigation and interpretation of data was performed by NK and TI. The original draft of the manuscript was written by NK, and all authors attributed reviewing and editing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This retrospective study was approved by the ethics committee of the Osaka International Cancer Institute (approval number: 21150).
Consent for publication
This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All patients signed informed consent for the use of their data for research purposes before starting fSRS.
Competing interests
Naoyuki Kanayama received an honorarium from Varian Medical Systems as a HyperArc consultant. Toshiki Ikawa received an honorarium from Varian Medical Systems as a HyperArc consultant. Koji Takano: None. Hideyuki Arita: None. Masahiro Morimoto: None. Takero Hirata: None. Kazuhiko Ogawa: None. Teruki Teshima: None. Koji Konishi: None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kanayama, N., Ikawa, T., Takano, K. et al. Association of increasing gross tumor volume dose with tumor volume reduction and local control in fractionated stereotactic radiosurgery for unresected brain metastases. Radiat Oncol 19, 95 (2024). https://doi.org/10.1186/s13014-024-02487-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13014-024-02487-6