Abstract
Background
Anatomical reduction and stable fixation of complex tibial plateau fractures remain challenging in clinical practice. This study examines the efficacy of using 3D printing technology combined with customized plates for treating these fractures.
Methods
We retrospectively analyzed 22 patients treated with 3D printing and customized plates at the Orthopedic Department of the Central Hospital affiliated with Shenyang Medical College from September 2020 to January 2023. These patients were matched with 22 patients treated with traditional plates with similar baseline characteristics. Patients were divided into an experimental group (3D-printed models and customized plates) and a control group (traditional plates). The control group underwent traditional surgical methods, while the experimental group had a preoperative 3D model and customized plates for surgical planning. We compared baseline characteristics and recorded various indicators, including preoperative preparation time, surgical time, intraoperative blood loss, number of intraoperative fluoroscopies, hospital stay duration, fracture healing time, complications, knee joint range of motion (ROM), Rasmussen anatomical and functional scores, and HSS scores.
Results
All surgeries were successful with effective follow-up. The experimental group had shorter surgical time, less intraoperative blood loss, and fewer intraoperative fluoroscopies (P < 0.05). At 6 months and 1 year postoperatively, the experimental group had better knee joint HSS scores than the control group. Preoperative preparation time and total hospital stay were shorter in the control group (P < 0.05). There were no significant differences in fracture healing time and follow-up duration between groups. The experimental group showed better knee joint flexion angles (P < 0.05). Rasmussen scores showed no statistical difference between groups (P > 0.05). The incidence of complications was slightly lower in the experimental group but not significantly different.
Conclusion
3D printing technology combined with customized plates for complex tibial plateau fractures enables precise articular surface reduction, significantly shortens surgical time, and reduces intraoperative blood loss. This method improves knee joint function, offering a more effective treatment option.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Tibial plateau fractures, as a common type of intra-articular knee fractures, account for approximately 1.66% of all fractures, often caused by high-energy trauma and more prevalent among patients with osteoporosis [1,2,3]. The knee joint, as one of the primary weight-bearing joints in the human body, is significantly affected by tibial plateau fractures, resulting in serious impairment of knee joint function [4]. The anatomical structure at the tibial plateau is complex, and high-energy trauma often leads to displacement of the tibial plateau fracture fragments, articular surface depression, resulting in bone defects, and involving surrounding soft tissues. Currently, the most commonly used classification for tibial plateau fractures in clinical practice is the Schatzker classification, proposed in 1979 by Canadian surgeon Marvin Schatzker. This classification is based on the anatomical location and severity of the fracture, providing a foundation for guiding treatment plans [5]. However, tibial plateau fractures present individualized differences, making comprehensive assessment of each patient’s personalized characteristics crucial for fracture treatment.
The primary goal of treating tibial plateau fractures is to restore the anatomical relationship of the articular surface, maintain stability of the knee joint, and reduce the occurrence of complications associated with articular surface fractures [6, 7]. However, fracture reduction at this site demands precision, and the surgical procedure is challenging. Therefore, preoperative fracture assessment is crucial for a thorough understanding of the fracture pattern and for selecting the optimal treatment approach. Clinical decision-making and preoperative planning primarily rely on conventional imaging modalities. However, due to the frequent multi-directional displacement and rotation of fracture fragments, traditional X-rays and CT three-dimensional reconstructions cannot provide comprehensive and intuitive visualization of the fracture situation. Therefore, using these methods makes it difficult to accurately assess the true extent of the fracture, posing significant challenges to surgical treatment [8,9,10]. If treated improperly, it may lead to dysfunction of the knee joint and the development of traumatic arthritis [11]. For complex tibial plateau fractures, open reduction and internal fixation surgery is the fundamental treatment method, requiring surgical procedures to meet anatomical reduction requirements. However, traditional implants such as plates and screws often have issues with poor fit and inadequate restoration of joint surface height, which can significantly impact the postoperative fracture recovery. Sassoon et al. [12] reported that the use of anterior lateral plates may not adequately protect the effective support of the posterior lateral plateau articular surface. Due to the complexity of tibial plateau fractures and individualized differences, the surgical objectives may not always be achieved. Research indicates that up to 30% of tibial plateau fracture patients undergoing surgical treatment do not achieve optimal reduction status [13]. Therefore, preoperative clarification of fracture fragment displacement and articular surface collapse, as well as selecting plates and screws with good compatibility with the fracture surface, are crucial for the treatment of tibial plateau fractures [14].
In recent years, with the rapid development of medical technology, the application of 3D printing technology in the field of orthopedics has been increasing, providing crucial support for the treatment of various orthopedic conditions [15, 16]. 3D printing technology, equipped with specialized software systems, can convert three-dimensional CT imaging data into model data and realize the printing of 1:1 solid models of the target area. Surgeons can observe the morphological information of the fracture site from any angle, better understanding the fracture pattern. Especially for complex fractures, personalized plates can be customized according to the specific situation of the patient, achieving precise, efficient, and safe treatment during surgery. This study aims to investigate the clinical efficacy of utilizing 3D printing models combined with personalized customized plates in the treatment of complex tibial plateau fractures.
Materials and methods
Inclusion and exclusion
Criteria From September 2020 to January 2023, a total of 22 patients who underwent 3D-printed personalized steel plate implantation at the Department of Orthopedics, affiliated with Shenyang Medical College Center Hospital, were included in the study. Simultaneously, 22 patients who received traditional steel plate treatment with matching baseline characteristics were included, totaling 44 patients in the study. Inclusion Criteria: 1.Age between 25 and 60 years old, with unilateral tibial plateau fracture combined with joint surface depression.2. Closed fracture, with normal limb function before injury.3. Time from fracture to surgery less than 2 weeks. Exclusion criteria:1. Open fractures.2. Severe concomitant injuries on the same limb.3. Patients with severe hepatic or renal dysfunction, cardiovascular or cerebrovascular diseases, and those lost to follow-up. Control group (traditional steel plate internal fixation treatment) consisting of 22 cases and experimental group (3D printed model combined with individualized customized steel plate internal fixation treatment) consisting of 22 cases. The control group adopted the conventional steel plate internal fixation method, while the experimental group underwent preoperative 3D printing of solid models and customization of individualized steel plates. The surgical procedures, radiographic evaluations, and physical examinations were performed by the same orthopedic surgical team. All patients provided informed consent for treatment and signed written informed consent forms. This trial was approved by the Ethics Committee of the Affiliated Central Hospital of Shenyang Medical College (Ethics No.2020018).
Preoperative preparation
After admission, all patients underwent comprehensive physical examinations and routine preoperative assessments. Preoperative X-rays (Netherlands, Philips digital radiography DR system) and three-dimensional CT scans (Netherlands, Philips 256-slice spiral CT machine, with a scanning layer thickness of 0.6 mm) were performed to assess the injuries. The injured lower limbs of the patients were temporarily immobilized in the extended position at 0° using plaster splints, with limb elevation and ice packs applied to reduce swelling. Routine preoperative pain relief and anticoagulant medications were administered. For the individualized steel plate group, fracture data were virtually simulated, 3D models were printed, and individualized steel plates were customized. All patients received one dose of antibiotic prophylaxis 30 min before surgery.
3D model and individualized steel plate production
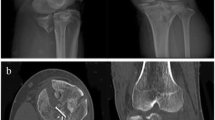
The CT data of the patient’s fracture site (Fig. 1) was imported into Mimics 20.0 software (Materialise, Belgium) workstation in DICOM format. Three-dimensional modeling of the tibial plateau fracture data was performed (Fig. 2A). The fracture fragments were separated and color-coded (Fig. 2B), followed by anatomical virtual reduction of the fracture fragments (Fig. 2C). Subsequently, the three-dimensional modeling and virtually reduced fracture data were exported as STL format and imported into FashPrint 5 software (FlashForge, China) to print out the three-dimensional physical models of the fracture after modeling and virtual reduction (Figs. 4 and 5). Through a collaboration between medical professionals and engineers, a customized individualized steel plate solution was designed (Fig. 3).
Engineers use Unigraphics NX software (Siemens PLM Software, USA) for detailed design of steel plates in the subsequent project. They utilize FashPrint 5 software to print a physical model of the steel plate (Fig. 5). The physical model of the steel plate is then imported into Mimics software through reverse scanning to confirm the placement, length, direction, and diameter of the screws (Fig. 3). Transparency processing is applied to the fracture model to ensure that the implanted screws do not enter the ankle joint cavity. Additionally, the recommended length of the screws is marked beside each screw hole on the steel plate (Fig. 3). Individualized custom steel plates are fabricated using pure titanium TA3 as the raw material at the manufacturing facility. Furthermore, detailed inspection and sterilization are required for the steel plates.
Based on the virtually reduced fracture model, a personalized plate scheme was customized, confirming the optimal placement of the plate and screws. The fracture model was made transparent to ensure that the implanted screws did not enter the joint cavity. The length of the fixation screws was measured, and the recommended length was marked next to the screw holes
Surgical procedure
Experimental Group: General anesthesia or lumbar epidural anesthesia is administered. After the anesthesia takes effect, the patient lies supine on the operating table, with a tourniquet placed at the root of the thigh of the affected limb. The surgical area is routinely disinfected, and sterile drapes are applied. Different surgical incisions are selected according to the type of fracture, and subcutaneous tissues and fascia are dissected layer by layer to expose the fracture ends. The fracture fragments are realigned according to the pre-printed 3D model, and bone grafting is performed at the bone defect site to fill in the gap. The collapsed fracture fragments are supported to restore the smoothness of the joint surface. Kirschner wires are temporarily used to fix the fracture fragments to maintain alignment. After the joint surface is restored to smoothness under C-arm fluoroscopy, individually customized steel plates are selected and placed in appropriate positions. Screws are then sequentially inserted. C-arm fluoroscopy is performed again to ensure the smoothness of the joint surface, good alignment of fracture lines, appropriate screw lengths, ensuring they do not penetrate the joint surface. The knee joint is flexed and extended, confirming firm fixation at the fracture site before suturing.
Control Group: Patients in this group undergo the same surgical procedure as the experimental group. However, the placement of steel plates and screws is based on the surgeon’s experience rather than predetermined by the 3D model.
Postoperative care
After surgery, the patient’s plaster fixation is removed. Within 24 h, a single dose of antibiotics is administered to prevent postoperative wound infection. Cold compresses are applied to the patient’s knee joint, and routine treatments for reducing swelling, relieving pain, and preventing lower limb deep vein thrombosis are performed. On the first day postoperatively, after reviewing the knee joint’s anteroposterior and lateral X-rays, exercises such as quadriceps isometric contractions, straight leg raises, and ankle pump exercises are initiated. The surgical incision dressing is changed every 2–3 days to observe the wound healing progress. Sutures are removed at 2 weeks postoperatively. At 4 weeks postoperatively, patients are allowed to bear light weight with the aid of crutches for protection and gradually perform knee flexion exercises. At 8 weeks postoperatively, patients begin full range of motion and partial weight-bearing exercises on the knee joint until full flexion is achieved. At 12 weeks postoperatively, based on the results of follow-up X-rays, gradual full weight-bearing training is initiated. For older patients or those with severe comminuted fractures or significant osteoporosis, the use of crutches may be extended as necessary.
Observation indicators
Comparison of the following parameters between the two groups of patients: preoperative preparation time, surgical time, intraoperative blood loss, number of intraoperative fluoroscopy sessions, fracture healing time, length of hospital stay, incidence of complications, knee joint range of motion (ROM), Rasmussen anatomical and functional scores, and Knee joint function HSS score. Postoperatively, knee joint anteroposterior and lateral X-rays are taken at day 1, 1 month, and 2 months postoperatively every two weeks to assess bone healing until fracture union. At 1 month, 2 months, 6 months, and 12 months postoperatively, knee joint function is evaluated according to the HSS scoring criteria.
Statistical methods
Analysis is conducted using SPSS 27.0 statistical software. Count data is represented by the number of cases, and analysis is performed using the chi-square test. Continuous data is presented as mean ± standard deviation. Continuous data is first assessed for normality using the Shapiro-Wilk test. For continuous variables that follow a normal distribution, independent samples t-test is utilized for analysis. For continuous variables that do not adhere to a normal distribution, Mann-Whitney U test is employed for analysis. In these analyses, a p-value less than 0.05 is considered statistically significant.
Results
Comparison of baseline data between two groups
A total of 44 patients were included in this study, with 22 cases in the experimental group and 22 cases in the control group. There were no significant differences between the two groups in baseline characteristics (age, gender, injured side, BMI, cause of injury) (Table 1).
Clinical data
The surgical time in the experimental group was significantly shorter than that in the control group, measuring 75.27 ± 15.98 min and 102.82 ± 21.11 min, respectively (p < 0.05). The intraoperative blood loss in the experimental group was also significantly less than that in the control group, measuring 129.32 ± 25.32 ml and 158.18 ± 33.61 ml, respectively (p < 0.05). The number of intraoperative fluoroscopy sessions in the experimental group was significantly lower than that in the control group, measuring 6.5 ± 2.09 and 11.32 ± 3.50, respectively (p < 0.001). Additionally, the accuracy of steel plate placement in the experimental group was higher than that in the control group. However, the preoperative preparation time and total length of hospital stay were superior in the control group compared to the experimental group (p < 0.05). Both groups also recorded fracture healing time and follow-up duration, with no statistical differences (Table 2).
Functional outcomes
The knee flexion angle in the control group was 125.68 ± 6.78, while in the experimental group it was 130.45 ± 4.86 (p<0.05). The knee extension motion in the control group was 1.59 ± 4.47°, and in the experimental group, it was − 0.45 ± 4.06° (p>0.05). There was statistical significance in the knee flexion angle between the two groups (p<0.05). The experimental group showed higher rates of excellent scores in Rasmussen’s anatomical and functional evaluations compared to the control group, but the difference between the two groups was not statistically significant. Additionally, there were no significant differences in the HSS scores between the two groups at the first and second months postoperatively (p>0.05). However, at 6 and 12 months postoperatively, there were statistically significant differences in the HSS scores between the two groups, with the experimental group performing better than the control group (Tables 3 and 4).
Complications
The overall incidence of complications was 19.0% in the control group and 9.1% in the experimental group, with no statistically significant difference between the groups (p = 0.473). In each group, one patient experienced a superficial infection, which was successfully treated with antibiotics and wound care. In the control group, one patient showed signs of post-traumatic osteoarthritis. After conservative treatment, the knee pain was alleviated, but there were still limitations during squatting. In the experimental group, one patient experienced joint stiffness, while in the control group, two patients experienced joint stiffness. After rehabilitation exercises, the joint function of these patients gradually recovered (Table 5).
Typical case
Case 1
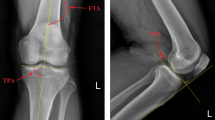
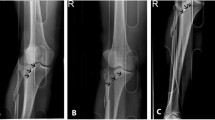
A 49-year-old male was admitted to the hospital due to left knee pain, swelling, and restricted movement caused by a traffic accident. Figure 1 shows the preoperative CT scan of the fracture. Using Mimics 20.0 software editing technology, the characteristics of the damaged tibial plateau were clearly displayed (Fig. 2). Designing personalized steel plates and recommending screw lengths based on the specific model (Fig. 3). Subsequently, a precise 1:1 model of the injured tibial plateau was 3D printed, and a personalized plate was fabricated (Figs. 4 and 5). Postoperative follow-up X-rays showed satisfactory fracture reduction and fixation (Fig. 6).
(A) Postoperative first-day follow-up knee anteroposterior and lateral X-ray images. (B, C) X-ray images taken at the postoperative 2nd and 3rd months follow-up. (D) X-ray image taken at the postoperative 8th month follow-up Case 2 A 56-year-old male patient was admitted to the hospital due to left knee pain, swelling, and restricted movement following a fall injury
Discussion
Characteristics of complex tibial plateau fractures include comminuted fracture fragments, joint surface compression, collapse, often accompanied by varying degrees of soft tissue damage such as cartilage and ligament injuries, which can easily lead to complications such as inadequate fixation and post-traumatic arthritis [17]. This type of collapse fracture is prone to occur at the posterior part of the lateral tibial plateau, which is not easily detected on anteroposterior and lateral X-rays, often leading to misdiagnosis [18]. As a weight-bearing joint, knee joint open reduction and internal fixation (ORIF) is a common treatment method aimed at restoring knee joint function. The surgical goal is to achieve anatomical reduction of the fracture, provide bone grafting and sturdy fixation, restore lower limb alignment, and allow early knee joint mobilization. However, surgical treatment is highly challenging, and improper management can lead to serious complications [10, 19, 20]. Goetz et al. [21] study indicated that accurate intra-articular reduction significantly improves postoperative knee joint function. For complex tibial plateau fractures, the quality of fracture reduction and the choice of surgical approach directly impact the recovery of knee joint function. To improve surgical outcomes and reduce postoperative complications, it is crucial to fully understand the fracture morphology preoperatively, clarify the sequence of fracture reduction, and select well-matched plate implants for fracture treatment [10]. Therefore, it is worthwhile to explore a safe and effective treatment approach.
In traditional surgery, achieving precise reduction markers for comminuted tibial plateau fractures is often challenging. Surgeons commonly rely on their experience for manipulation. Due to individual anatomical variations among patients, difficulties may arise during surgery, such as poor plate adaptation to bone surfaces and time-consuming plate molding. These drawbacks can prolong surgical duration and increase risks of wound infection and flap necrosis. Ultimately, achieving satisfactory anatomical reduction remains elusive [9]. These issues pose numerous challenges for traditional surgical approaches in the treatment of complex tibial plateau fractures, highlighting the urgent need for a more precise and efficient treatment strategy to address them.
In recent years, the application of 3D printing technology in surgical procedures has become increasingly widespread, particularly in the field of orthopedics [22]. 3D printing technology has numerous advantages in the production of fracture models, preoperative surgical planning, and the customization of personalized internal fixation devices [23]. For complex intra-articular fractures, it is often challenging to expose and fix the fracture fragments during surgery. Utilizing 3D printing technology, a life-sized 1:1 scale model can be created, allowing the surgeon to directly observe the morphology and displacement of the fracture. This also aids in accurately determining the fracture classification [24]. Preoperatively, individualized internal fixation plates are custom-made based on the specific fracture location of the patient. These plates are pre-shaped to better fit the fracture morphology and bone quality of the patient. This approach can effectively reduce the risk of instability during fracture reduction and fixation, ensure that the screws are placed in the optimal position and direction, enhance the precision of intraoperative reduction and fixation, reduce the number of intraoperative fluoroscopies, shorten the surgery time, consequently reduce blood loss, and lower surgical risks [25, 26]. Additionally, using 3D-printed fracture models to explain the fracture morphology, surgical objectives, and procedures to the patient and their family can enhance communication and understanding between medical staff and patients, thereby improving patient compliance [27].
Our research results indicate that in the surgical treatment of complex tibial plateau fractures, patients in the experimental group demonstrated significant advantages compared to the control group. Compared to traditional surgery, the experimental group had notably shorter operation times, significantly reduced intraoperative blood loss, and fewer fluoroscopy instances. These advantages have also been confirmed in other types of fractures, especially in complex periarticular fractures, such as pelvic fractures [28]and complex trimalleolar fractures [29]. Preoperative virtual surgical planning combined with fracture model simulation allows for minimizing excessive soft tissue dissection during surgery. Pre-designed customized individual plates and screw placements better match the patient’s fracture condition, ensuring smooth fracture reduction and fixation intraoperatively. In this study, the experimental group exhibited a higher anatomical reduction rate compared to the control group, indicating that the use of 3D-printed models combined with customized individual plates assists in achieving anatomical reduction of fractures. During the 1-month and 2-month follow-ups postoperatively, there was no statistically significant difference in Knee Society Score (HSS) between the two groups, which may be attributed to the fractures still being in the healing phase, and knee joint function not yet being fully restored. However, during the 6-month and 1-year follow-up postoperatively, patients in the experimental group exhibited significantly better knee joint function than those in the control group, with a lower incidence of complications. This indicates that patients in the experimental group had superior recovery of knee joint function in the late stage of fracture healing compared to the control group. This study introduces personalized plate customization for different types of fractures, overcoming the limitations of solely relying on 3D-printed models for fracture reduction and fixation. Through the collaboration of medical and engineering disciplines, personalized plates can be designed according to the actual fracture condition of the patient, facilitating more effective individualized treatment. Sterilized models can be referenced intraoperatively, aiding surgeons in comprehensively understanding various fracture details and avoiding the limitations of traditional two-dimensional images [30]. Therefore, the combination of 3D printing technology with personalized plate fabrication has to some extent improved the success rate of surgery and reduced the incidence of surgical complications.
The limited number of cases in this study necessitates the expansion of sample size in future research. Prospective randomized controlled trials are needed to further validate the reliability and generalizability of the research results. It is worth noting that the process of 3D printing involves software processing and printing out bone models without soft tissue, which may introduce some errors. Additionally, 3D printing technology requires specialized software, personnel, and dedicated equipment, which can increase the cost for patients in routine medical care.
Conclusions
In summary, the combination of 3D printing technology with customized individualized plates holds promising potential for the treatment of tibial plateau fractures. This technology can reduce surgical time, improve the accuracy of implant placement and matching to the fracture surface, and facilitate postoperative knee joint function recovery. It provides better understanding and communication between doctors and patients, offering significant convenience to orthopedic surgeons. Therefore, it is worthy of clinical application and promotion.
Data availability
Data Availability StatementAll data generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Jiang L, Zheng Q, Pan Z. Comparison of extended anterolateral approach in treatment of simple/complex tibial plateau fracture with posterolateral tibial plateau fracture. J Orthop Surg Res. 2018;13(1):303.
Blain H, Masud T, Dargent-Molina P, Martin FC, Rosendahl E, van der Velde N, et al. A comprehensive fracture prevention strategy in older adults: the European Union Geriatric Medicine Society (EUGMS) statement. Aging Clin Exp Res. 2016;28(4):797–803.
Gosch M, Kammerlander C, Neuerburg C. [Osteoporosis-epidemiology and quality of care]. Z Gerontol Geriatr. 2019;52(5):408–13.
Millar SC, Arnold JB, Thewlis D, Fraysse F, Solomon LB. A systematic literature review of tibial plateau fractures: what classifications are used and how reliable and useful are they? Injury. 2018;49(3):473–90.
Kfuri M, Schatzker J. Revisiting the Schatzker classification of tibial plateau fractures. Injury. 2018;49(12):2252–63.
Adams JDJ, Loeffler MF. Soft tissue Injury considerations in the treatment of Tibial Plateau fractures. Orthop Clin North Am. 2020;51(4):471–9.
Yan Z, Zou C, Kenmegne GR, Pan X, Ghimire N, Silva KMN, et al. Newly designed plate for the treatment of posterolateral tibial plateau fractures: a finite element analysis. J Orthop Surg Res. 2024;19(1):201.
Krause M, Krüger S, Müller G, Püschel K, Frosch K-H. How can the articular surface of the tibial plateau be best exposed? A comparison of specific surgical approaches. Arch Orthop Trauma Surg. 2019;139(10):1369–77.
Lou Y, Cai L, Wang C, Tang Q, Pan T, Guo X, et al. Comparison of traditional surgery and surgery assisted by three dimensional printing technology in the treatment of tibial plateau fractures. Int Orthop. 2017;41(9):1875–80.
Prat-Fabregat S, Camacho-Carrasco P. Treatment strategy for tibial plateau fractures: an update. EFORT Open Rev. 2016;1(5):225–32.
Mthethwa J, Chikate A. A review of the management of tibial plateau fractures. Musculoskelet Surg. 2018;102(2):119–27.
Sassoon AA, Torchia ME, Cross WW, Cass JR, Sems SA. Fibular shaft allograft support of posterior joint depression in tibial plateau fractures. J Orthop Trauma. 2014;28(7):e169–75.
Meulenkamp B, Martin R, Desy NM, Duffy P, Korley R, Puloski S, et al. Incidence, risk factors, and location of articular malreductions of the Tibial Plateau. J Orthop Trauma. 2017;31(3):146–50.
Bizzotto N, Tami I, Santucci A, Adani R, Poggi P, Romani D, et al. 3D printed replica of articular fractures for surgical planning and patient consent: a two years multi-centric experience. 3D Print Med. 2015;2(1):2.
Marongiu G, Prost R, Capone A. Use of 3D modelling and 3D printing for the diagnostic process, decision making and preoperative planning of periprosthetic acetabular fractures. BMJ Case Rep. 2020;13(1).
Marongiu G, Prost R, Capone A. A New Diagnostic Approach for Periprosthetic Acetabular fractures based on 3D modeling: a study protocol. Diagnostics (Basel). 2019;10(1).
McGonagle L, Cordier T, Link BC, Rickman MS, Solomon LB. Tibia plateau fracture mapping and its influence on fracture fixation. J Orthop Traumatol. 2019;20(1):12.
Salari P, Busel G, Watson JT. A radiographic zone-based approach to predict meniscus injury in lateral tibial plateau fracture. Injury. 2021;52(6):1539–43.
Kandemir U, Maclean J. Surgical approaches for tibial plateau fractures. J Knee Surg. 2014;27(1):21–9.
Tscherne H, Lobenhoffer P. Tibial plateau fractures. Management and expected results. Clin Orthop Relat Res. 1993(292).
Goetz JE, Fredericks D, Petersen E, Rudert MJ, Baer T, Swanson E, et al. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23(10):1797–805.
Shen S, Wang P, Li X, Han X, Tan H. Pre-operative simulation using a three-dimensional printing model for surgical treatment of old and complex tibial plateau fractures. Sci Rep. 2020;10(1):6044.
Wu W-Y, Xu W-G, Wan C-Y, Fang M. Preoperative plan with 3D Printing in Internal and external fixation for Complex Tibial Plateau fractures. Orthop Surg. 2019;11(4):560–8.
Samaila EM, Negri S, Zardini A, Bizzotto N, Maluta T, Rossignoli C, et al. Value of three-dimensional printing of fractures in orthopaedic trauma surgery. J Int Med Res. 2020;48(1):300060519887299.
Dong X-P, Zhang Y-W, Wang Z, Deng L. Clinical application of three-dimensional printing assisted percutaneous guide plate in minimally invasive reduction and internal fixation of tibial plateau fracture. Asian J Surg. 2020;43(9):921–3.
Diment LE, Thompson MS, Bergmann JHM. Clinical efficacy and effectiveness of 3D printing: a systematic review. BMJ Open. 2017;7(12):e016891.
Bizzotto N, Tami I, Tami A, Spiegel A, Romani D, Corain M, et al. 3D printed models of distal radius fractures. Injury. 2016;47(4):976–8.
Xu M, Zhang L-H, Zhang Y-Z, Zhang L-C, He C-Q, Wang Y, et al. Custom-made locked plating for acetabular fracture: a pilot study in 24 consecutive cases. Orthopedics. 2014;37(7):e660–70.
Liang H, Zhang H, Chen B, Yang L, Xu R, Duan S, et al. 3D printing technology combined with personalized plates for complex distal intra-articular fractures of the trimalleolar ankle. Sci Rep. 2023;13(1):22667.
Yang P, Du D, Zhou Z, Lu N, Fu Q, Ma J, et al. 3D printing-assisted osteotomy treatment for the malunion of lateral tibial plateau fracture. Injury. 2016;47(12):2816–21.
Acknowledgements
We wish to thank all patients and medical staff for their co-operation.
Funding
This study was supported by the Natural Science Foundation of Liaoning Province (2024-MS-222), the Liaoning Provincial Department of Education Fund Project (JYTMS20231396), and the Science and Technology Plan Project of Shenyang City (Grant no. 22-321-32-13).
Author information
Authors and Affiliations
Contributions
Z-CC designed the study and interpreted the data. HW, X-TZ and H-FL collected the data. MS analyzed the data. S-YD,H-RL and R-DX wrote the paper. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Affiliated Central Hospital of Shenyang Medical College (Ethics No. 2020018). All procedures were carried out in accordance with relevant guidelines and regulations. Written informed consent was obtained from all patients.
Consent for publication
Informed consent for publication has been obtained from all participants.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Duan, S., Xu, R., Liang, H. et al. Study on the efficacy of 3D printing technology combined with customized plates for the treatment of complex tibial plateau fractures. J Orthop Surg Res 19, 562 (2024). https://doi.org/10.1186/s13018-024-05051-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-05051-w