Abstract
Background
Aortic dissection is a severe cardiovascular condition associated with high mortality rates, particularly in cases of Stanford type A aortic dissection (TAAD). Myocardial infarction with non-obstructive coronary arteries (MINOCA) following surgery for TAAD is rare but potentially fatal.
Case presentation
: A 69-year-old woman presented with sudden chest pain and was diagnosed with acute TAAD. Emergency surgery was performed, during which complications arose, including significant hemodynamic instability. Despite efforts to manage the patient’s condition postoperatively, she developed hemodynamic instability and myocardial infarction, leading to cardiogenic shock. MINOCA was diagnosed based on clinical presentation, echocardiographic findings, and coronary angiography ruling out significant stenosis or occlusion. The patient’s condition deteriorated despite aggressive treatment, ultimately resulting in death.
Conclusion
MINOCA following surgery for TAAD is a rare but serious complication. Vigilant postoperative monitoring and timely intervention are essential for identifying and managing acute cardiac dysfunction in these patients. Further research is required to improve outcomes in this challenging clinical scenario.
Similar content being viewed by others
Background
Aortic dissection occurs when there is a breach in the intimal layer of the aorta, leading to the infiltration of blood into the media layer and the subsequent formation of both true and false lumens within the aortic wall. This condition is among the most prevalent and fatal cardiovascular diseases, with Stanford type A aortic dissection (TAAD) representing approximately 58–62% of all aortic pathologies and boasting a mortality rate of up to 30% within 48 h [1, 2]. Acute myocardial infarction arises from acute ischemia and hypoxia of myocardial cells due to coronary artery stenosis, occlusion, or an imbalance in oxygen supply and demand, leading to myocardial necrosis and impaired cardiac function. When coronary angiography reveals no significant obstruction, this condition is classified as myocardial infarction with non-obstructive coronary arteries (MINOCA) [3]. The occurrence of MINOCA following surgery for Stanford TAAD is exceedingly rare, as detailed in the following text.
Case presentation
A 69-year-old female patient with no history of hypertension or connective tissue disorders predisposing her to aortic dissection presented with sudden, tearing chest pain for 1 day before admission. Emergency computed tomography angiography (CTA) revealed Stanford TAAD involving the aortic arch, left subclavian artery, superior mesenteric artery, left renal artery, left common iliac artery, and left external iliac artery, with severe stenosis at the origin of the celiac trunk. Notably, no significant stenosis was observed in either the left or right coronary artery. Furthermore, emergency echocardiography indicated moderate aortic valve regurgitation with an area of 7.2 cm² and a left ventricular ejection fraction (LVEF) of 61%. With the patient’s consent, an emergency Bentall procedure; total aortic arch replacement with the deployment of an elephant trunk stent graft (Sun’s procedure); and ascending aorta to the innominate artery, brachiocephalic artery, and left subclavian artery bypass grafts were performed under general anesthesia with cardiopulmonary bypass (CPB). A Cabrol anastomosis was additionally performed due to difficulty in mobilizing the left coronary artery. The right coronary artery was directly anastomosed in situ. The duration of the CPB was 328 min, the aortic cross-clamp time was 137 min, and deep hypothermic circulatory arrest lasted for 25 min, with the transfusion of 1100 mL of red blood cells. The patient received a single 2000-mL histidine–tryptophan–ketoglutarate cardioplegia infusion during the surgery. Following surgery, she was transferred to the surgical intensive care unit at 18:45 h, supported by low-dose vasopressor (norepinephrine 0.02 µg/kg/min), with a heart rate of 65 bpm, blood pressure of 125/80 mmHg, central venous pressure of 10 mmHg, and blood lactate level of 6.8 mmol/L.
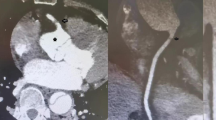
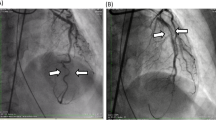
Around 02:15 h on the day after surgery (approximately 7.5 h after transfer), the patient experienced a sudden drop in blood pressure to 85/62 mmHg, her heart rate increased to 102 bpm, and her central venous pressure rose to 15 mmHg. The norepinephrine infusion was increased to 0.08 µg/kg/min to maintain blood pressure. The patient exhibited central fever, peripheral circulatory failure with decreased skin temperature, rectal temperature elevated to 38.8 °C, and a significant decrease in urine output, and bedside echocardiography showed marked systolic dysfunction at the apical segment of the left ventricle, ruling out acute cardiac tamponade. Subsequent bedside electrocardiography revealed Q-wave formation with ST-segment elevation in lateral leads (I, aVL), ST-segment elevation in anterior leads (V2–3), and a QS pattern in leads V4–6, indicative of myocardial infarction, with notable changes observable compared with the preoperative electrocardiogram (Fig. 1A). Urgent coronary angiography showed aneurysmal dilation of the left main coronary artery, irregular intimal surface of the mid-left anterior descending artery, 30% stenosis of the proximal left circumflex artery, and 30% stenosis of the proximal right coronary artery. No coronary artery occlusion or subtotal occlusion was observed during the procedure (Fig. 1C–E). Repeat echocardiography revealed a significant decrease in left ventricular systolic function (LVEF, 11%) and suggested the presence of a left ventricular aneurysm. Additionally, a 2.8-mm defect in the interventricular septum (Fig. 1B), indicative of an interventricular septal rupture, was detected using a transapical color flow Doppler probe.
(A) Electrocardiography revealed Q-wave formation with ST-segment elevation in lateral leads (I, aVL), ST-segment elevation in anterior leads (V2–3), and a QS pattern in leads V4–6. (B) Echocardiography indicated interventricular septal perforation. (C–E) Coronary angiography ruled out severe stenosis or occlusion due to surgical factors or atherosclerosis
At this juncture, the patient’s hemodynamics became increasingly unstable, prompting the initiation of emergency veno-arterial extracorporeal membrane oxygenation (VA-ECMO) support (flow rate, approximately 3.5 L/min; rotational speed, 3025 rpm; and FiO2, 60%). Over the following 3 days, her creatine kinase-MB (CK-MB) and troponin levels were continuously monitored, peaking at 452.2 U/L for CK-MB and consistently exceeding 10,000 pg/mL for troponin. On the third postoperative day, repeat echocardiography revealed reduced left ventricular systolic function (LVEF, 35%) and the formation of a left ventricular aneurysm with interventricular septal perforation. The patient received support with VA-ECMO, coronary vasodilators, nutritional support, anticoagulation, and continuous renal replacement therapy. Despite ECMO support, however, the patient’s condition deteriorated, leading to multi-organ dysfunction. On the morning of the fourth postoperative day, the patient’s family signed a document requesting withdrawal of ECMO support, and the patient succumbed to cardiogenic shock at 11:20 h on the same day.
Discussion and conclusions
Our patient, without a history of hypertension or familial diseases, presented with sudden, tearing chest pain. The aortic CTA scan revealed Stanford TAAD involving the aortic arch, left subclavian artery, superior mesenteric artery, left renal artery, left common iliac artery, and left external iliac artery, with severe stenosis at the origin of the celiac trunk. The clinical presentation was highly typical, indicating a clear diagnosis and necessitating emergent surgical intervention [4]. Our institution’s most experienced cardiothoracic surgeon performed emergency surgery, followed by specialized postoperative management, with an anticipated favorable prognosis. However, shortly after surgery, the patient experienced rapid hemodynamic deterioration and severe left ventricular dysfunction, ultimately leading to cardiogenic shock and death.
The use of CPB and aortic cross-clamping can cause additional myocardial damage, leading to increases in blood markers such as CK-MB and troponin. Both systemic and local inflammatory responses are mediated by ischemia/reperfusion injury to the heart and lungs during CPB [5]. Furthermore, in TAAD, organ malperfusion, particularly involving the gut or limbs, can result in low cardiac output, mimicking myocardial infarction due to the release of inflammatory mediators [4]. It is essential to differentiate between low cardiac output syndrome caused by an inflammatory response to CPB and aortic dissection malperfusion and that caused by myocardial infarction. In our patient, there were no signs of acute abdominal organ or lower limb ischemia postoperatively. Troponin levels consistently exceeded 10,000pg/mL, and the CKMB trajectory was consistent with myocardial infarction.
Based on our patient’s clinical presentation, persistent increase in CK-MB and troponin levels, echocardiographic evidence of interventricular septal perforation and ventricular wall aneurysm formation, and coronary angiography ruling out severe stenosis or occlusion due to surgical factors or atherosclerosis, the diagnosis of MINOCA was established.
MINOCA refers to acute myocardial infarction without obstructive coronary artery disease on angiography. The reported incidence varies widely, affecting approximately 1–14% of patients with acute coronary syndromes undergoing coronary angiography [6]. The Fourth Universal Definition of Myocardial Infarction released by the European Society of Cardiology in 2018 specifically added MINOCA as a type of myocardial infarction [7]. For a diagnosis of MINOCA, the patient must meet the following three criteria [6, 8]: (i) clear diagnosis of acute myocardial infarction (similar to myocardial infarction caused by obstructive coronary artery disease); (ii) non-obstructive coronary artery disease shown on coronary angiography (no major coronary artery stenosis of ≥ 50% in any potentially infarct-related vessel, including normal coronary arteries [no stenosis < 30%] and mild coronary atherosclerosis [stenosis > 30% and < 50%]); and (iii) absence of other specific diseases causing acute myocardial infarction, such as myocarditis and pulmonary embolism. The present case meets the diagnostic criteria for MINOCA.
According to the 2019 American Heart Association Scientific Statement on the Diagnosis and Management of MINOCA [9], the etiology of MINOCA includes myocardial necrosis caused by factors related to coronary atherosclerosis (such as plaque rupture, plaque erosion, intramural thrombus, and spontaneous dissection) and factors unrelated to coronary atherosclerosis (such as epicardial coronary artery spasm, coronary microvascular dysfunction, and myocardial oxygen supply–demand imbalance). Coronary artery spasm and microvascular dysfunction are considered the primary mechanisms underlying MINOCA occurrence [8].
Coronary artery vasospasm (CAV) denotes the transient intense constriction of the epicardial branches of the coronary arteries, resulting in partial or complete vessel occlusion and precipitating myocardial ischemia or myocardial infarction [10]. CAV represents a rare and perilous complication linked to cardiac surgery, which is predominantly observed after coronary artery bypass grafting, with an incidence ranging from 0.43 to 1.3% [10], and less frequently observed following valve surgery. Predisposing factors include increased catecholamine levels, manipulation of coronary arteries during surgery, release of vasoconstrictive mediators by platelets, respiratory alkalosis, hypothermia, and sympatho-adrenergic neural stimulation [10,11,12]. Although coronary angiography performed during emergency evaluation did not reveal CAV in this case, intraoperative manipulation of both coronary arteries, particularly the left coronary artery during Cabrol anastomosis; deep hypothermic circulatory arrest during surgery; and subsequent postoperative administration of low-dose epinephrine all constitute risk factors for CAV. Intraoperative or postoperative transient CAV may have substantially contributed to myocardial ischemia in this case.
Myocardial ischemia is not solely dictated by significant stenosis in major coronary arteries but also by coronary microvascular disease and myocardial microcirculatory dysfunction [8]. In more than half of women with stable angina, coronary angiography unveils normal or non-obstructive stenosis [9]. Indices such as fractional flow reserve and index of microcirculatory resistance are indispensable tools for microcirculatory function assessment. Our patient likely harbored pre-existing microvascular disease or microcirculatory dysfunction before surgery. Regrettably, due to the abrupt onset of a life-threatening condition necessitating emergency surgery, a detailed preoperative assessment of microcirculatory function was unattainable. Potential microcirculatory dysfunction might have exacerbated severe myocardial ischemia in this case.
Additionally, spontaneous coronary artery dissection and coronary artery thrombosis are significant factors in MINOCA occurrence. In this case, emergency coronary angiography did not uncover significant intracoronary thrombus or coronary artery dissection, suggesting a low likelihood of these factors contributing to acute myocardial infarction. However, it remains plausible that the patient might have experienced postoperative intracoronary acute thrombosis resulting in acute myocardial infarction, followed by spontaneous reperfusion.
Regarding treatment, the American Heart Association recommends the following [6]: (i) urgent supportive therapy for cardiogenic shock; (ii) cardiac protection with antiplatelet drugs, statins, and/or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, with beta-blockers cautioned against in suspected CAV patients; and (iii) etiological treatment. Our patient rapidly deteriorated into cardiogenic shock postoperatively, culminating in the subsequent development of ventricular septal perforation and ventricular aneurysm formation, indicative of a very extensive infarction. Although emergency treatment involving VA-ECMO support, coronary artery dilation, anticoagulation, and other therapies was initiated, the response was limited. Literature reports suggest a more favorable prognosis for MINOCA than acute myocardial infarction caused by obstructive coronary artery disease [9]. However, this patient, being older and presenting with ventricular septal perforation and ventricular aneurysm formation, represents one of the most severe and intricate clinical scenarios, which is associated with a markedly increased mortality rate.
In conclusion, acute TAAD cases remain one of the most critical and hemodynamically complex patient cohorts. Patients undergoing coronary artery manipulation, hypothermic procedures, and catecholamine administration require vigilant monitoring for changes in cardiac function and careful surveillance for MINOCA occurrence postoperatively. MINOCA constitutes an exceedingly rare yet fatal complication following Bentall and Sun’s surgery in patients with TAAD. This case stands as the first documented instance of such a complication, characterized by unfavorable treatment outcomes. Presently, there remains a dearth of clinical experience and research concerning the management of such patients. Based on the pathogenesis of MINOCA and the diagnostic and therapeutic trajectory of this case, we underscore the importance of postoperative vigilance for acute cardiac dysfunction; timely completion of electrocardiography, echocardiography, and myocardial injury marker assessments; and correction of reversible factors.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- AHA:
-
American Heart Association
- ARB:
-
Angiotensin receptor blockers
- CAV:
-
Coronary artery vasospasm
- CK-MB:
-
Creatine Kinase-MB
- CTA:
-
Computed tomography angiography
- CRRT:
-
Continuous renal replacement therapy
- CPB:
-
Cardiopulmonary bypass
- CVP:
-
Central venous pressure
- ECMO:
-
Extracorporeal membrane oxygenation
- ESC:
-
European Society of Cardiology
- FFR:
-
Fractional flow reserve
- IMR:
-
Index of microcirculatory resistance
- LVEF:
-
Left ventricular ejection fraction
- MINOCA:
-
Myocardial infarction with non-obstructive coronary arteries
- SICU:
-
Surgical intensive care unit
- TAAD:
-
Stanford type A aortic dissection
- UDMI:
-
Fourth Universal Definition of Myocardial Infarction
- VA-ECMO:
-
Veno-Arterial Extracorporeal Membrane Oxygenation
References
Feng W, Li H, Wang Q, Li C, Wu J, Yang J, et al. Prognostic significance of neutrophil count on in-hospital mortality in patients with acute type a aortic dissection. Front Cardiovasc Med. 2023;10:1095646. https://doi.org/10.3389/fcvm.2023.1095646.
Harris KM, Nienaber CA, Peterson MD, Woznicki EM, Braverman AC, Trimarchi S, et al. Early Mortality in Type A Acute Aortic dissection: insights from the International Registry of Acute Aortic Dissection. JAMA Cardiol. 2022;7(10):1009–15. https://doi.org/10.1001/jamacardio.2022.2718.
Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–826. https://doi.org/10.1093/eurheartj/ehad191.
Yu W, Liang Y, Gao J, Xie D, Xiong J. Surgical choice for the treatment of partial intestinal ischemic necrosis caused by acute type a aortic dissection combined with malperfusion of superior mesenteric artery. J Cardiothorac Surg. 2024;19(1):286. https://doi.org/10.1186/s13019-024-02790-z.
Banz Y, Rieben R, Zobrist C, Meier P, Shaw S, Lanz J, et al. Addition of dextran sulfate to blood cardioplegia attenuates reperfusion injury in a porcine model of cardiopulmonary bypass. Eur J cardio-thoracic Surgery: Official J Eur Association Cardio-thoracic Surg. 2008;34(3):653–60. https://doi.org/10.1016/j.ejcts.2008.05.024.
Parwani P, Kang N, Safaeipour M, Mamas MA, Wei J, Gulati M, et al. Contemporary diagnosis and management of patients with MINOCA. Curr Cardiol Rep. 2023;25(6):561–70. https://doi.org/10.1007/s11886-023-01874-x.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138(20):e618–51. https://doi.org/10.1161/CIR.0000000000000617.
Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, et al. An EAPCI Expert Consensus Document on Ischaemia with non-obstructive coronary arteries in collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation endorsed by Coronary Vasomotor Disorders International Study Group. Eur Heart J. 2020;41(37):3504–20. https://doi.org/10.1093/eurheartj/ehaa503.
Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American heart association. Circulation. 2019;139(18):e891–908. https://doi.org/10.1161/CIR.0000000000000670.
Mussie E, DeWitte N, Goulet M, Garfield J. Coronary artery vasospasm after mechanical aortic valve replacement: a case report. Cureus. 2023;15(11):e49747. https://doi.org/10.7759/cureus.49747.
Ahmad T, Kishore KS, Maheshwarappa NN, Pasarad AK. Postoperative diffuse coronary spasm after two valve surgery - a rare phenomenon. Indian Heart J. 2015;67(5):465–8. https://doi.org/10.1016/j.ihj.2015.06.007.
Rahmouni El Idrissi K, Chauvette V, Lamarche Y, El-Hamamsy I. Recurrent coronary vasospasm after cardiac surgery. Ann Thorac Surg. 2020;110(6):e481–3. https://doi.org/10.1016/j.athoracsur.2020.04.082.
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
M.C.: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Conceptualization. J.H.: Writing – review & editing, Methodology, Investigation, Research design. C.Z.: Writing – review & editing, Methodology, Research design, Investigation, Data analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study involving human participant was reviewed and approved by Ethics Committee of Guangdong Provincial People’s Hospital.
Consent for publication
Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, M., Hao, J. & Zhang, C. Exceptionally rare MINOCA: a case of acute myocardial infarction following surgery for Stanford type A aortic dissection. J Cardiothorac Surg 19, 485 (2024). https://doi.org/10.1186/s13019-024-02979-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13019-024-02979-2