Abstract
Background
Dry Afromontane forests play a vital role in mitigating climate change by sequestering and storing carbon, as well as reducing greenhouse gas emissions. Despite previous research highlighting the importance of carbon stocks in these ecosystems, the influence of canopy cover and environmental factors on carbon storage in dry Afromontane forests has been barely assessed. This study addresses this knowledge gap by investigating the effects of environmental factors and vegetation cover on carbon stocks in Desa’a forest, a unique and threatened Afromontane dry forest ecosystem in northern Ethiopia. Data on woody vegetation, dead litter, grass biomass, and soil samples were collected from 57 plots. A one-way analysis of variance (ANOVA) was performed at a 95% confidence level (α = 0.05) to examine the influence of canopy cover and environmental factors on the carbon stocks of various pools.
Results
Among the 35 woody species identified, Juniperus procera was the most dominant, while Carissa edulis Vahl and Eucalyptus globulus were the least dominant. The average total carbon stock was 92.89 Mg ha−1, with contributions from aboveground carbon, below-ground carbon, litter carbon, grass carbon, and soil organic carbon. Among the carbon pools, soil organic carbon had the highest carbon stock, accounting for 76.8% of the total, followed by above-ground biomass carbon at 17.7%. Significant variations in carbon stocks were found across altitude class and canopy level but not slope and aspect factors.
Conclusions
In summary, altitude and canopy level were found to significantly influence carbon stocks in Desa’a forest, providing valuable insights for conservation and climate change mitigation efforts in dry Afromontane forests. Forest intervention planning and management strategies should consider the influence of different environmental variables and tree canopy levels.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Forests play a crucial role in addressing global climate change by absorbing carbon dioxide (CO2) from the atmosphere, which has a positive impact on biodiversity, local economies, human health, and recreational activities [1]. As global warming is increasing, there is growing interest in using forests to mitigate climate change through carbon sequestration [2]. Forests are instrumental at storing carbon and act as a natural “brake” against climate change [3]. However, when forests are cleared, the carbon stored within them is released back into the atmosphere as in the form of CO2 [4]. Therefore, the effectiveness of forests in mitigating climate change depends on their carbon sequestration potential and management strategies [5].
Tropical forests cover approximately 45% of the world’s forest [6] and store around 25% of the world’s carbon [7]. Dry forests, which account for nearly half of the world’s tropical and subtropical forests, play a crucial role in supporting the livelihoods of millions of people, particularly in regions with high population densities and associated energy and land demands [8, 9]. Dry forests provide essential ecosystem services that help communities adapt to climate change impacts, such as droughts and other extreme events [10]. These forests are home to a high diversity of plant and animal species, which need to be protected for maintaining ecosystem balance and resilience [11]. The conservation and sustainable management of dry forests are essential for maintaining resilient and multi-functional landscapes and for countering the increasing vulnerability of people, forest ecosystems, and species in these fragile ecosystems [12].
Dry forests play a crucial role in climate change mitigation by sequestering and storing carbon and reducing greenhouse gas emissions [10]. As terrestrial ecosystems, forests are major sinks of carbon, sequestering about one-third of biotic emissions [13]. Dry forests, which are distributed across extensive geographical ranges in Africa, Latin America, and the Asia Pacific, have high potential for carbon storage [14]. However, the potential of dry forests to sequester and store carbon is poorly understood, and past attempts to estimate carbon stocks have ignored the drylands ecosystem heterogeneity [15]. The mitigation role of dry forests, including dry Afromontane forests, is influenced by various environmental factors. These factors play a significant role in determining the carbon sequestration potential and overall contribution to climate change mitigation.
Environmental factors such as altitude, slope, and aspect can significantly affect the carbon sequestration potential of dry Afromontane forests [13, 16, 17]. For example, higher altitudes can lead to cooler temperatures, which can negatively impact the growth and health of trees and, consequently, their ability to sequester carbon [5, 16]. Furthermore, a literature review on forest carbon sequestration found that environmental factors such as soil quality, temperature, and precipitation significantly influence the carbon sequestration potential of forests [18]. These factors influence the amount of carbon that can be stored in the biomass and soils of these ecosystems, which in turn, impacts their ability to mitigate climate change.
Canopy cover has a significant impact on the carbon storage capacity of dry forests, with varying levels of canopy density influencing the accumulation and sequestration of carbon in these ecosystems. For example, a higher canopy cover can lead to increased interception of precipitation, which can result in more water being held in the canopy, foliage, and soil, thereby enhancing carbon sequestration [17]. Canopy cover also affects the amount of sunlight that reaches the forest floor, which can impact the growth and development of understory plants and, consequently, the overall carbon sequestration potential of the ecosystem [19]. Understanding these factors and their influence on carbon sequestration can help guide the development of effective management strategies and conservation efforts to enhance the mitigation role of these ecosystems in addressing climate change.
However, quantitative information on how canopy cover and other environmental factors affect carbon sequestration potential of Desa’a dry afromontane forest is lacking. In Desa’a forest, previous works have documented studies on the impact of fencing on regeneration, tree Growth and carbon stock potential [20], regeneration response of various species [21], tree dieback effect on climate change mitigation potential of a dry afromontane forest [22] and allometric models for predicting aboveground biomass of trees [23]. Understanding the interplay between canopy cover, environmental factors, and the mitigative potential of dry afromontane forests is crucial for effective management, allowing these ecosystems to maximize their climate change mitigation potential while also conserving their ecological and socio-economic values [8]. Therefore, the aim of this study is (i) to analyze the vegetation structure of Desa’a forest; (ii) to assess the relationship between canopy cover, and environmental factors, and with biomass and soil carbon stock; and (iii) to identify the key environmental factors influencing biomass and soil carbon stocks in Desa’a forest, northern Ethiopia.
Materials and methods
Study area
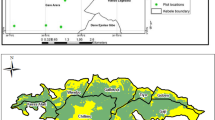
Geographically, Desa’a forest is situated between 13° 20′ and 14° 10′ north latitude; 39° 32′ and 39° 55′ east longitude (Fig. 1). The size of the forest is 154,071 ha. The forest area is 60 km far away from northeast of Mekelle, the capital city of Tigray region, northern Ethiopia. The altitude of the forest area ranges from 900 at the lower limit up to 3100 m above sea level at the plateau [24]. Over a five-year period from 2015 to 2020, temperature data collected from the Atsbi district meteorological station, which is situated adjacent to the study area, revealed that the average minimum and maximum temperatures in the study area were 9.2 ℃ and 19.9 ℃, respectively. The average annual temperature was 18 ℃. Besides, the area has a uni-modal rainfall pattern with the peak rainy season being from July to August and hence, the amount of rainfall ranges between 406 mm and 692 mm with a mean annual rainfall of 602 mm.
Desa’a forest is dominated by Juniperus procera and Olea europaea tree species and the dominant soil types are Lepthosols, Cambisols, Vertisols and Arenosols. The local farming community surrounding Desa’a forest practices a mixed farming system, where crops and livestock are integrated into the same agricultural operation. Wheat and barley are the primary crops grown in the study area, indicating a significant focus on cereal production in the region [24].
Sampling techniques and data collection
A random selection of forest areas was made within the Desa’a forest, from three forest sites, namely Kaal Amin, Felegewayne, and Hawile. A systematic grid sampling method was used to identify plots within the area, with a 500-m gap between transects and 500 m between plots within each transect. A total of 11 transects (4 in Kaal Amin, 4 in Hawile and 3 in Felegewayne) were established, with five plots per transect in most cases, except for one transect that had seven plots. This resulted in a total of 57 sample plots, evenly spaced at 500 m intervals along the transects.
The number of main plots were determined using Pearson et al. [25] equation;
where: E = allowable error or the desired half-width of the confidence interval. Calculated by multiplying the mean carbon stock by the desired precision (that is, mean carbon stock x 0.1, for 10% precision), t = the sample statistic from the t-distribution for the 95% confidence level; t is usually set at 2 as the sample size is unknown at this stage, Ni = number of sampling units for land cover type i (= area of land cover type in hectares), n = number of sampling units in the population, si = standard deviation of land cover i.
Each plot consisted of two nested subplots: a main plot measuring 20 m by 20 m, a subplot measuring 3 m by 3 m, and a sub-subplot measuring 1 m by 1 m. The 3 m × 3 m sub-plot were established at the center of the main plot while the 1 m × 1 m sub-plots were established, with one in the center and four in the corners. Various physical characteristics were recorded for each plot, including canopy cover, aspect, altitude, and slope. These data were categorized according to the guidelines outlined in Table 1. The forest canopy levels were classified in accordance with the Ethiopian forestry definition standards [26].
All trees and shrubs were identified in the main plots. A botanist supported by the local people was engaged to confirm scientific names and local names of the plant species. Diameter at breast height (DBH) and height (H) of all species within the plot was measured using a caliper and or a tape meter and a 5 m pole graduated with 10 cm markings respectively from each main plot. Trees taller than 5 m were measured using clinometer positioned at 10 m distance from the base of the tree and focused on the highest point of the tree. However, trees and shrubs with DBH ≥ 4.2 cm were considered for the biomass and carbon estimation. Sapling and seedlings were recorded from sub-plots measuring 3 m × 3 m. Soil, grass and dead litter samples were collected from the 1 m × 1 m sub-plots. All grass biomass and dead litter within the sub-plots were collected. The total fresh weight was measured using a spring balance in the field, and a composite sample was taken to the laboratory for carbon analysis. The samples were oven dried at 72 ℃ for 48 h [27]. The subsample is used to determine oven-dry-to-wet mass ratios to convert the total wet mass to oven-dry mass. Soil samples were collected from each sub-plot at two depths (0–10 cm and 10–20 cm) using a core sampler [28]. The soil samples were mixed properly in their respective layers, and composite soil samples were placed in plastic bags and labeled. Within each plot, we collected two undisturbed soil cores from the 0–10 cm and 10–20 cm soil layers at the plot’s center, carefully sealing them in airtight containers to preserve their integrity for subsequent measurements of bulk density. In the laboratory, the soils were oven dried at 105 ℃ for 24 h to remove moisture, allowing for the determination of organic carbon percentage and bulk density.
Analysis of vegetation structure
The structure of the vegetation was analyzed by computing species density, relative density DBH and height. Tree density was computed by converting the count from the sample plot to a hectare basis as in Eq. 2.
Relative density of trees was calculated as the number of individual species divided to the total number of individuals and multiplied by 100 (Eq. 3).
The DBH and H of the trees and shrubs were categorized into six classes with 5 cm intervals, following the classification system used by Mucheye and Yemata [29]. The percentage distribution of trees and shrubs in each class was then calculated to provide a comprehensive overview of the size structure of the vegetation.
Carbon quantification
Estimation of aboveground biomass carbon (AGC)
The aboveground biomass of the trees and shrubs was estimated using a specific equation (Eq. 4) developed by Tetemke et al. [23] in the Desa’a forest, which is the same location where the current study took place.
where, AGB is aboveground biomass (kg), DBH is diameter at breast height (cm); whereas “a” and “b” are coefficients (i.e. a = 0.298 and b = 2.034).
Aboveground carbon stock was estimated using the following equation (Eq. 5)
where, AGC is aboveground carbon and AGB is aboveground biomass and 0.47 is conversion factor for carbon content [30].
Estimation of belowground biomass carbon (BGC)
Belowground biomass was estimated from root–shoot ratios by taking into account the 26% of aboveground biomass of woody species according to IPCC [30] (Eq. 6):
where, BGB is belowground biomass, AGB is aboveground biomass and 0.26 is conversion factor.
Belowground carbon was calculated using the following formula (Eq. 7)
where, BGC is belowground carbon, BGB is belowground biomass and 0.47 is conversion factor for carbon content.
Estimation of litter biomass carbon (LC)
The amount of biomass in the litter was analyzed as follows [31] (Eq. 8);
where: LB = litter biomass (Mg ha−1).
Wfield = fresh weight of litter sampled (g) within the subplot;
A = size of the area in which litter were collected (ha);
Wsub-sample (dry) = weight of the oven-dried sub-sample (g) and Wsub-sample (fresh) = weight of the fresh sub-sample of litter taken to the laboratory (g).
Litter carbon stock was estimated as follow (Eq. 9):
Where, LC is litter carbon and LB is litter biomass and 0.47 is default carbon fraction value, as recommended by IPCC [30].
Estimation of grass biomass carbon (GC)
Grass biomass carbon stock was calculated as follows [28] (Eq. 10);
where: GB = Grass biomass (Mg ha−1), Wfield = weight of wet field sample of grass sampled (g) within an area of size 1 m2; A = Size of the area in which grass was collected (ha); Wsub-sample (dry) = weight of the oven-dry sub-sample of grass taken to the laboratory to determine moisture content (g), Wsub-sample (fresh) = weight of the fresh sub-sample of grass taken to the laboratory (g).
Grass carbon stock was estimated as follow (Eq. 11):
where, GC is grass carbon and GB is grass biomass 0.47 is the default carbon fraction value, as recommended by IPCC [30].
Estimation of soil organic carbon (SOC)
Soil organic carbon (SOC) was estimated using Pearson et al. [31] (Eq. 12).
where, SOC is soil organic carbon per unit area (Mg ha−1), Bulk density (g/cm3) = Oven dry mass (g)/Volume (cm−3), D is the depth at which the soil sample was taken (20 cm) and % carbon is carbon concentration (%) determined in the laboratory using the Walkley and Black [32] method.
Estimation of total carbon stock (CT)
The total carbon stock was calculated by summing the carbon stock of the individual carbon pools [31] as in Eq. 13.
where, CT = total carbon stock for all carbon pools (Mg ha−1), AGC = aboveground carbon stock (Mg ha−1), BGC = belowground carbon stock (Mg ha−1), LC = litter carbon stock (Mg ha−1), GC = grass carbon stock (Mg ha−1) and SOC = soil organic carbon stock (Mg ha−1).
Statistical analysis
Prior to ANOVA, data were tested for normality and equality of variance. One-way analysis of variances (ANOVA) with 95% confidence interval (α = 0.05) was applied to see the effect of canopy cover and environmental factors on carbon stocks of different pools. Tukey honestly significant difference (HSD) post-hoc tests were performed to separate means across the different levels of environmental variables. Moreover, standard deviation of means were calculated to quantify the precision of the mean values. Pearson correlation test was also used to analyze the relationship between the environmental factors and carbon pools. To identify change in carbon stock along the environmental variables, linear regression analyses were done. Carbon stocks of different pools were considered as the dependent variable, while altitude, slope and aspect were used as the independent variables. Statistical tests were performed using minitab software version16.
Results
Vegetation characteristics
The total numbers of plant species recorded in the study area were 35, which belong to 22 different families. The total numbers of stems used to determine their carbon stock were 1323 trees and shrubs. The most dominant tree species was Juniperus procera Hochst. ex Endl. and covers a relative density of 76.0%. The second dominant plant species was Dodonaea angustifolia (2675 stems ha−1) which covers a relative density of 8.1%. However, the least dominant plant species were Carissa edulis Vahl and Eucalyptus globulus (totally each with 1 stem). Based on tree life form, among the total woody species recorded 67.5% (1161 total stems) were trees, whereas the remaining 12.5% (162 total stems) were shrubs. The total numbers of saplings were 1849 and the seedlings were 2365. From the total 35 woody species, only 4 species (11.5% relative density) were exotic, whereas the remaining 31 species (88.5% relative density) were indigenous.
40% of the total woody species had a DBH below 5 cm (Fig. 2). Another 30% fell within the DBH range of 5 to 10 cm. There were only a small number of woody species with a larger diameter, specifically in the DBH class between 20.1 and 25 cm, which made up 5% of the total stem density. Generally, as the diameter of woody species increased, the density of trees and shrubs decreased. The smallest recorded DBH value was 4.2 cm, while the largest value was 69 cm.
From Fig. 3, it can be observed that the highest tree height density category was between 1 and 5 m, accounting for approximately 50% of the total count of stems. 26.4% of the total stems were found in the second height class category. There were only a few woody species found in the height class above 20 m, making up approximately 2.6% of the total count of stems.
Carbon stocks
The mean total carbon stock from the five carbon pools was 92.89 Mg ha−1 (Table 2). Among the carbon pools, soil organic carbon had the highest carbon stock, accounting for 76.8% of the total, followed by above-ground biomass carbon at 17.7%.
Carbon pools under different environmental factors
Carbon stocks at different canopy levels
Aboveground and belowground carbon significantly varied across the canopy levels (Table 3). Significantly highest aboveground and belowground carbon stocks were recorded in the dense forest while lowest aboveground and belowground carbon stocks were recorded in the bare land.
Carbon pools along altitudinal gradients
The variation in altitudinal gradient affected carbon stock in all carbon pools (Table 4; Fig. 4). Significantly highest aboveground carbon stock was recorded in the middle altitude, while lowest carbon stock was recorded in the upper altitude. Similarly, significantly highest litter carbon stock was recorded in the middle altitude, while lowest carbon stock was recorded in the upper altitude. In case of grass carbon, significantly highest carbon stock was recorded in the lower altitude class.
Carbon stocks across slope classes
While the highest aboveground carbon stock was recorded in the gentle slope, the lowest was recorded in the moderate slope (Table 5; Fig. 5). However, carbon stocks in all pools were not significantly different across the slope classes.
Carbon stocks across aspect categories
The difference on the direction of landscape (aspect) did not significantly affect carbon stocks across all carbon pools (Table 6; Fig. 6). However, the highest aboveground and belowground carbon stock was found on the northwest direction and the lowest was found on the northeast direction of the study area. The highest SOC was recorded in the west direction, while the lowest was recorded in the northwest direction.
Relationship between the environmental factors and carbon pools
Correlations between SOC stock and environmental factors
There was both positive and negative relationship between environmental factors and carbon stock variables (Table 7). The Pearson correlation result ranges from the minimum value (r = 0.03) almost no relationship between slope with and both above and belowground carbon pools to a maximum value (r = − 0.42) moderate negative relationship between altitude and litter carbon pool.
Regression models of soil organic carbon stock
Links between litter carbon stock, soil carbon stock and environmental factors remained significant (Table 8), indicating that environmental factors do seem to be a factor governing carbon stock. The results of the analysis also indicated that altitude is the most significant predictor of litter carbon and soil organic carbon (p = 0.03 and p = 0.02, respectively).
Discussion
Carbon stocks under different carbon pools
The carbon stock along different carbon pools is affected by anthropogenic and environmental variables [17]. The pools can have spatial and temporal variations [33]. The mean total carbon stock for the Desa’a forest in northern Ethiopia was 92.89 Mg ha−1. This value is lower than the mean total carbon stock of dry Afromontane forests in Ethiopia, which was estimated to be 113.0 Mg ha−1 [26]. The lower current result of 92.89 Mg ha−1 compared to the national average of 113.0 Mg ha−1 could be explained by the differences in the disturbance level and the environmental gradients of the forests. The disturbance level and the edaphic variables were responsible for the variation in the carbon stocks among forests in dry Afromontane forests in Ethiopia [17, 34, 35]. The degree of disturbances and topographic factors in the forests causes variations in carbon storage at different scales [36,37,38]. The methods followed and the allometric equation used to estimate vegetation biomass is another important factor that causes significant variation in carbon stock among forests [39, 40].
Soil organic carbon had the highest carbon stock, accounting for 76.8% of the total carbon stocks, followed by aboveground carbon contributing 17.7%. The high amount of soil organic carbon in the study area can be attributed to several reasons. One possible reason is that the study area had high forest density in the past, and the recent inventory data from the “WeForest” project (2018) indicated that 74% of the forest density is degraded. Soils can store carbon for up to 500 years [41], which could explain the high soil organic carbon levels. Belay et al. [42] also reported high soil carbon stocks in Afromontane forests in the highlands of Ethiopia, resulting from long-lasting biomass accumulation. Consistent with the present study, Chinasho et al. [43] reported that 74% and 14% of the total carbon stock were stored in soil and above-ground carbon stocks, respectively.
On the other hand, the present study recorded the lowest carbon stock in the litter carbon pool compared to other study results. For example, the mean total litter carbon of tropical dry forests was reported to be 2.10 Mg ha−1 [30]. Other dry Afromontane forests in Ethiopia have reported higher values, such as 2.34 Mg ha−1 [44], 2.72 Mg ha−1 [45], and 1.28 Mg ha−1 [46], but higher than the value of 0.02 Mg ha−1 reported by Aregie [47]. The lower carbon stock in the litter carbon pool in this study could be attributed to climate factors. It could also be due to a lower litterfall accumulation caused by the evergreen nature of the tree species throughout the year. Additionally, high decomposition rates and free grazing of animals can affect litter fall [48], might contribute to the lower litter carbon stock. Furthermore, since the study area has a mountainous and hilly landscape, litter fall could be easily washed out by erosion and other external factors [17]. Removing biomass from the forest floor by harvesting through herbivory, or residue or fuelwood could significantly reduce soil carbon stocks [49].
Effect of environmental factors on carbon stock in dry afromontane forests
The variation in forest canopy levels affects the carbon stock of different carbon pools in the forest. The variation in canopy levels has an impact on the carbon stock of different carbon pools. The dense forest canopy level exhibited the highest mean carbon stock and is consistent with the findings of Solomon et al. [14] in the Wujig Mahgo Waren dry afromontane forest. Dense tree and woody vegetation coverage in the dense canopy level may contribute to its high carbon stock potential.
Altitude affected the carbon stocks in all carbon pools within the forest area. Altitude has a major effect on the variety, biomass, and carbon stock in forest ecosystems [50]. Climatic factors and differences in soil water regime along elevation gradients can influence forest carbon stock, as mentioned by Gedefaw et al. [51]. The middle altitude class exhibited the highest carbon stock, while the upper altitudinal class had the lowest carbon stock, with the lower class falling in between, which is consistent with Kassahun et al. [44]. Total carbon stock was higher at the middle altitude than the upper and lower altitudes in Yegof montane forest, in Ethiopia [52]. The highest value at the middle altitudinal class in the present study might be due to the optimal climatic and soil conditions for tree growth and survival. The altitudinal categorization is not standard and is site-dependent, making it difficult to compare the carbon stock between forests. Forests at higher altitudes are exposed to high wind and low temperatures and have low carbon stocks compared to forests at lower and middle altitudes with higher biomass production due to higher photosynthesis and net primary production [53]. Variations in tree morphology with altitude were observed, with trees at high elevations displaying stunted growth, characterized by short stature and slender profiles. In contrast, trees at lower elevations exhibited a more bushy appearance, featuring short trunks and abundant branching. Meanwhile, trees at intermediate altitudes showed a more robust profile, with taller canopies and wider diameters, which will have a substantial impact on the estimation of biomass and carbon stocks.
Regarding the grass carbon pool, the study found that the lower altitude class had the highest carbon stock, while the upper altitude class had the lowest. This was attributed to the high accumulation of grass biomass in the slightly flattened landscape of the lower altitude class, which benefits from good availability of water and soil nutrients [54]. Additionally, the use of grass biomass for livestock feed by local farmers was noted as a factor contributing to the increased vegetative capacity over time.
In terms of slope classes, the present study did not find a significant difference in carbon stock across the carbon pools, like the findings of Yohannes et al. [55], Simegn and Soromessa [48], and Kassahun et al. [44]. The similar species composition and soil type throughout the slope gradient of the forest may account for this statistically not significant result.
Regarding aspects, the present study did not find a significant effect on carbon stock across the carbon pools. This contrasts with the findings of Kassahun et al. [44], who reported a significant difference. The highest carbon stock was recorded in the south (S) and southeast (SE) aspects, while the lowest was in the northeast (NE) aspects. In contrast, other studies have shown different results. For example, Gedefaw et al. [51], found the highest carbon stock in the western aspect of Tara Gedam forest in northwestern Ethiopia, and Shiferaw [56] indicated higher AGC and BGC in the eastern aspect. Microclimatic differences induced by topographic aspects may contribute to the variation in soil carbon sequestration and the distribution of plant communities, as suggested by previous studies.
Correlation between environmental factors and carbon pools
The Pearson correlation analysis revealed both positive and negative relationships between vegetation cover, environmental factors, and carbon pools. Altitude demonstrated a significant inverse correlation with aboveground, belowground, and litter carbon, but a positive significant correlation with soil carbon stock. This finding is consistent with the study conducted by Fang et al. [57], which reported similar results. Therefore, as elevation increases, there is a significant decrease in aboveground, belowground, and litter carbon, while soil organic carbon stock increases. This relationship can be attributed to various factors that vary with altitude, such as geomorphology, soil composition, humidity, cloudiness, rainfall, and temperature, as suggested by Gedefaw et al. [51] and Wodajo et al. [45].
Supporting this notion, a recent study conducted by Wodajo et al. [45] in the Gara-muktar dry Afromontane forest found a weak negative correlation between above and belowground carbon stocks with altitude, but a weak positive correlation with litter carbon and soil organic carbon (SOC). However, Tilahun [58] reported different results, indicating a positive correlation between elevation and aboveground and belowground carbon pools.
In terms of slope, there was almost no relationship observed between slope and aboveground and belowground biomass carbon stocks. However, a significant positive relationship was found, indicating a significant increase in litter carbon stock as slope increases. It is important to note that this current study’s findings differ from those of Kassahun et al. [44], who reported a direct relationship between carbon stock and both altitude and slope.
Conclusion
In conclusion, this study provides a comprehensive overview of the woody vegetation composition, density, and structure in the Desa’a forest, as well as its carbon stock. The results reveal a dominance of certain tree species, with Juniperus procera Hochst. ex Endl. standing out as a prominent feature. The study also highlights the prevalence of small-diameter trees and shrubs, which form the majority of the forest’s woody cover.
Notably, the study finds that canopy level and altitude have significant effects on carbon storage in the forest. The dense forest canopy is characterized by higher aboveground and belowground carbon stocks compared to open forests and bare land. Altitude emerges as a key predictor of carbon stocks, with middle-altitude areas exhibiting higher values. In contrast, slope class and aspect do not exhibit a significant impact on carbon storage. Instead, altitude is found to be the most important factor governing carbon stock in the study area.
The findings of this study underscore the importance of considering environmental factors when assessing carbon stocks in forest ecosystems. The results can inform conservation and management strategies for maintaining and increasing carbon stocks in the Desa’a forest and similar ecosystems. Moreover, the study highlights the need for further research on the dynamics of carbon storage in these ecosystems and their responses to environmental changes. Overall, this study provides valuable insights into the complex relationships between vegetation structure, environmental conditions, and carbon storage in the Desa’a forest.
Data availability
Data is available with a reasonable request.
References
Waring B, Neumann M, Prentice IC, Adams M, Smith P, Siegert M. What role can forests play in tackling climate change. London: Imperial College London; 2020.
Aneseyee AB. Vegetation composition and deforestation impact in Gambella National Park, Ethiopia. J Energy Nat Resour. 2016;5(3):30–6.
Mildrexler DJ, Berner LT, Law BE, Birdsey RA, Moomaw WR. Large Trees Dominate Carbon Storage in Forests East of the Cascade Crest in the United States Pacific Northwest. Front Forests Global Change. 2020;3:594274.
Gasparri NI, Grau HR, Manghi E. Carbon pools and emissions from Deforestation in extra-tropical forests of Northern Argentina between 1900 and 2005. Ecosystems. 2008;11(8):1247–61.
Belay S, Amsalu A, Abebe E. Land Use and Land Cover changes in Awash National Park, Ethiopia: impact of decentralization on the Use and Management of resources. Open J Ecol. 2014;04:15:11.
Food Nations AOotU. Global forest resources assessment 2020—Key findings. Rome: Food NAOU; 2020.
Bonan GB. Forests and climate change: Forcings, Feedbacks, and the climate benefits of forests. Science. 2008;320(5882):1444–9.
Siyum ZG. Tropical dry forest dynamics in the context of climate change: syntheses of drivers, gaps, and management perspectives. Ecol Processes. 2020;9(1):25.
Tesfaye M, Manaye A, Tesfamariam B, Mekonnen Z, Eshetu SB, Löhr K, et al. Contribution of dry forests and Forest Products to Climate Change Adaptation in Tigray Region. Ethiopia Forests. 2022;13(12):2026.
Amanuel W, Tesfaye M, Worku A, Seyoum G, Mekonnen Z. The role of dry land forests for climate change adaptation: the case of Liben Woreda, Southern Oromia, Ethiopia. J Ecol Environ. 2019;43(1):11.
Sola P. Tropical dry forests under threat & under-researched. Bogor: Center for International Forestry Research (CIFOR)-CGIAR; 2014.
Khosravi Mashizi A, Sharafatmandrad M. Dry forests conservation: a comprehensive approach linking ecosystem services to ecological drivers and sustainable management. Global Ecol Conserv. 2023;47:e02652.
Yadav VS, Yadav SS, Gupta SR, Meena RS, Lal R, Sheoran NS, et al. Carbon sequestration potential and CO2 fluxes in a tropical forest ecosystem. Ecol Eng. 2022;176:106541.
Solomon N, Pabi O, Annang T, Asante IK, Birhane E. The effects of land cover change on carbon stock dynamics in a dry afromontane forest in northern Ethiopia. Carbon Balance Manag. 2018;13(1):14.
Leley NC, Langat DK, Kisiwa AK, Maina GM, Muga MO. Total carbon stock and potential carbon sequestration economic value of Mukogodo Forest-landscape ecosystem in drylands of Northern Kenya. Open J Forestry. 2021;12(1):19–40.
Mendelsohn R, Sedjo R, Sohngen B, Mooij RAD, Keen M, Parry IWH. Fiscal Policy to Mitigate Climate Change: A Guide for Policymakers. Chapter 5 Forest Carbon Sequestration*: International Monetary Fund; 2012. p. ch05.
Gebeyehu G, Soromessa T, Bekele T, Teketay D. Carbon stocks and factors affecting their storage in dry afromontane forests of Awi Zone, northwestern Ethiopia. J Ecol Environ. 2019;43(1):7.
Athamrie A, Lemi T. The role of forest ecosystems for carbon sequestration and poverty alleviation in Ethiopia. Int J For Res. 2023;2013:3838404.
Solomon N, Birhane E, Tadesse T, Treydte AC, Meles K. Carbon stocks and sequestration potential of dry forests under community management in Tigray, Ethiopia. Ecol Processes. 2017;6(1):20.
Ruo G, Weldegebrial B, Yohannes G, Yohannes G. The impact of fencing on regeneration, tree growth and carbon stock in Desa Forest, Tigray, Ethiopia. Biomedical J. 2018;1:17.
Aynekulu E, Denich M, Tsegaye D. Regeneration response of < span class=genus-species>Juniperus procera and < span class=genus-species>Olea europaea subsp < span class=genus-species>cuspidata to Exclosure in a dry afromontane forest in Northern Ethiopia. Mt Res Dev. 2009;29(2):143–52. 10.
Mokria M, Gebrekirstos A, Aynekulu E, Bräuning A. Tree dieback affects climate change mitigation potential of a dry afromontane forest in northern Ethiopia. For Ecol Manag. 2015;344:73–83.
Tetemke BA, Birhane E, Rannestad MM, Eid T. Allometric models for Predicting Aboveground Biomass of Trees in the dry afromontane forests of Northern Ethiopia. Forests. 2019;10(12):1114.
Abegaz A. Farm management in mixed crop-livestock systems in the Northern Highlands of Ethiopia. Wageningen University and Research; 2005.
Pearson T, Walker S, Brown S. Sourcebook for land use, land-use change and forestry projects. Arlington, USA: Winrock International and the Bio-carbon fund of the World Bank; 2005.
EFRL, Ethiopia’s. Forest reference level submission to the UNFCCC. 2017.
UNFCCC. Measurements for estimation of carbon stocks in afforestation and reforestation project activities under the clean development mechanism: a field manual. Bonn: UNFCCC; 2015.
Hairiah K, Sonya D, Fahmuddin A, Meine vNa SR. Measuring Carbon stocks across Land Use systems: a manual Bogor, Indonesia.: World Agroforestry Centre (ICRAF), SEA Regional Office. Brawijaya University and ICALRRD (Indonesian Center for Agricultural Land Resources Research and Development); 2011.
Mucheye G, Yemata G. Species composition, structure and regeneration status of woody plant species in a dry afromontane forest, Northwestern Ethiopia. Cogent Food Agric. 2020;6(1):1823607.
IPCC. IPCC guidelines for national greenhouse gas inventories. Geneva: IPCC; 2006. p. 2006.
Pearson T, Walker S, Brown S. Source book for land use, land-use change and forestry. VA, USA: Projects Winrock International. 2005:35.
Walkley A, Black IA. An examination of the Degtjareff method for determining soil organic matter, and aproposed modification of the chromic acid titration method. Soil Sci. 1934;37(1):29–38.
Tesfaye MA, Gardi O, Bekele T, Blaser J. Temporal variation of ecosystem carbon pools along altitudinal gradient and slope: the case of Chilimo dry afromontane natural forest, Central Highlands of Ethiopia. J Ecol Environ. 2019;43(1):17.
Daba DE, Dullo BW, Soromessa T. Effect of Forest Management on Carbon Stock of Tropical Moist Afromontane Forest. Int J Forestry Res. 2022;2022(1):3691638.
Asrat F, Soromessa T, Bekele T, Kurakalva RM, Guddeti SS, Smart DR, et al. Effects of Environmental factors on Carbon stocks of dry Evergreen afromontane forests of the Choke Mountain Ecosystem, Northwestern Ethiopia. Int J Forestry Res. 2022;2022(1):9447946.
Ahmed S, Lemessa D. Patterns and drivers of the above- and below-ground carbon stock in Afromontane forest of southern Ethiopia: implications for climate change mitigation. Trop Ecol. 2024;65:508–516. https://doi.org/10.1007/s42965-024-00334-z.
Tanner LH, Wilckens MT, Nivison MA, Johnson KM. Biomass and Soil Carbon stocks in Wet Montane Forest, Monteverde Region, Costa Rica: assessments and challenges for quantifying Accumulation Rates. Int J Forestry Res. 2016;2016(1):5812043.
Willcock S, Phillips OL, Platts PJ, Balmford A, Burgess ND, Lovett JC, et al. Quantifying and understanding carbon storage and sequestration within the Eastern Arc mountains of Tanzania, a tropical biodiversity hotspot. Carbon Balance Manag. 2014;9(1):2.
Araujo ECG, Sanquetta CR, Dalla Corte AP, Pelissari AL, Orso GA, Silva TC. Global review and state-of-the-art of biomass and carbon stock in the Amazon. J Environ Manage. 2023;331:117251.
Vashum K. Methods to estimate above-ground biomass and carbon stock in natural forests - a review. J Ecosyst Ecogr. 2012;2:1–7.
Brevik EC, Burgess LC. Soils and human health. Boca Raton: CRC; 2012.
Belay B, Pötzelsberger E, Sisay K, Assefa D, Hasenauer H. The Carbon Dynamics of Dry Tropical Afromontane Forest Ecosystems in the Amhara Region of Ethiopia. Forests. 2018;9(1):18.
Chinasho A, Soromessa T, Bayable E. Carbon stock in woody plants of Humbo forest and its variation along altitudinal gradients: the case of Humbo district, Wolaita Zone, southern Ethiopia. Int J Environ Prot Policy. 2015;3(4):97–103.
Kassahun K, Soromessa T, Belliethathan S. Forest carbon stock in woody plants of ades forest, Western Hararghe Zone of Ethiopia and its variation along environmental factors: implication for climate change mitigation. Forest. 2015;5(21):4.
Wodajo A, Mohammed M, Tesfaye MA. Carbon stock variation along altitudinal and slope gradients in Gara-Muktar forest, West Hararghe Zone, Eastern Ethiopia. Forestry Res Engineering: Int J. 2020;4:1–2020.
Kenea LM. Estimation of biomass and soil carbon stock along altitudinal gradient of Anchebbi dry afromontane forest in Danno district West Shewa zone, Ethiopia. 2020.
Aregie AM. Carbon stock estimation along altitudinal gradient in Sekele-Mariam dry evergreen montane forest, North-Western Ethiopia. Forestry and Natural Resources. Wondo Genet, Ethiopia, Hawassa University. Master of Sciences 2018;53.
Simegn TY, Soromessa T. Carbon stock variations along altitudinal and slope gradient in the forest belt of Simen Mountains National Park, Ethiopia. Am J Environ Prot. 2015;4(4):199–201.
Mayer M, Prescott CE, Abaker WEA, Augusto L, Cécillon L, Ferreira GWD, et al. Tamm review: influence of forest management activities on soil organic carbon stocks: a knowledge synthesis. For Ecol Manag. 2020;466:118127.
Luo J-J, Masson S, Behera S, Shingu S, Yamagata T. Seasonal climate predictability in a coupled OAGCM using a different approach for ensemble forecasts. J Clim. 2005;18(21):4474–97.
Gedefaw M, Soromessa T, Belliethathan S. Forest carbon stocks in woody plants of Tara Gedam forest: implication for climate change mitigation. Sci Technol Arts Res J. 2014;3(1):101–7.
Eshetu EY, Hailu TA. Carbon sequestration and elevational gradient: the case of Yegof mountain natural vegetation in North East, Ethiopia, implications for sustainable management. Cogent Food Agric. 2020;6(1):1733331.
Chimdessa T. Forest carbon stock variation with altitude in bolale natural forest, Western Ethiopia. Global Ecol Conserv. 2023;45:e02537.
Adnew W, Asmare B. Agronomic performance, yield, and Nutritional Value of grasses affected by agroecological settings in Ethiopia. Adv Agric. 2023;2023:9045341.
Yohannes H, Soromessa T, Argaw M. Carbon stock analysis along slope and slope aspect gradient in Gedo Forest: implications for climate change mitigation. J Earth Sci Clim Change. 2015;6(09):6–11.
Shiferaw G. Carbon stocks in different pools in natural and plantation forests of Chilimo, central highland of Ethiopia. Unpublished M Sc thesis, Addis Ababa University Addis Ababa. 2012.
Fang H, Ji B, Deng X, Ying J, Zhou G, Shi Y, et al. Effects of topographic factors and aboveground vegetation carbon stocks on soil organic carbon in Moso bamboo forests. Plant Soil. 2018;433:363–76.
Tilahun BL. Effects of elevations on woody species diversity and carbon stocks of Kella natural forests in Konso zone, Southern Ethiopia. 2019.
Acknowledgements
We would like to thank ‘WeForest’ project for their financial support and providing valuable data for this study. Moreover, our gratitude goes to Mr. Beyene who is laboratory technician of the department of Land Resources Management and Environmental Protection, Mekelle University. We acknowledge the Institute of International Education-Scholars Rescue Fund (IIE-SRF), Norwegian University of Life Sciences (NMBU), Faculty of Environmental Sciences and Natural Resource Management (MINA), and NORGLOBAL 2 project “Towards a climate-smart policy and management framework for conservation and use of dry forest ecosystem services and resources in Ethiopia [grant number: 303600]” for supporting the research stay of Emiru Birhane at NMBU.
Funding
This study was financially supported by WeForest’ project.
Author information
Authors and Affiliations
Contributions
“N.S., M.T., E.B., A.N. and T.G. conceived and designed the study; M.T. collected the data; N.S., M.T. and T.G. analyzed the data and wrote the paper; N.S., M.T., E.B., A.N. and T.G critically reviewed the paper and provided comments on the contents and structure of the paper. The authors read and approved the final manuscript.“”.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors gave their informed consent to this publication and its content.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Solomon, N., Birhane, E., Teklay, M. et al. Exploring the role of canopy cover and environmental factors in shaping carbon storage in Desa’a forest, Ethiopia. Carbon Balance Manage 19, 30 (2024). https://doi.org/10.1186/s13021-024-00277-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13021-024-00277-x