Abstract
Background
At present, preterm infants with respiratory distress syndrome (RDS) in China present higher mortality and morbidity rates than those in high-income countries. The aim of this nationwide survey was to assess the clinical management of RDS in China.
Methods
A nationwide cross-sectional survey to assess adherence to RDS management recommendations was performed. One neonatologist per hospital was randomly selected. The primary outcome was the key care of RDS management.
Results
Among the 394 participating hospitals, 88·3% were birthing centres. The number of doctors and nurses per bed were 0·27 and 0·72, respectively. Antenatal corticosteroids (any dose) were administered to 90% of the women at risk of preterm birth at < 34 weeks of gestation (90·0% inborn vs. 50·0% outborn, p < 0·001). The median fraction of inspired oxygen (FiO2) for initial resuscitation was 0·30 for babies born at ≤ 32 weeks of gestation and 0·25 for those born at > 32 weeks. T-piece resuscitators were available in 77·8% of delivery rooms (DRs) (tertiary hospitals: 82·5% vs. secondary hospitals: 63·0%, p < 0·001). Surfactant was used in 51·6% of the DRs. Less invasive surfactant administration (LISA) was used in 49·7% of the hospitals (tertiary hospitals: 55·3% vs. secondary hospitals: 31·5%, p < 0·001). Primary non-invasive ventilation was initiated in approximately 80·0% of the patients. High-frequency oscillation ventilation was primarily reserved for rescue after conventional mechanical ventilation (MV) failure. Caffeine was routinely used during MV in 59·1% of the hospitals. Bedside lung ultrasonography was performed in 54·3% of the health facilities (tertiary hospitals: 61·6% vs. secondary hospitals: 30·4%, p < 0·001). Qualified breast milk banks and Family Integrated Care (FICare) were present in 30·2% and 63·7% of the hospitals, respectively.
Conclusions
Significant disparities in resource availability and guidelines adherence were evident across hospitals. Future strategies should address DR facilities and medication access, technical training, staff allocation, and ancillary facility development for a better management of RDS patients in China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Neonatal respiratory distress syndrome (RDS) is a prevalent pulmonary condition observed in preterm infants, and is primarily linked to insufficient pulmonary surfactant (PS) or underdeveloped lung structures [1, 2]. RDS affects approximately 30% of infants born between 28 and 34 weeks of gestation, with the prevalence increasing to approximately 60% for those born before 28 weeks [3]. The Chinese Neonatal Network (CHNN) reported a survival rate of 87·6%, with 51·8% of infants born at < 32 weeks of gestation surviving without major morbidities [4]. However, survival rates vary with socioeconomic status. In low- and middle-income countries (LMICs), more than 90% of extremely preterm infants (EPIs, less than 28 weeks of gestation) do not survive beyond the first few days of life, with RDS being one of the most common causes of death, compared with the mortality rate of less than 10% in high-income countries [5]. Even in high-income countries, critical conditions associated with significant morbidity and mortality still exist in specific high-risk perinatal situations (e.g., specific social or geographic reasons) [6]. Efforts to standardize RDS management, including prenatal care, stabilization in the delivery room (DR), surfactant administration, and ventilation strategies, have led to the establishment of various guidelines and consensuses [7,8,9].
Perinatal and neonatal care in China has significantly progressed, with neonatal mortality rate of 3·1‰ in 2021, closely aligning with the rates in high-income countries [10, 11]. Local guidelines and consensuses in China contribute to defining the criteria for RDS care and neonatal intensive care unit (NICU) construction [7, 12]. National and regional neonatal networks are actively engaged in fostering quality improvements in participating hospitals [4, 12,13,14]. Additionally, the development of regional neonatal transportation systems development reflects improved management for critically ill neonates.
Nevertheless, numerous lower-tier hospitals in China continue to face challenges in providing timely and effective treatment, particularly for EPIs [15]. Given the vast size and population of China, the Medical sUrvey of NICU Insight in CHina (MUNICH) was conducted to comprehensively explore current RDS management across the country.
Methods
Study design and participants
In China, general hospitals and maternity-child healthcare hospitals are birthing centres where pregnant women give birth. In contrast, all neonates in children’s hospitals are outborn and transferred from birthing centres not only due to respiratory diseases, but also due to other congenital defects or perinatal diseases [16,17,18]. Maternity-child healthcare hospitals and children’s hospitals are usually referred to as paediatric specialty hospitals, which provide treatment for children as well as neonates. Physicians may work as specialists in neonatology departments or as paediatricians responsible for both neonates and children.
In China, tertiary hospitals provide high-level specialized medical services to several areas where most high-risk pregnant women and preterm babies are treated. In parallel, secondary hospitals are regional hospitals that provide comprehensive health services to multiple communities. Although most preterm babies born in secondary hospitals are transferred to designated tertiary hospitals, physicians in secondary hospitals may occasionally deal with preterm birth. Cities were categorized into 1st-tier, 2nd-tier, and 3rd-tier and lower-tier cities. 1st-tier and 2nd-tier cities were defined as well-developed cities, including municipalities directly under the Central Government, capital cities, or regional centres (Table S1). Mainland China was stratified according to a traditional seven-region partition (Northeast, North, East, South, Central, Northwest, and Southwest China). The provinces/autonomous regions/centrally administered municipalities in each region are listed in Table S2. Doctors’ titles were divided into chief and associate chief physician and attending and resident physicians.
On the basis of the above background, a nationwide, multicentre, cross-sectional survey was designed. We used the online MedSci database, covering 31 provincial administrative regions, municipalities, and autonomous regions in China, with 95,444 registered doctors. A total of 2,881 hospitals with neonatal units were identified in the MedSci database.
The MUNICH was conducted with stratified convenience sampling according to the different regions and types of hospitals. The sample size was allocated to eligible hospitals in each region, and only one doctor was recruited at each hospital. The adjusted Yamane formula in the equation was used for sample size calculation.
where
n = sample size
N = population size = 1500
e = the degree of accuracy expressed as a proportion = 0.03
\(\rho\) = the number of standard deviations that would include all possible values in the range of 4
t = t-value for the selected alpha level or confidence level at 99% = 2.58
ɛ = adjust margin of error [(ε = \(\frac{\rho e}{t}\))]
The estimated effective sample size of 354 participants was required to achieve a 99% confidence interval (CI), assuming a target hospital number of 1500 (200–250 hospitals in each of the seven district regions). The estimated questionnaire response rate was 90%, and 90% of the responses was valid, for a total sample size of 437. Considering the diverse development across various regions in China and the variability in hospital types, a total sample size of 450 cases was selected in this study. 50–70 doctors from public hospitals in each region were planned to be recruited, with a total of 450 doctors in seven regions (nine doctors for the presurvey). The sample was divided into general hospitals, maternity-child healthcare hospitals and children’s hospitals at a ratio of 6:6:1, and into tertiary and secondary hospitals at a ratio of 3:1 in each region (Fig. 1). Physicians who worked in the neonatology department of the selected hospitals were randomly chosen. If the first doctor did not respond, a second doctor from the same hospital was contacted. If no doctor in the selected hospital responded, a doctor at a backup hospital was contacted. Data collection spanned from October to December 2022.
In line with the principles of the Declaration of Helsinki, the study was approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (Approval Number: 2022–376). All the physicians provided informed consent before participating in the survey.
Questionnaire preparation
The questionnaire was collaboratively developed by the MUNICH Study Group, which comprised specialists with extensive expertise in RDS management. The questionnaire, in alignment with established guidelines, underwent rigorous assessment. A preliminary survey involving nine neonatologists was performed to ensure data clarity and precision. The questionnaire was composed of six dimensions: general information (11 questions), prenatal and perinatal conditions (20 questions), non-invasive ventilation (eight questions), invasive ventilation (eight questions), pulmonary surfactant administration (21 questions), and others (15 questions) (Supplemental 1).
Questionnaire distribution and data collection
A third-party online survey platform was used to manage the distribution and collection of questionnaires. Doctors received the questionnaire via a web link or QR code. The exclusion criteria were as follows: (1) survey with the same answer option selected for all questions or answer options selected with apparent regularity (e.g., ABAB or AAAA); (2) surveys for which the respondent failed to follow the instructions for the question or answered outside the scope of the question; and (3) survey responses from doctors at private hospitals and nurses.
Statistical analyses
Categorical data were represented as frequencies (N) and percentages (%), and intergroup differences were assessed via the chi-square test. Continuous data normality was determined via the Shapiro‒Wilk method. Normally distributed continuous data were expressed as the mean ± standard deviation (mean ± SDs) and were analyzed via t tests or analysis of variance. Nonnormally distributed continuous data were presented as the median (median), first quartile (Q1), and third quartile (Q3), with intergroup comparisons conducted via the Mann‒Whitney U test or Kruskal‒Wallis H test. All the statistical tests were two-tailed, adhering to a predetermined significance level of α = 0·05. The average composite score for the ranking questions was calculated as follows: composite score = (Σ frequency × weight)/number of people who answered the question. The frequency was the number of people who chose each option to be ranked in different positions. The weight was determined by the ranking of the options. For example, if there were three options involved, the weight of the first position in the ranking was three, the second was two, and the third was one. Statistical analysis and boxplots were performed via R (Version 4·2·2). All the data were analysed in five dimensions: hospital level, hospital type, city tier, geographical region, and doctor title.
Results
Profiles of the included physicians and hospitals
Of the 449 questionnaires distributed, 398 responses were obtained (nine presurvey questionnaires were not included). Four questionnaires were excluded (two from nurses and two from doctors at private hospitals), resulting in 394 valid responses from 30 provinces across China (Table S1). The response rate was 88·9% (407/458), and 99·0% of the responses were valid (394/398). Among the respondents, 378 were from birthing centres and 16 were from children’s hospitals. Among all the physicians, 97·4% (384/394) were from the neonatology department or neonatal intensive care unit. Tertiary hospitals constituted 60·9% (298/394) of the sample. Further details on the participating doctors and hospitals are provided in Table 1.
Bed capacity and human resources
The median numbers of NICUs and total beds were 10·0 (5·0, 25·0) and 30·0 (20·0, 50·0), respectively (Table 2). Compared with secondary hospitals, tertiary hospitals had more beds (20·0 (12·0, 25·0) vs. 35·0 (22·3, 60·0) beds, p < 0·001). There were more beds in paediatric specialty hospitals than in general hospitals (40·0 (25·0, 80·0) vs. 25·0 (17·0, 40·0) beds, p < 0·001).
The median numbers of doctors per bed and nurses per bed were 0·27 and 0·72, respectively. Paediatric specialty hospitals had higher ratios than general hospitals did (doctors per bed, 0·26 vs. 0·29, p = 0·002; nurses per bed, 0·66 vs. 0·77, p < 0·001). Comparisons of bed capacity and human resources among different regions are shown in Fig. 2 and Table S3.
Main points of RDS care
Antenatal corticosteroids
Antenatal corticosteroids (any dose) were administered to approximately 90% of the women at risk of preterm birth at < 34 weeks of gestation. Among the 176 hospitals with both outborn and inborn babies, fewer outborn babies than inborn babies received antenatal corticosteroids (50·0% vs. 95·0%, p < 0·001). The reasons for the lack of antenatal corticosteroid use are shown in Fig. S1A, with precipitous labour exhibiting the highest score of 4·64 points.
Delivery rooms
Data related to DRs were obtained from 378 birthing centres.
Oxygen therapy in DRs
Oxygen blenders were present in 82·0% (310/378) of the DRs in the birthing centres. A median fraction of inspired oxygen (FiO2) of 0·30 for babies born at < 28 weeks of gestation, 0·30 for those born at 28–31 weeks of gestation, and 0·25 for those born at > 32 weeks of gestation was used for initial resuscitation. Notably, general hospitals preferred to set higher initial FiO2 values than maternity-child healthcare hospitals did for preterm babies born between 28–31 weeks of gestation (0·30 (0·30, 0·40) vs. 0·30 (0·25, 0·35), p = 0·023) and > 32 weeks of gestation (0·30 (0·21, 0·40) vs. 0·21 (0·21, 0·30), p = 0·001). The designated lower and upper limits for target oxygen saturation after the first 10 min postnatally were 89% and 95%, respectively. Compared with secondary hospitals, tertiary hospitals exhibited higher limits (88·0%-95·0% vs. 90·0%-95·0%, p < 0·05). The initial FiO2 and target oxygen saturation limits among the different groups of hospitals are shown in Table 3.
T-piece resuscitators
T-piece resuscitators (TPRs) were available in 77·8% (294/378) of the DRs (tertiary hospitals: 82·5% vs. secondary hospitals: 63·0%, p < 0·001; general hospitals: 73·9% vs. maternity-child healthcare hospitals: 84·7%, p = 0·015). Forty-eight (48/378, 12·7%) respondents had only bag-valve-mask resuscitators for positive pressure ventilation in their DRs, with equipment deficiency being the most common cause (41/48, 85·4%). In Northwest China, access to TPRs was comparatively better, at a rate of 90·4%, which was significantly higher than the rates in other regions (p = 0·019) (Table S4).
PS administration in DRs
A total of 195 (51·6%) hospitals could use PS in the DR. Maternity-child healthcare hospitals had greater accessibility to PS than general hospitals did (61·3% vs. 46·1%, p = 0·004). Compared with 3rd tier and below cities, more hospitals in 1st-tier cities and 2nd-tier cities could use PS in the DR (41·8% vs. 64·7% vs. 60·8%, p < 0·001). The inability to obtain surfactant from the hospital pharmacy, lack of reimbursement before birth, and potential refusal to pay for medication (ranking scores: 4·15, 3·64, and 3·01, respectively) were the top three reasons for the absence of PS in the DRs (Fig. S1B).
PS administration
A total of 60·4% of the respondents used FiO2 (41·6% chose > 0·30, 41·2% > 0·40 and 15·5% > 0·50) as an indicator for PS administration in patients receiving non-invasive ventilation (NIV). PS was routinely administered to patients receiving mechanical ventilation (MV) according to 79·7% of the respondents.
As shown in Table 4, the INtubation-SURfactant-Extubation (INSURE) method was used in 341 (341/394, 86·5%) hospitals. Moreover, less invasive surfactant administration (LISA) and/or minimally invasive surfactant therapy (MIST) could be used in 49·7% (196/394) of the hospitals. Compared with secondary hospitals, tertiary hospitals presented a greater capacity for LISA/MIST (31·5% vs. 55·3%, p < 0·001). LISA/MIST was utilized in approximately 30·0% of RDS patients receiving NIV in hospitals performing LISA/MIST. Furthermore, feeding tubes were used for LISA/MIST in nearly half (50·5%) of the hospitals, whereas peripheral vein catheters and umbilical venous catheters were available in 28·2% and 19·8% of the hospitals, respectively.
Non-invasive ventilation
NIV was initiated as primary support in approximately 80·0% of the RDS patients (Central China had a higher rate (85·0%) than other regions did, p = 0·013). Continuous positive airway pressure (CPAP) was more frequently used as an initial (ranking score: 5·07 points) and postextubation (ranking score: 4·49 points) support modality than other NIV modes were (Fig. S1C-D).
Mechanical ventilation
Among the different MV modes, synchronized intermittent mandatory ventilation combined with pressure support ventilation and volume guarantee (SIMV + PSV + VG), synchronized intermittent mandatory ventilation combined with pressure support ventilation (SIMV + PSV), and pressure-controlled assist-control combined with volume guarantee (PC-AC + VG) were the top three modes used (ranking scores: 4·47, 4·09, and 3·92 points, respectively) (Fig. S1E). High-frequency oscillation ventilation (HFOV) was employed as a rescue therapy after conventional MV failure by 91·7% of the physicians, whereas only 36·9% chose it as the initial support modality for EPIs (Fig. S1F).
Caffeine therapy
A total of 59·1% (233/394) of the respondents reported routinely using caffein in patients receiving MV, and 29·2% (115/394) of them preferred to initiate caffeine therapy before weaning patients from ventilators. A total of 82·5% (325/394) of the doctors chose to start caffeine treatment as early as possible for preterm babies whose gestational age was less than the median gestational age of 32 weeks and whose birth weight was less than 1500 g.
Bedside lung ultrasound
Bedside lung ultrasound (LUS) was performed in 54·3% (214/394) of the hospitals (Table 4). Tertiary hospitals had great access to bedside LUS than secondary hospitals did (61·6% vs. 30·4%, p < 0·001). The primary applications of LUS are shown in Fig. S2. In the 180 hospitals without bedside LUS, lower-tier cities faced ultrasound machine shortages (1st-tier 36·2% vs. 2nd-tier cities 45·0% vs. 3rd-tier and lower-tier cities 61·8%, p = 0·006).
Ancillary facility construction
Only 30·2% (119/394) of the hospitals possessed a qualified breast milk bank equipped to perform testing, sterilization, storage, and distribution. There was a greater proportion of tertiary hospitals than secondary hospitals (33·4% vs. 19·6%, p = 0·011), and there were fewer general hospitals than paediatric specialty hospitals (26·1% vs. 36·6%, p = 0·028). Home-like wards were available in only 36·0% (142/394) of the hospitals, with more paediatric specialty hospitals than general hospitals having these wards (48·4% vs. 28·2%, p < 0·001). Family Integrated Care (FICare) was available in 63·7% of the hospitals. A greater number of tertiary hospitals and paediatric specialty hospitals implemented FICare in their daily care (tertiary hospitals: 67·9% vs. secondary hospitals: 50·0%, p = 0·002). Among all these regions, central China had the highest proportion of these hospitals (81·8%) (Table S4). The comparison data are shown in Table 4.
Medical insurance
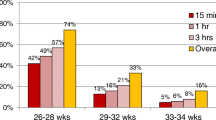
Approximately 90% of RDS patients in the hospitals were eligible for medical insurance reimbursement. The highest percentage (95%) was observed in Central China (Fig. 3A). The percentage of medical insurance reimbursement expenses concerning overall hospitalization expenses was approximately 60%, with Southern China having the highest proportion at 65% (Fig. 3B).
Discussion
The MUNICH is a cross-sectional, nationwide online survey that aimed to provide a comprehensive overview of the current landscape of RDS care by collecting data from 394 neonatologists (hospitals) across China. The survey explored numerous facets of RDS management and concluded that neonatologists in China are well equipped with essential expertise for the effective treatment of RDS patients. As a result, several areas needing further improvement were identified, including low numbers of doctors and nurses per bed, a lower antenatal corticosteroid utilization rate among outborn infants, relatively conservative oxygen therapy use in DRs, less use of the LISA method, insufficient infrastructure support, and considerable inconsistencies in RDS care among hospitals. To our knowledge, this is the first national survey considering various aspects of RDS treatment in China.
In the survey, we observed median doctors per bed and nurses per bed ratios of 0·27 and 0·72, respectively. These figures closely align with previously published data (doctors per bed, 0.26; nurses per bed, 0.70) [19]. Disparities among different types of hospitals pose additional challenges. Paediatric specialty hospitals, which had more patients, experienced lower staffing ratios for both doctors and nurses. This imbalance may result in increased workload, potentially leading to lower job satisfaction and staff turnover, creating a detrimental cycle. A recent review revealed that the ratio of nurses per bed in neonatology departments in LMICs varies significantly, indicating an insufficient and inequitable distribution of health workers and a heavy workload in LMICs [20]. Presently, there are no globally accepted recommendations for staffing ratios in neonatology departments. However, UK standards by Bliss suggest that there should be a minimum nurse-to-baby ratio of 1:1 for intensive care [21]. A 1:1 NICU nursing staffing ratio has been associated with reduced in-hospital mortality, whereas understaffing increases the risk of nosocomial infections in very-low-birth-weight babies [21, 22]. Improving staff allocation and reducing imbalances may be pivotal steps in China.
Approximately 90·0% of preterm infants born at < 34 weeks of gestation received antenatal corticosteroids (any dose). The CHNN reported antenatal corticosteroid use in 75·6% of infants born at < 32 weeks of gestation [4]. In the US, 88·1% of mothers with extremely preterm babies receive antenatal corticosteroids [23]. Notably, there has been increasing emphasis on antenatal corticosteroid use over time [24,25,26]. However, our data highlight a potential concern: a lower antenatal corticosteroid utilization rate among outborn infants, with emergency labour being a primary factor. Therefore, mothers at high risk of preterm birth require systematic pregnancy management and should be transferred to experienced perinatal centres.
Our data revealed that 82·0% of the DRs in the hospitals had an oxygen blender, which was slightly lower than the 91% reported in Europe [27]. The initial FiO2 setting was 0·30 for babies born at ≤ 32 weeks of gestation and 0·25 for those born at > 32 weeks of gestation, which appeared more conservative than what has been recommended in guidelines and consensuses [7, 8]. Notably, general hospitals tended to have higher initial FiO2 settings than paediatric specialty hospitals did. Regarding target oxygen saturation after the first 10 min postnatally, tertiary hospitals tended to be more consistent with the guidelines and consensuses than secondary hospitals did (90·0%-95·0% vs. 88·0%-95·0%, p < 0·05). The overall availability rate of TPRs in the DRs was 77·8%. Disparities were identified among hospitals and across regions. Despite the common perception of greater development in East and South China, less developed Northwest China exhibited better leadership in this respect. This could be attributed to the efforts and emphasis placed on the regional neonatal network and neonatal societies in Northwest China. Moreover, only 12% of infants born at < 32 weeks of gestation received CPAP in the DR according to CHNN data, which is significantly lower than 79% reported in European data [27]. This highlights a substantial gap between possession and utilization, underscoring the urgent need for future TPR promotion and training [4]. Of the 48 respondents who had only bag-valve-mask resuscitators in the DR, the first step to improvement is to have the right equipment in place.
There are certain inconsistencies concerning where, when, and how to administer PS. Surfactant was not available in the DRs in 48·4% of the birthing centres, and maternity-child healthcare hospitals had greater access to surfactant than general hospitals did. The reasons cited included the inability to obtain surfactant from the hospital pharmacy, lack of reimbursement before birth, and potential refusal by parents to cover the cost of PS. These issues underscore the challenges of medication access in DRs and prenatal communication, necessitating multidisciplinary collaboration for resolution. Doctors have displayed a variety of approaches in which FiO2 is used as an indicator for PS administration in patients receiving NIV, despite many studies suggesting that a FiO2 of 0·30 predicts CPAP failure and the need for PS [28,29,30,31,32]. According to our data, 49·7% of the hospitals performed LISA. A previous survey indicated that the LISA adoption rate was 52% in Europe, and a recent survey in Turkey reported that it was 81·6%, emphasizing the growing prevalence of LISA/MIST techniques in recent years [33, 34]. In China, the LISA/MIST utilization rate is relatively low, and more training is needed.
The survey revealed significant variations and imbalances among different hospitals, with tertiary hospitals and paediatric specialty hospitals having greater access to advanced medical resources. Less developed regions face more technical barriers, and medical insurance policies vary across different districts. This situation is a result of both socioeconomic and medical factors. Drawing upon the data from the MUNICH, the following inferences can be made: (1) NICU admission criteria for hospitals of various levels and types and centralized management of extremely and very preterm infants or newborns requiring advanced life support are needed. Ideally, in utero transfers can be performed for high-risk pregnant mothers. (2) Training sessions such as DR resuscitation strategies, LISA/MIST techniques, and ventilation strategies are needed. (3) The promotion of ancillary facilities, including qualified breast milk banks, home-like wards, and FICare is needed to enhance medical care in key hospitals. (4) Improvements in medical insurance policies and reimbursement rates are requested to provide solid support for the treatment of preterm infants. Next, we need to work with the Subspecialty Group of Neonatology and CHNN to widely disseminate the latest RDS guidelines and consensuses, and a future survey with a larger sample size is advisable. Today, the paediatricians’ responsibility is not only to heal, but also to promote the child’s well-being (mental, physical and social) [35]. Through these efforts, it is hoped that neonatologists will be better able to assist families in making the right decisions from a medical, social, political and economic perspective.
This survey has several limitations. First, collecting data online does not ensure the accuracy of the numerical values obtained. Second, discrepancies may arise between the perspectives of physicians and the actual information. Third, the sample size of paediatric specialty hospitals included in this survey was relatively limited, which may restrict the generalizability of the findings to the broader population of neonatal RDS patients. Notably, the sample distribution was not fully aligned with what was planned due to response variations. Moving forward, our next step involves collaborating closely with the Subspecialty Group of Neonatology and CHNN to ensure broad dissemination of the updated RDS guidelines and consensuses. Additionally, an expanded survey with a larger cohort is expected to enrich our insights.
Conclusions
Chinese medical practitioners possess the necessary expertise to address the diverse requirements associated with RDS care effectively. However, certain deficiencies and significant variations exist. Enhancing staff allocation, upgrading DR facilities and medications, overcoming gaps in key techniques, fostering multidisciplinary collaboration, and developing ancillary facilities will contribute to the overall improvement in RDS management.
Availability of data and materials
The datasets generated during and/or analyzed during the current survey are available from the corresponding author upon reasonable request.
Abbreviations
- CHNN:
-
Chinese Neonatal Network
- CI:
-
Confidence interval
- CPAP:
-
Continuous positive airway pressure
- DR:
-
Delivery room
- EPI:
-
Extremely preterm infant
- FICare:
-
Family integrated care
- FiO2 :
-
Fraction of inspired oxygen
- HFOV:
-
High-frequency oscillation ventilation
- INSURE:
-
INtubation-SURfactant-Extubation
- LISA:
-
Less invasive surfactant administration
- LMICs:
-
Low- and middle-income countries
- LUS:
-
Lung ultrasound
- MIST:
-
Minimally invasive surfactant therapy
- MUNICH:
-
Medical sUrvey of NICU Insight in China
- MV:
-
Mechanical ventilation
- NIV:
-
Non-invasive ventilation
- NICU:
-
Neonatal intensive care unit
- PC-AC:
-
Pressure-controlled assist-control
- PS:
-
Pulmonary surfactant
- PSV:
-
Pressure support ventilation
- RDS:
-
Respiratory distress syndrome
- SIMV:
-
Synchronized intermittent mandatory ventilation
- TPR:
-
T-piece resuscitator
- VG:
-
Volume guarantee
References
Wu J, Wang Y, Zhao A, Wang Z. Lung ultrasound for the diagnosis of neonatal respiratory distress syndrome: a meta-analysis. Ultrasound Q. 2020;36(2):102–10.
Reuter S, Moser C, Baack M. Respiratory distress in the newborn. Pediatr Rev. 2014;35(10):417–28.
You H, Huang X. Effect of pulmonary surfactant on the prevention of neonatal respiratory distress syndrome in premature infants. Am J Transl Res. 2021;13(4):3642–9.
Cao Y, Jiang S, Sun J, Hei M, Wang L, Zhang H, et al. Assessment of neonatal intensive care unit practices, morbidity, and mortality among very preterm infants in China. JAMA Netw Open. 2021;4(8):e2118904.
Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller A-B, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–72.
Serra G, Miceli V, Albano S, Corsello G. Perinatal and newborn care in a two years retrospective study in a first level peripheral hospital in Sicily (Italy). Ital J Pediatr. 2019;45(1):152.
Sweet DG, Carnielli VP, Greisen G, Hallman M, Klebermass-Schrehof K, Ozek E, et al. European consensus guidelines on the management of respiratory distress syndrome: 2022 update. Neonatology. 2023;120(1):3–23.
The Subspecialty Group of Neonatology tSoP, Chinese Medical Association, the Editorial Board, Chinese Journal of Pediatrics. Consensus for pulmonary surfactant therapy in neonates in China (2021). Chin J Pediatr. 2021;59(8):627–32.
Kim HS, Lee S, Kim JH. Real-world evidence versus randomized controlled trial: clinical research based on electronic medical records. J Korean Med Sci. 2018;33(34):e213.
National Health Commission of the People’s Republic of China. Statistical bulletin of China’s health development in 2021. Chin Pract J Rural Dr. 2022;29(9):1–11.
Li J, Yang J, Shou H, Zhang L, Huang X, Tang X, et al. Real-world outcomes of niraparib treatment in patients with ovarian cancer: a multicenter non-interventional study in China. Cancer Commun (Lond). 2023;43(6):716–9.
National Health and Family Planning Commission of the People’s Republic of China. Guidelines on the construction and management of critical newborn treatment center. J Dev Med. 2018;6(1):7–14.
Hei M, Li X, Shi Y, Cao Y, Sun J, Wu H, et al. Chinese Neonatal Network: a national protocol for collaborative research and quality improvement in neonatal care. BMJ Open. 2022;12(5):e051175.
Wu W, Gu XY, Shi JY, GP Z. The mortality of very preterm infant with intrauterine operations and/or invasive prenatal interventions in Chinese neonatal network in 2019: a cohort study. Chin J Evid Based Pediatr. 2022;17(5):325–30.
Li QP, Feng ZC, DJ C. Current situation and challenges in management of extremely premature infants. Chin J Perinat Med. 2021;24(11):801–5.
Piro E, Serra G, Schierz IAM, Giuffrè M, Corsello G. Neonatal ten-year retrospective study on neural tube defects in a second level University Hospital. Ital J Pediatr. 2020;46(1):72.
Giuffrè M, Verso CL, Serra G, Moceri G, Cimador M, Corsello G. Portal vein thrombosis in a preterm newborn with mutation of the MTHFR and PAI-1 genes and sepsis by Candida parapsilosis. Am J Perinatol. 2016;33(11):1099–103.
Schierz IAM, Serra G, Antona V, Persico I, Corsello G, Piro E. Infant developmental profile of Crisponi syndrome due to compound heterozygosity for CRLF1 deletion. Clin Dysmorphol. 2020;29(3):141–3.
Li Q, Han T, Zhang Y, Zhang Q, Kong X, Yang Y, et al. A nationwide survey on neonatal medical resources in mainland China: current status and future challenges. BMC Pediatr. 2019;19(1):436.
Bolan N, Cowgill KD, Walker K, Kak L, Shaver T, Moxon S, et al. Human resources for health-related challenges to ensuring quality newborn care in low- and middle-income countries: a scoping review. Glob Health Sci Pract. 2021;9(1):160–76.
Watson S, Arulampalam W, Petrou S, Marlow N, Morgan A, Draper E, et al. The effects of a one-to-one nurse-to-patient ratio on the mortality rate in neonatal intensive care: a retrospective, longitudinal, population-based study. Arch Dis Child Fetal Neonatal Ed. 2016;101(3):F195-200.
Rogowski JA, Staiger D, Patrick T, Horbar J, Kenny M, Lake ET. Nurse staffing and NICU infection rates. JAMA Pediatr. 2013;167(5):444–50.
Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013–2018. JAMA. 2022;327(3):248–63.
Jiang S, Yan W, Li S, Zhang L, Zhang Y, Shah PS, et al. Mortality and morbidity in infants <34 weeks’ gestation in 25 NICUs in China: a prospective cohort study. Front Pediatr. 2020;8:33.
Collaborative Study Group for Extremely Preterm and Extremely Low Birth Weight Infants, Collaborative Study Group for Extremely Preterm Extremely Low Birth Weight Infants. [The morbidities of extremely preterm and extremely low birth weight infants during hospitalization]. Chin J Pediatr. 2015;53(5):334–40.
Kong X, Xu F, Wu R, Wu H, Ju R, Zhao X, et al. Neonatal mortality and morbidity among infants between 24 to 31 complete weeks: a multicenter survey in China from 2013 to 2014. BMC Pediatr. 2016;16(1):174.
Trevisanuto D, Gizzi C, Gagliardi L, Ghirardello S, Di Fabio S, Beke A, et al. Neonatal resuscitation practices in Europe: a survey of the Union of European neonatal and perinatal societies. Neonatology. 2022;119(2):184–92.
Dargaville PA, Aiyappan A, De Paoli AG, Dalton RGB, Kuschel CA, Kamlin CO, et al. Continuous positive airway pressure failure in preterm infants: incidence, predictors and consequences. Neonatology. 2013;104(1):8.
Gulczyńska E, Szczapa T, Hożejowski R, Borszewska-Kornacka MK, Rutkowska M. Fraction of inspired oxygen as a predictor of CPAP failure in preterm infants with respiratory distress syndrome: a prospective multicenter study. Neonatology. 2019;116(2):171–8.
Kruczek P, Krajewski P, Hożejowski R, Szczapa T. FiO2 before surfactant, but not time to surfactant, affects outcomes in infants with respiratory distress syndrome. Front Pediatr. 2021;9:734696.
Dell’Orto V, Nobile S, Correani A, Marchionni P, Giretti I, Rondina C, et al. Early nasal continuous positive airway pressure failure prediction in preterm infants less than 32 weeks gestational age suffering from respiratory distress syndrome. Pediatr Pulmonol. 2021;56(12):3879–86.
Multicenter Study Collaborative Group for Evaluation of Outcomes in Very Low Birth Weight Infants. Failure of non-invasive continuous positive airway pressure as the initial respiratory support in very preterm infants: a multicenter prospective cohort study. Chin J Pediatr. 2021;59(4):273–9.
Öncel MY, Erdeve Ö. A national survey on use of less invasive surfactant administration in Turkey. Turk J Pediatr. 2020;62(5):787–94.
Klotz D, Porcaro U, Fleck T, Fuchs H. European perspective on less invasive surfactant administration-a survey. Eur J Pediatr. 2017;176(2):147–54.
Serra G, Giuffrè M, Piro E, Corsello G. The social role of pediatrics in the past and present times. Ital J Pediatr. 2021;47(1):239.
Acknowledgements
We would like to thank all the physicians who participated in the MUNICH survey. This survey was supported by Chiesi China. Chiesi China had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Funding
This survey was supported by Chiesi China. Chiesi China had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Consortia
Contributions
Long Chen: Writing – review & editing, Writing – original draft, Methodology, Data curation, Formal analysis. Yong Ji: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Rong Ju: Writing – review & editing, Methodology, Data curation, Formal analysis. Jiang-Qin Liu: Writing – review & editing, Methodology, Data curation, Formal analysis. Ling Liu: Writing – review & editing, Methodology, Investigation, Formal analysis. Jingyun Shi: Writing – review & editing, Writing – original draft, Investigation, Formal analysis. Hui Wu: Writing – review & editing, Methodology, Data curation, Supervision/oversight. Lili Wang: Writing – review & editing, Writing – original draft, Data curation, Investigation. Falin Xu: Writing – review & editing, Writing – original draft, Data curation, Formal analysis. Chuanzhong Yang: Writing – review & editing, Conceptualization/design, Methodology, Supervision/oversight. Huayan Zhang: Writing – review & editing, Conceptualization/design, Methodology, Supervision/oversight. Yuan Shi: Writing – review & editing, Conceptualization/design, Methodology, Supervision/oversight. All authors reviewed the results and approved the final version of the manuscript. All the authors contributed equally.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of the Children’s Hospital of Chongqing Medical University (Approval Number: 2022–376). All the physicians provided informed consent before participating in the survey.
Consent for publication
Not applicable.
Competing interests
All authors declared no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, L., Ji, Y., Ju, R. et al. A nationwide survey on the management of neonatal respiratory distress syndrome: insights from the MUNICH survey in 394 Chinese hospitals. Ital J Pediatr 50, 168 (2024). https://doi.org/10.1186/s13052-024-01741-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13052-024-01741-7