Abstract
Background
The profile of changes in airway driving pressure (dPaw) induced by positive-end expiratory pressure (PEEP) might aid for individualized protective ventilation. Our aim was to describe the dPaw versus PEEP curves behavior in ARDS from COVID-19 patients.
Methods
Patients admitted in three hospitals were ventilated with fraction of inspired oxygen (FiO2) and PEEP initially adjusted by oxygenation-based table. Thereafter, PEEP was reduced from 20 until 6 cmH2O while dPaw was stepwise recorded and the lowest PEEP that minimized dPaw (PEEPmin_dPaw) was assessed. Each dPaw vs PEEP curve was classified as J-shaped, inverted-J-shaped, or U-shaped according to the difference between the minimum dPaw and the dPaw at the lowest and highest PEEP. In one hospital, hyperdistention and collapse at each PEEP were assessed by electrical impedance tomography (EIT).
Results
184 patients (41 including EIT) were studied. 126 patients (68%) exhibited a J-shaped dPaw vs PEEP profile (PEEPmin_dPaw of 7.5 ± 1.9 cmH2O). 40 patients (22%) presented a U (PEEPmin_dPaw of 12.2 ± 2.6 cmH2O) and 18 (10%) an inverted-J profile (PEEPmin_dPaw of 14,6 ± 2.3 cmH2O). Patients with inverted-J profiles had significant higher body mass index (BMI) and lower baseline partial pressure of arterial oxygen/FiO2 ratio. PEEPmin_dPaw was associated with lower fractions of both alveolar collapse and hyperinflation.
Conclusions
A PEEP adjustment procedure based on PEEP-induced changes in dPaw is feasible and may aid in individualized PEEP for protective ventilation. The PEEP required to minimize driving pressure was influenced by BMI and was low in the majority of patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Hypoxemic respiratory failure is the leading cause of intensive care unit (ICU) admission in COVID-19, the majority of subjects meeting Acute Respiratory Distress Syndrome criteria (C-ARDS) [1]. Initially, it was observed that many patients presented a disparity between well-preserved lung mechanics and severe hypoxemia [2] and 2 different phenotypes in C-ARDS were proposed, which should be managed with different ventilatory strategies [2]. However, this was not confirmed in posterior published data, remaining recommendations to treat C-ARDS accordingly ARDS ventilation evidence-based [3]. Several hypotheses were proposed to the wide range of respiratory system compliance (Crs) observed in many C-ARDS series, including hypoxemia due to impaired perfusion in patients with higher compliance or lungs with high recruitability and lower compliance [2].
Optimal positive end-expiratory pressure (PEEP) has been pursued [4] and the question of how to recognize patients that get benefit from higher PEEP levels has led to new technologies like Electrical Impedance Tomography (EIT), a bedside tool to monitor ventilation distribution, allowing PEEP titration to reduce both collapse and hyperdistention [5].
Airway driving pressure (dPaw) is a simple parameter to monitor on the ventilator and, when diminished with increased PEEP was associated with reduced mortality risk in ARDS [6]. In C-ARDS, a lower dPaw was associated with better survival [7, 8].
In the present study, we aim to describe the profile of PEEP-induced changes in dPaw during a PEEP adjustment procedure as aid for individualized protective ventilation, including a group where it was done together with an EIT monitor.
Methods
Patients
In this prospective observational physiologic study, adults patients admitted to the ICU of three hospitals with C-ARDS confirmed by positive nasopharyngeal polymerase chain reaction for SARS-CoV-2 and receiving invasive mechanical ventilation (MV) ≤ 48 h were analyzed. Patients with barotrauma assessed by computed tomography (CT), chronic pulmonary disease, and increased intracranial pressure were excluded.
Mechanical ventilation settings
After analgesia and sedation adjustment, all subjects were initially ventilated in volume-controlled ventilation, tidal volume of 6 mL/kg with constant inspiratory flow, plateau pressure ≤ 30 cmH2O, FiO2 and PEEP adjusted to keep SaO2 > 90% based on the ARDSNetwork table [9] and respiratory rate to maintain normal partial pressure of carbon dioxide (PaCO2). Fluids and vasopressors were provided to maintain mean arterial pressure above 60 mmHg and, neuromuscular blocking used to avoid ventilatory asynchronies.
PEEP adjustment procedure
After initial ventilatory settings, PEEP was reduced, 2 cmH2O every thirty seconds [10], from 20 until 6 cmH2O while dPaw was assessed in each step, and the lowest PEEP that minimized dPaw (PEEPmin_dPaw) was identified. The posterior PEEP adjustment was at the discretion of the clinical team responsible for patient care.
EIT assessment
In one of the hospitals, patients were investigated by EIT (Enlight 1800, Timpel, São Paulo, Brazil) during the PEEP adjustment procedure. Regional variations in impedance (∆Z) during ventilation, map the Vt distribution in the lung and creates a PEEP titration tool which was used to assess PEEP-induced pulmonary hyperdistention and collapse and its effects on dPaw during the PEEP adjustment procedure. The EIT optimal PEEP (PEEPEIT) was defined as the PEEP that represents the best compromise between hyperdistention and collapse estimated [5, 11].
Evaluation of dPaw vs PEEP curve profile
After the PEEP adjustment procedure, each dPaw vs PEEP curve was recorded and retrospectively classified into one of three categories according to the difference between the minimum dPaw [12] and the dPaw at the lowest (ΔdPlow) and highest (ΔdPhigh) PEEP [4]. If ΔdPlow < 0.2 × ΔdPhigh, the curve was classified as J-shaped; if ΔdPhigh < 0.2 × ΔdPlow, the curve was classified as inverted-J-shaped; otherwise, the curve was U-shaped.
Statistical analysis
Results are reported without imputation as mean (standard deviation), or count (percentage), after testing for normality using the Shapiro–Wilk test. One-way ANOVA was used for the comparison between the three groups. A Bonferroni-Holm post hoc test was applied to correct multiple testing. Hyperdistention and collapse curves at different PEEP levels were assessed by computing areas under the curves (AUCs) [13] by adding the areas under each pair of consecutive observations:
where Y was the estimated hyperdistention or collapse. The AUCs were compared only between U-shaped and J-shaped PEEP vs dPaw groups, because just one patient with Inverted-J shape had EIT measurement.
Statistical analysis was performed in R (The R Foundation, Vienna, Austria), and a p < 0.05 was considered significant.
Results
Between Jul 27th, 2020, and Feb 24th, 2021, a total of 184 patients were included, and a PEEP adjustment procedure was performed before 48 h on invasive MV. Table 1 shows clinical characteristics in each curve profile dPaw vs PEEP. Patients with inverted J-Shaped dPaw versus PEEP profile presented significantly higher body mass index (BMI) (Table 1) and lower partial pressure of arterial oxygen and fraction of inspired oxygen ratio (PaO2/FiO2) and Crs at baseline (Table 2).
Respiratory mechanics and PEEP titration
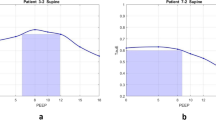
Based on the analysis of the dPaw vs PEEP profile, most of the COVID-19 patients (n = 126) exhibited a J-shaped dPaw vs PEEP profile with dPaw starting to increase for PEEPs ≥ 7.5 ± 1.9 cmH2O, only a few COVID-19 patients had mostly inverted-J profiles (n = 18), usually requiring higher levels of PEEP (PEEPmin_dPaw ranging from 14 to 20 cmH2O) (Table 2, Fig. 1). Only 21.7% of COVID-19 patients presented the U-shaped profile with the PEEPmin_dPaw ranging from 10 to 14 cmH2O.
Respiratory system mechanics associated with the percentage of collapse and hyperdistention at different levels of PEEP. In panels A, D, and G, data were obtained by electrical impedance tomography, where ● is the respiratory system compliance; Δ is the percentage of collapse and □ is the percentage of overdistension. Panels B, C, E, F, H, and I show the percentage change in driving pressure obtained by a mechanical ventilator for a representative patient (B, E, H) and all patients (C, F, I). Panels A–C correspond to the category of patients with J-shaped curves; panels D–F correspond to the category of patients with U-shaped curves, and panels G–I correspond to the category of patients with inverted J-shaped curves
The J-shaped dPaw vs PEEP profile was associated with increased hyperdistention, and collapse reduction as PEEP increased and, in this group, PEEPmin_dPaw was lower than PEEP based on the ARDSNetwork table (Table 2). At the range of the PEEPmin_dPaw both hyperdistention and collapse were minimized independent of the dPaw vs PEEP profile (Table 2, Fig. 1).
Discussion
Our study interpreted the dPaw vs PEEP curve profile among C-ARDS patients. The main findings were: (1) 90% of C-ARDS-19 patients presented a J- or U-shaped dPaw vs PEEP curve profile usually requiring PEEPs < 12 cmH2O to minimize dPaw; (2) PEEPs > 15 cmH2O would be necessary in only 10% of C-ARDS, and those patients presented an inverted-J dPaw vs PEEP curve profile and higher BMI; and (3) PEEPmin_dPaw was associated with a reduction of both alveoli collapse and hyperdistention. All these patients averaged PaO2/FiO2 below 150 which there is evidence of benefit from using higher levels of PEEP in ARDS [14].
ARDS and C-ARDS are heterogeneous conditions with uncertainty about to set PEEP [2, 3] commonly based by oxygenation targets [9]. However in C-ARDS this strategy frequently resulted in worse lung mechanics [15], and cardiac output impairment [16].
Our EIT data and an experimental CT study [4] show that, at constant VT, dPaw and compliance respond to both hyperdistention and collapse. 126/184 of our patients presented a J-shaped curves, with the largest hyperdistention AUC, where increasing PEEP to improve oxygenation may not work. In U-shaped curves the balanced risk of collapse and hyperdistention was obtained with about 12 cmH2O PEEP. In these two groups, higher PEEPs would carry a greater risk of iatrogenesis. Finally, patients with an inverted-J-shaped required higher PEEPs to minimize dPaw and presented higher BMI and lower initial PaO2/FiO2 ratio. In the only patient with this profile on EIT, PEEP decreased collapsed areas without increasing hyperdistention up to 20 cmH2O. The interpretation of the PEEP with respiratory system mechanics or with the amount of recruitment and overdistension on EIT seems to give the same information.
At least one-third of patients were obese in C-ARDS different cohorts [3, 7, 8], even though the effect of obesity on respiratory mechanics is well known, a relationship between BMI and compliance has not been described as an explanation, at least in part, for the COVID-19 different phenotypes. Obesity reduce Crs with the major contribution coming from the lung and not the chest wall [17] in spite of no significant association between compliance and BMI has been detected in a large cohort study of C-ARDS [18]. Mezidi et al. comparing a group of obese vs non-obese in C-ARDS patients monitoring esophageal pressure in a decremental PEEP trial demonstrated a significant difference in PEEP level for the same transpulmonary driving pressure (∆PL) and dPaw [19]. ∆PL also did not enhance significant information concerning the prediction of outcome in ARDS patients compared to dPaw itself [20].
Limitations
The observational nature of this study is its major limitation, and although data were acquired prospectively, they were interrogated retrospectively. The heavy workload upon COVID-19 pandemic made impossible to perform a clinical trial comparing clinical outcomes considering the observed profiles. The small proportion of patients investigated with EIT did not allow an appropriate comparison between the two methods, but data suggest a similar result to obtain the best PEEP for protective ventilation with a much simpler bedside procedure.
Conclusion
The dPaw vs PEEP curve is a feasible method and provides individualized information. A range of compliance and PEEPmin_dPaw was observed in all 3 groups and its interpretation suggested that just in a minority of C-ARDS patients, higher PEEP improves compliance, and even in these cases, it appears that obesity, together with disease severity, determines this behavior. The overall influence of personalizing PEEP on clinical outcomes remains to be determined.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ARDS:
-
Acute Respiratory Distress Syndrome
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- C-ARDS:
-
ARDS from COVID-19
- COVID-19:
-
Corona Virus Disease-19
- Crs:
-
Respiratory system compliance
- ∆P L :
-
Transpulmonary driving pressure
- dPaw:
-
Airway driving pressure
- EIT:
-
Electrical impedance tomography
- FiO2 :
-
Fraction of inspired oxygen
- IBW:
-
Ideal body weight
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- PEEP:
-
Positive end-expiratory pressure
- PEEPEIT :
-
EIT optimal PEEP
- PEEPmin_dPaw :
-
The lowest PEEP that minimized dPaw
- PaO2/FiO2 :
-
Partial pressure of arterial oxygen and fraction of inspired oxygen ratio
- V t :
-
Tidal volume
- SOFA:
-
Sequential organ failure assessment score
- ∆Z :
-
Regional variation in impedance
- ΔdPlow:
-
Driving pressure at lowest PEEP
- ΔdPhigh:
-
Driving pressure at highest PEEP
References
Rubenfeld GD, Thompson T, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute Respiratory Distress Syndrome: the Berlin definition. JAMA. 2012. https://doi.org/10.1001/jama.2012.5669.
Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099–102.
Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, Hernández M, Gea A, Arruti E, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med. 2020;46(12):2200–11.
Carvalho AR, Spieth PM, Pelosi P, Vidal Melo MF, Koch T, Jandre FC, et al. Ability of dynamic airway pressure curve profile and elastance for positive end-expiratory pressure titration. Intensive Care Med. 2008;34(12):2291–9.
Costa ELV, Borges JB, Melo A, Suarez-Sipmann F, Toufen C, Bohm SH, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009;35(6):1132–7.
Amato MBP, Meade MO, Slutsky AS, Brochard L, Costa ELV, Schoenfeld DA, et al. Driving pressure and survival in the Acute Respiratory Distress Syndrome. N Engl J Med. 2015;372(8):747–55.
Ferreira JC, Ho YL, Besen BAMP, Malbouisson LMS, Taniguchi LU, Mendes PV, et al. Protective ventilation and outcomes of critically ill patients with COVID-19: a cohort study. Ann Intensive Care. 2021;11(1):92.
Estenssoro E, Loudet CI, Ríos FG, Kanoore Edul VS, Plotnikow G, Andrian M, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9(9):989–98.
The Acute Respiratory Distress Syndrome Network. Ventilation with Lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
Bergamini BC, Carvalho NS, Medeiros DM, Bozza FA, Giannella-Neto A, Carvalho AR. Time-Dependence influence on mechanical properties of the respiratory system in two strategies for decremental positive end-expiratory pressure titration. Em: A48 mechanical ventilation [Internet]. American Thoracic Society; 2012 [citado 17 de janeiro de 2023]. p. A1685–A1685. Disponível em. https://doi.org/10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A1685
Perier F, Tuffet S, Maraffi T, Alcala G, Victor M, Haudebourg AF, et al. Electrical impedance tomography to titrate positive end-expiratory pressure in COVID-19 Acute Respiratory Distress Syndrome. Crit Care Lond Engl. 2020;24(1):678.
Bergamini BC, Carvalho NS, Medeiros DM, Bozza FA, Giannella-Neto A, Carvalho AR. Time-dependence influence on mechanical properties of the respiratory system in two strategies for decremental positive end-expiratory pressure titration. Em: A48 mechanical ventilation [Internet]. American Thoracic Society; 2012 [citado 13 de janeiro de 2023]. p. A1685–A1685 (American Thoracic Society International Conference Abstracts). Disponível em. https://doi.org/10.1164/ajrccm-conference.2012.185.1_MeetingAbstracts.A1685
Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–5.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, et al. Higher vs lower positive end-expiratory pressure in patients with acute lung injury and Acute Respiratory Distress Syndrome: systematic review and meta-analysis. JAMA. 2010;303(9):865–73.
Protti A, Santini A, Pennati F, Chiurazzi C, Cressoni M, Ferrari M, et al. Lung response to a higher positive end-expiratory pressure in mechanically ventilated patients with COVID-19. Chest. 2022;161(4):979–88.
Dell’Anna AM, Carelli S, Cicetti M, Stella C, Bongiovanni F, Natalini D, et al. Hemodynamic response to positive end-expiratory pressure and prone position in COVID-19 ARDS. Respir Physiol Neurobiol. 2022;298:103844.
Fumagalli J, Berra L, Zhang C, Pirrone M, Santiago RRDS, Gomes S, et al. Transpulmonary pressure describes lung morphology during decremental positive end-expiratory pressure trials in obesity. Crit Care Med. 2017;45(8):1374–81.
Li Bassi G, Suen JY, Dalton HJ, White N, Shrapnel S, Fanning JP, et al. An appraisal of respiratory system compliance in mechanically ventilated covid-19 patients. Crit Care. 2021;25(1):199.
Mezidi M, Daviet F, Chabert P, Hraiech S, Bitker L, Forel JM, et al. Transpulmonary pressures in obese and non-obese COVID-19 ARDS. Ann Intensive Care. 2020;10(1):129.
Chen L, Grieco DL, Beloncle F, Chen GQ, Tiribelli N, Madotto F, et al. Partition of respiratory mechanics in patients with Acute Respiratory Distress Syndrome and association with outcome: a multicentre clinical study. Intensive Care Med. 2022;48(7):888–98.
Acknowledgements
The authors thank the management of Instituto Nacional de Infectologia Evandro Chagas/Fundacão Oswaldo Cruz and Hospital Universitário Pedro Ernesto/Universidade do Estado do Rio de Janeiro for their support of the study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES). In addition, we thank the physiotherapy team, mainly Érica Paixão da Costa, for her contribution to this study.
Funding
This research was supported by Instituto Nacional de Infectologia Evandro Chagas, Fundação Oswaldo Cruz (INI/Fiocruz), Rio de Janeiro, Brasil. Brazilian Council for Scientific and Technological Development (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grants Nos. 302702/2017-2 and 302839/2017-8) and the Rio de Janeiro State Research Supporting Foundation (Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, Grants Nos. E26/211.867/2016, E-26/202.785/2017, E-26/203.001/2018). Institutional Incentive Program for Scientific, Technological and Artistic-Cultural Production—PROCIÊNCIA 2019-2020, from the Federal Institute of Education, Science and Technology, Rio de Janeiro (IFRJ).
Author information
Authors and Affiliations
Contributions
MRC developed the acquisition and monitoring software for the bedside PEEP titration curve profile, collected, analyzed, and interpreted the patient data regarding PEEP titration, respiratory mechanics, and blood gas and was a major contributor to writing the manuscript. LMC developed the acquisition and monitoring software for the bedside PEEP titration curve profile, analyzed and interpreted the patient data regarding PEEP titration, and respiratory mechanics, and was a major contributor to writing the manuscript. GMR and TCX developed the acquisition and monitoring software for the bedside PEEP titration curve profile, analyzed and interpreted the patient data regarding PEEP titration, and respiratory mechanics, and was a major contributor to writing the manuscript. AMJ analyzed, and interpreted the patient data regarding PEEP titration, respiratory mechanics, blood gas, and clinical features. DMM analyzed, and interpreted the patient data regarding PEEP titration, respiratory mechanics, blood gas, and clinical features and was a major contributor to writing the manuscript. LFR collected, analyzed, and interpreted the patient data regarding PEEP titration, respiratory mechanics, blood gas, and clinical features. BG collected, analyzed, and interpreted the patient data regarding PEEP titration in Electrical Impedance Tomography. ARC analyzed and interpreted all patient data and was a major contributor to writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Protocols were approved by institutional review boards of the National Institute of Infections Diseases (CAAE 31050420.8.2001.5262), Pedro Ernesto University Hospital (CAAE 31050420.8.1001.5259), Brazilian clinical trials (RBR-2z3f7k).
Consent for publication
Not applicable.
Competing interests
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
da Cruz, M.R., Camilo, L.M., da Costa Xavier, T.B. et al. Positive end-expiratory pressure induced changes in airway driving pressure in mechanically ventilated COVID-19 Acute Respiratory Distress Syndrome patients. Crit Care 27, 118 (2023). https://doi.org/10.1186/s13054-023-04345-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-023-04345-5