Abstract
Background
High glucose levels are key factors and key contributors to several cardiovascular diseases associated with cardiomyocyte injury. Ferroptosis, which was identified in recent years, is a mode of cell death caused by the iron-mediated accumulation of lipid peroxides. Neuregulin-4 (Nrg4) is an adipokine that has protective effects against metabolic disorders and insulin resistance. Our previous study revealed that Nrg4 has a protective effect against diabetic myocardial injury, and the aim of this study was to investigate whether Nrg4 could attenuate the occurrence of high glucose-induced ferroptosis in cardiomyocytes.
Methods
We constructed an in vivo diabetic myocardial injury model in which primary cardiomyocytes were cultured in vitro and treated with Nrg4. Changes in ferroptosis-related protein levels and ferroptosis-related indices in cardiomyocytes were observed. In addition, we performed back-validation and explored signalling pathways that regulate ferroptosis in primary cardiomyocytes.
Results
Nrg4 attenuated cardiomyocyte ferroptosis both in vivo and in vitro. Additionally, the AMPK/NRF2 signalling pathway was activated during this process, and when the AMPK/NRF2 pathway was inhibited, the beneficial effects of Nrg4 were attenuated.
Conclusion
Nrg4 antagonizes high glucose-induced ferroptosis in cardiomyocytes via the AMPK/NRF2 signalling pathway.
Similar content being viewed by others
Background
High glucose levels are key factors and key contributors to several cardiovascular diseases associated with cardiomyocyte damage [1]. Long-term diabetes leads to structural and functional alterations in cardiomyocytes, referred to as diabetic cardiomyopathy (DCM) [2]. DCM is a serious and underrecognized complication of diabetes that leads to ventricular diastolic dysfunction and eventually to the development of heart failure [3]. Therefore, treatment strategies for DCM need to be further explored [4].
Discovered in recent years, ferroptosis is a mode of cell death caused by the iron-mediated accumulation of lipid peroxides; the main mechanism is the catalysis of unsaturated fatty acids on the cell membrane in the presence of divalent iron or lipoxygenase (LOX), which induces lipid peroxidation and thus cell death. This process also manifests as a decrease in GPX4, the regulatory core enzyme of the glutathione system [5]. In terms of cell morphology, ferroptosis is mainly characterized by a decrease in mitochondrial volume, an increase in the density of bilayer membranes, and a decrease in or disappearance of mitochondrial cristae [6]. The maintenance of iron homeostasis is essential for normal cardiac function. A growing body of evidence suggests that iron imbalances are common in many cardiovascular disease subtypes. Ferroptosis has been shown to be involved in the pathogenesis of numerous cardiovascular diseases, including atherosclerosis, myocardial ischaemia‒reperfusion injury, and arrhythmias [7]. Emerging literature suggests a key role for ferroptosis in high glucose-induced cardiac injury [8,9,10].
Neuregulin-4 (Nrg4) is a novel adipokine secreted mainly by brown adipose tissue [11, 12]. A growing body of literature suggests that Nrg4 plays an important role in regulating energy metabolism, modulating glucolipid metabolism, and reducing insulin resistance [13, 14]. Lowering the serum Nrg4 concentration may play an important role in the development of diabetes [15]. In addition, recent studies have identified a role for Nrg4 in the cardiovascular system, i.e., in regulating vascular function and improving atherosclerosis [16]. Our previous study revealed that Nrg4 has a protective effect against diabetic myocardial injury; however, whether Nrg4 attenuates high glucose-induced ferroptosis in cardiomyocytes is unclear.
AMPK, a serine-threonine kinase, is often referred to as the cellular "master switch of metabolism". AMPK plays a key role in DCM as an important kinase regulating energy homeostasis [17]. Nuclear transcription factor erythroid 2–related factor 2 (NRF2) is a key regulator of cellular antioxidant responses and plays an important role in antioxidant responses [18]. Emerging evidence suggests that sulforaphane (SFN) can help ameliorate diabetic cardiomyopathy by inhibiting ferroptosis through the modulation of the AMPK/NRF2 pathway [8]. Moreover, our previous study revealed that Nrg4 alleviated diabetic cardiomyopathy by activating autophagy through the AMPK/mTOR signalling pathway [19]. Therefore, we hypothesized that Nrg4 may inhibit cardiomyocyte ferroptosis in a high-glucose environment through the AMPK/NRF2 pathway.
Methods
Experimental animals
Eight-week-old male C57BL/6 J mice were purchased from SPF Biotechnology Co., Ltd. (Beijing, China). All mice were housed under standard conditions (22 ± 1 °C and 12 h light/dark cycle) with free access to food and water. The experiments were approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University and conducted in accordance with the Principles of Laboratory Animal Welfare established by Hebei Medical University.
Model establishment and experimental groups
A type 1 diabetes model was established by the intraperitoneal injection of 50 mg/kg freshly prepared streptozotocin (STZ; Sigma, St. Louis, MO, USA) dissolved in 0.1 mM sodium citrate buffer (pH 4.5) for 5 consecutive days. The control mice received the same volume of sodium citrate buffer. One week after injection, tail vein blood was collected randomly. Glucose concentrations were measured by a glucometer (Accu-Chek, Roche Diagnostics, Basel, Switzerland), and concentrations ≥ 16.7 mmol/L confirmed the successful establishment of the model [20].
Mice were randomly divided into four groups (n = 6 in each group): nondiabetic control (CON) group, type 1 diabetes (DM) group, Nrg4-treated type 1 diabetes (DM + Nrg4) group and Nrg4-treated (CON + Nrg4) group. The DM + Nrg4 and CON + Nrg4 groups were injected intraperitoneally with recombinant Nrg4 (Sino Biological, Inc., Beijing, China) for 4 weeks after successful modelling. The dose (100 μg/kg, 3 times a week) was selected based on the results of previous studies [19, 21]. The same volume of PBS was injected into mice in the CON and DM groups. Fig. S1 illustrates the changes in the metabolic indicators in each group of mice during the experiment. Next, echocardiography was performed to measure cardiac function. Then, the animals were euthanized, and heart tissue was collected.
Cell culture
Primary cardiomyocytes were isolated from neonatal (1 to 3 days) C57BL/6 J mice (3 to 5 mice eath). These cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum and 1% penicillin/streptomycin and then placed in a 95% O2 and 5% CO2 incubator at 37 °C. When the cardiomyocyte population reached a confluence of 50–60%, the cells were exposed to normal glucose (NG) or high glucose (HG) medium. The HG medium was complete DMEM (5.5 mM glucose) supplemented with D-glucose (final concentration, 30 mM) [22]. The NG medium was complete DMEM (5.5 mM glucose) supplemented with 24.5 mM mannitol. The in vitro model of DCM was validated by measuring the protein level of GLUT4, a specific molecular marker of glucose metabolism (Fig. S2). Cell experiments were performed in three steps. In the first step, the cells were incubated in HG or NG for 24 h and then incubated for 12 h in the presence or absence of Nrg4 (100 ng/ml) [21]. In the second step, the cells were incubated in HG or HG supplemented with erastin (MCE; Monmouth Junction, NJ, USA) (10 μM) [23] for 24 h and then incubated for 12 h in the presence or absence of Nrg4 (100 ng/ml). Erastin is a ferroptosis inducer that acts on mitochondria in a ROS- and iron-dependent manner. In the third step, the cells were incubated in HG or HG supplemented with Compound C (10 μM, Comp C; MCE) [24] for 24 h and then incubated for an additional 12 h in the absence or presence of Nrg4 (100 ng/ml). Comp C is a selective, ATP-competitive inhibitor of AMPK.
Echocardiography
The mice were shaved with a small animal shaver to remove chest hair, and the forehead was fully exposed. Mice were anaesthetized via inhalation of isoflurane gas. The mice were fixed on a thermostatically heated plate with the limbs extended, and the ultrasound gel mixture was applied evenly to the chest. An RMV710B ultrasound probe instrument was used for left ventricular short-axis views; left ventricular short-axis two-dimensional ultrasound images were saved, and the left ventricular ejection fraction (LVEF), left ventricular shortening fraction (LVFS), and left ventricular end-diastolic volume (LVEDV) were calculated using M. At least 3 measurements were performed for each mouse, and the mean of 3 cardiac cycles was recorded.
Histological staining
Heart samples were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin and cut into 5-μm thick sections. Haematoxylin and eosin (HE) staining and Masson's trichrome staining were performed. Morphological changes and collagen content of myocardial tissue were assessed via light microscopy.
Transmission electron microscopy (TEM)
First, animal hearts and primary cardiomyocytes were fixed with 4% glutaraldehyde. Next, the tissues or cells were postfixed with osmium tetroxide (1%) and dehydrated in a graded ethanol series (70%, 90% and 100%). Finally, the samples were embedded and cut into ultrathin Sects. (70–80 nm). These sections were examined with an electron microscope operating at 80 kV.
Western blot analysis
Proteins were obtained from heart or cell extracts and analysed using protein blot analysis as previously described [25]. The following primary antibodies were used: anti-TFR1 (1:5000; Abcam), anti-GPX4 (1:1000; ABclonal), anti-ACSL4 (1:1000; ABclonal), anti-SLC7A11 (1:5000; Abcam), anti-AMPK (1:3000; Arigo), anti-p-AMPK (1:3000; CST), anti-NRF2 (1:1000; Affinity), and anti-histone H3 (1:10,000; Bioeasy). ImageJ software was used to analyse the greyscale values. The ratio of the detected protein band density to the GAPDH protein band density was used for statistical analysis.
Determination of tissue iron content
The tissue iron content in the animal heart tissue was measured using a Tissue Iron Content Assay Kit (Solarbio BC4355, China) according to the manufacturer's instructions.
Determination of lipid peroxidation
Lipid peroxidation levels in animal hearts and primary cardiomyocytes were measured using a Lipid Peroxidation (MDA) Assay Kit (Solarbio BC0025, China) according to the manufacturer's instructions.
Determination of GSH and oxidized GSH (GSSG) levels
Glutathione and GSSG levels were measured in animal hearts and primary cardiomyocytes using a Glutathione Content Assay Kit (Solarbio BC1175 and BC1185, China) according to the manufacturer's instructions.
Fe2⁺ detection
Fe2⁺ was detected in primary cardiomyocytes using the FerroOrange probe (Dojindo F374, Japan) according to the manufacturer's instructions.
Statistical analysis
All the statistical analyses were performed using GraphPad Prism 9 software. All the data are expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) and Bonferroni's multiple comparison test were used for comparisons between groups. p < 0.05 was considered to indicate statistical significance.
Results
Nrg4 attenuates myocardial injury in type 1 diabetic mice
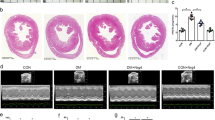
In this study, no unexpected deaths were observed in any group during the experiment. Nrg4 treatment significantly prevented the development of cardiac insufficiency in type 1 diabetic mice. Eight weeks after the successful establishment of the diabetes model, echocardiography revealed cardiac dilatation with dysfunction in the DM group. LVEDV was greater in the DM group than in the CON group, and Nrg4 significantly inhibited this increase. LVEF and LVFS were decreased in the DM group, indicating impaired cardiac function in the diabetic mice. However, treatment with Nrg4 normalized the impaired cardiac function (Fig. 1A–D). In addition, we also examined the pathological structures of myocardial tissues. HE staining revealed the neat and regular arrangement of myocardial fibres and clearly visible nuclei in the CON group. Myocardial cells in the DM group were extensively oedematous with enlarged cell gaps and unclear or even fused nuclei. After Nrg4 treatment, the myocardial cell changes normalized, and the cells were more regularly arranged (Fig. 1E). Masson staining revealed that the myocardial collagen fibres in the CON group were less abundant and more evenly distributed. In contrast, myocardial collagen fibres were increased and unevenly distributed in the DM group, with significant collagen accumulation (stained blue‒purple) occurring mainly in the interstitial and perivascular regions of the myocardium. Treatment with Nrg4 significantly reduced the degree of interstitial fibrosis (Fig. 1F).
Nrg4 attenuates cardiac dysfunction and cardiac fibrosis. A Representative echocardiographic images. B Left ventricular ejection fraction (LVEF). C Left ventricular fraction shortening (LVFS). D Left ventricular end-diastolic volume (LVEDV). E Left ventricle stained with HE (Original magnification × 200) F Interstitial fibrosis of the left ventricles was assessed by Masson staining. The collagen fibres are stained blue (with arrows, original magnification × 200). The data are presented as the means ± SDs; n = 6; *P < 0.05, **P < 0.01, and ***P < 0.0001
High glucose conditions promote ferroptosis, and Nrg4 attenuates ferroptosis
The above results demonstrated the cardioprotective effect of Nrg4 on diabetic cardiomyopathy. We next explored the potential mechanisms of Nrg4. Since ferroptosis is crucial for diabetic cardiomyopathy [8], we wanted to assess whether Nrg4 attenuates myocardial injury by regulating ferroptosis. TEM revealed changes in mitochondrial ultrastructure in the myocardial tissue from mice in each group, and the results showed that the mitochondria in the CON group were regular in shape, with clear boundaries. In the DM group, the size of mitochondria decreased, the density of the bilayer membrane of mitochondria increased, and the number of mitochondrial cristae decreased, but mitochondrial damage was less severe after Nrg4 treatment (Fig. 2A). We assessed the expression of ferroptosis marker proteins in the heart tissue of mice in each group by western blot analysis (Fig. 2B). Compared with those in the CON group, the expression of TFR1 and ACSL4 in the heart tissue of mice in the DM group was significantly higher, and the expression of GPX4 and SLC7A11 was significantly lower. After Nrg4 treatment, TFR1 and ACSL4 expression was significantly lower in the DM + Nrg4 group than in the DM group, and GPX4 and SLC7A11 were significantly higher (Fig. 2C–F). We also measured the Fe2⁺ concentration, MDA concentration, GSH content, and GSH/GSSG ratio in the heart tissue of the mice in each group. Compared with those in the CON group, the contents of Fe2⁺ and MDA in the heart tissues of mice in the DM group were significantly higher, and the GSH content and the GSH/GSSG ratio were significantly lower. After Nrg4 treatment, the contents of Fe2⁺ and MDA in the DM + Nrg4 group were significantly lower than those in the DM group, and the GSH content and GSH/GSSG ratio were significantly higher (Fig. 2G–J). The above results suggest that Nrg4 attenuates ferroptosis in DCM mice.
Nrg4 attenuates ferroptosis in DCM mice. A Representative TEM images depicting mitochondria in myocardial tissue, with arrows indicating mitochondria. B–F Western blot and quantitative analysis of ferroptosis-related proteins (TFR1, GPX4, ACSL4 and SLC7A11) in each group. G Comparison of Fe2 + in each group. H The content of the lipid peroxidation product MDA in each group. I The content of GSH in each group. J GSH/GSSG ratios in each group. The data are presented as the means ± SDs; n = 6; *P < 0.05, **P < 0.01, and ***P < 0.0001; ns, not significant
To further confirm the protective effect of Nrg4 on high glucose-induced ferroptosis in cardiomyocytes, we performed in vitro experiments in cultured primary cardiomyocytes. We examined changes in inflammatory markers (IL-1β, IL-6, and TNF-α) in an in vitro model. The results indicated that Nrg4 still played a protective role in the in vitro model (Fig. S3). We used FerroOrange as a fluorescent probe to fluorescently image intracellular Fe2⁺. The results showed that, compared with those in the NG, the fluorescence intensity was significantly greater and the Fe2⁺ content was higher in the HG group. After Nrg4 treatment, the Fe2⁺ fluorescence intensity was significantly lower and the Fe2⁺ content was lower in the HG + Nrg4 group than in the HG group (Fig. 3A). Ultrastructural changes in the mitochondria of the cells in each group were assessed via TEM, and the results showed that the mitochondria in the NG group were regular in shape with clear boundaries; mitochondria in the HG group were smaller in size, with increased bilayer density and fewer mitochondrial cristae, but mitochondrial damage was less severe after Nrg4 treatment (Fig. 3B). Then, we assessed the expression of ferroptosis marker proteins in each group of cells by western blot (Fig. 3C). The expression of TFR1 and ACSL4 significantly increased in primary cardiomyocytes after high glucose treatment, and the expression of GPX4 and SLC7A11 significantly decreased. After Nrg4 treatment, the expression of TFR1 and ACSL4 significantly decreased, while the expression of GPX4 and SLC7A11 increased (Fig. 3D–G). In addition, we also assessed the MDA and GSH levels and the GSH/GSSG ratio in each group of cells. The MDA content significantly increased in primary cardiomyocytes treated with high glucose, and the GSH content and GSH/GSSG ratio significantly decreased. Treatment with Nrg4 significantly decreased the MDA content and increased the GSH content and the GSH/GSSG ratio (Fig. 3H–J). The above results suggest that Nrg4 attenuates high glucose-induced ferroptosis in primary cardiomyocytes.
Nrg4 attenuates high glucose-induced ferroptosis in primary cardiomyocytes. A Representative immunofluorescence images with Fe2 + indicator (red) staining (original magnification × 200). B representative TEM images depicting mitochondria in vitro, with arrows indicating mitochondria. C–G Western blot and quantitative analysis of ferroptosis-related proteins (TFR1, GPX4, ACSL4 and SLC7A11) in vitro. H The content of the lipid peroxidation product MDA in vitro. I The content of GSH in vitro. J GSH/GSSG ratios in vitro. The data are presented as the means ± SDs; n = 3; *P < 0.05, **P < 0.001, and ***P < 0.0001; ns, not significant
Nrg4-mediated cardiac protection is associated with the attenuation of ferroptosis
Our previous study showed that Nrg4 attenuates ferroptosis induced by high glucose in cardiomyocytes. To determine whether the attenuation of cardiomyocyte injury by Nrg4 inhibition is dependent on ferroptosis, we applied a ferroptosis inducer in the second step of the cellular assay for reverse verification. We used FerroOrange as a fluorescent probe for the fluorescence imaging of intracellular Fe2⁺. The results showed that the fluorescence intensity of Fe2⁺ significantly decreased and Fe2⁺ content decreased after Nrg4 treatment. Compared with Nrg4 treatment alone (HG + Nrg4 group), erastin coadministration inhibited ferroptosis, which was attenuated by Nrg4, as evidenced by the significant increase in Fe2⁺ fluorescence intensity and increase in Fe2⁺ content (Fig. 4A). We subsequently confirmed these findings by TEM, which revealed reduced mitochondrial damage after Nrg4 treatment and increased mitochondrial damage after erastin coadministration (Fig. 4B). We then assessed the expression of ferroptosis marker proteins in each group of cells by western blot (Fig. 4C). Treatment with Nrg4 decreased the levels of TFR1 and ACSL4 and increased the levels of GPX4 and SLC7A11. Compared to those in the HG + Nrg4 group, the protein levels of TFR1 and ACSL4 and the protein levels of GPX4 and SLC7A11 were higher in the erastin coadministration group (Fig. 4D–G). In addition, we assessed the MDA and GSH levels and the GSH/GSSG ratio in the cells of each group. Nrg4 treatment decreased the MDA level and increased the GSH level and the GSH/GSSG ratio. Compared to those in the HG + Nrg4 group, the MDA content and the GSH/GSSG ratio increased and decreased, respectively, in the erastin coadministration group (Fig. 4H–J). The ferroptosis inducer-mediated inhibition of ferroptosis was attenuated by Nrg4, suggesting that Nrg4 attenuates high glucose-induced cardiomyocyte injury by antagonizing ferroptosis.
Erastin attenuates the protective effect of Nrg4 in cardiomyocytes. A Representative immunofluorescence images with Fe2 + indicator (red) staining (original magnification × 200). B Representative TEM images depicting mitochondria in vitro, with arrows indicating mitochondria. C–G Western blot and quantitative analysis of ferroptosis-related proteins (TFR1, GPX4, ACSL4 and SLC7A11) in vitro. H The content of the lipid peroxidation product MDA in vitro. I The content of GSH in vitro. J GSH/GSSG ratios in vitro. The data are presented as the means ± SDs; n = 3; *P < 0.05, **P < 0.01, and ***P < 0.0001
Nrg4 attenuates ferroptosis through the AMPK/NRF2 signalling pathway
Next, we tested the hypothesis that the protective effect of Nrg4 is mediated by the AMPK/NRF2 pathway. We coadministered Comp C (an AMPK inhibitor) and Nrg4 to high glucose–treated cardiomyocytes. Compared with HG, Nrg4 administration increased p-AMPK and NRF2 levels (Fig. 5A–C). These findings indicate that Nrg4 treatment activates the AMPK/NRF2 signalling pathway. The addition of Comp C inhibited AMPK and NRF2 activation; increased the protein levels of TFR1 and ACSL4; and decreased the protein levels of GPX4 and SLC7A11 (Fig. 5D–H). Moreover, the assay results indicated an increase in the MDA content and a decrease in the GSH content and in the GSH/GSSG ratio (Fig. 5I–K). These results suggest that Nrg4 ameliorates ferroptosis at least in part through the AMPK/NRF2 signalling pathway. Subsequently, we performed fluorescence imaging of intracellular Fe2⁺ by using FerroOrange as a fluorescent probe. Compared with Nrg4 treatment alone (HG + Nrg4 group), Comp C coadministration inhibited ferroptosis, which was attenuated by Nrg4, as evidenced by a significant increase in the fluorescence intensity of Fe2⁺ and an increase in the Fe2⁺ content (Fig. 6A). We confirmed these findings by observing mitochondria via TEM. Mitochondrial damage normalized after Nrg4 treatment, whereas damage increased after the coadministration of Comp C and Nrg4 (Fig. 6B). Collectively, these results suggest that AMPK inhibitors attenuate ferroptosis downregulated by Nrg4 and inhibit the protective effect of Nrg4 against myocardial injury.
Nrg4 attenuates ferroptosis through the AMPK/NRF2 signalling pathway. A–C Western blot and quantitative analysis of the expression of p-AMPK and NRF2 in vitro. D–H Western blot and quantitative analysis of ferroptosis-related proteins (TFR1, GPX4, ACSL4 and SLC7A11) in vitro. I The content of the lipid peroxidation product MDA in vitro. J The content of GSH in vitro. K GSH/GSSG ratios in vitro. The data are presented as the means ± SDs; n = 3; *P < 0.05, **P < 0.01, and ***P < 0.0001
Discussion
In this study, we found that Nrg4 can antagonize high glucose-induced ferroptosis in cardiomyocytes through the AMPK/NRF2 signalling pathway.
Nrg4 is a member of the extracellular ligand epidermal growth factor (EGF) family and is particularly enriched in brown adipose tissue (BAT) [26]. Nrg4 plays a key role in the regulation of glucose and lipid metabolism and energy homeostasis [27]. Previous studies have shown that Nrg4 is highly expressed in metabolic organs or tissues to inhibit hepatic adipogenesis and antagonize obesity-related hepatic steatosis and insulin resistance [28, 29]. Its biological activity also plays an important role in ameliorating diabetes [15], alleviating diabetic nephropathy [30], and regulating gluconeogenesis [31]. Recently, it has been shown that Nrg4 also plays a protective role in the cardiovascular system and that Nrg4 attenuates endothelial inflammation and atherosclerosis in male mice [16]. Our findings suggest that Nrg4 has potential therapeutic effects on high glucose-induced cardiomyocyte injury.
We specifically investigated the potential mechanisms by which Nrg4 exerts its therapeutic effects. The role of Nrg4 in metabolic diseases has been confirmed in numerous studies; however, in recent years, an increasing number of studies have suggested that ferroptosis may play a role in the development of various metabolic diseases, such as diabetes and its complications (e.g., diabetic nephropathy, diabetic cardiomyopathy, diabetic myocardial ischaemia/reperfusion injury, and atherosclerosis [AS]), and play important roles in the development of several metabolic diseases, such as diabetic nephropathy, diabetic cardiomyopathy, diabetic myocardial ischaemia/reperfusion injury and atherosclerosis [AS] [32]. For example, emerging studies have demonstrated that canagliflozin attenuates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy [33]. Therefore, the present study focused on ferroptosis, and our findings suggest that ferroptosis was promoted in both type 1 diabetic cardiomyopathy mice and primary cardiomyocytes cultured in high glucose medium. This effect included the appearance of a series of microstructural changes, such as mitochondrial shrinkage and a reduction in mitochondrial cristae; increases in the expression of the ferroptosis marker proteins TFR1 and ACSL4; decreases in the expression of GPX4 and SLC7A11; an increase in the MDA level; and decreases in the GSH level and the GSH/GSSG ratio. In addition, the Fe2⁺ content increased in mouse heart tissue, and the Fe2⁺ fluorescence intensity increased, as did the Fe2⁺ content in primary cardiomyocytes. Moreover, we observed that Nrg4 intervention attenuated these changes, suggesting that Nrg4 mitigates high glucose-induced ferroptosis. According to the results of our follow-up experiments, the attenuation of ferroptosis was partially eliminated when erastin was coadministered with Nrg4. These results suggest that ferroptosis mediates, at least in part, the amelioration of cardiomyocyte injury through Nrg4. Thus, we demonstrated that Nrg4 ameliorates high glucose-induced cardiomyocyte injury by antagonizing ferroptosis.
Finally, we explored the molecular signalling pathways that antagonize ferroptosis through Nrg4. AMPK has many beneficial effects on myocardial inflammation, oxidative stress, and other cardiac metabolic abnormalities [34]. NRF2 is one of the most important antioxidative stress regulators in vivo [35]. AMPK can activate NRF2 through the AMPK/AKT/GSK3β signalling pathway [36], and NRF2 is a key regulator of ferroptosis [35] (Fig. 7). Recently, the therapeutic drug SFN was shown to ameliorate diabetic cardiomyopathy by inhibiting ferroptosis through the modulation of the AMPK/NRF2 pathway [8]. Interestingly, Nrg4 can upregulate AMPK expression levels in diabetic cardiomyopathy [19], and in our study, we demonstrated that Nrg4 administration increased p-AMPK and NRF2 levels, thereby attenuating high glucose-induced ferroptosis in cardiomyocytes, whereas the concomitant administration of Nrg4 and Comp C attenuated the level of ferroptosis that was downregulated by Nrg4. These findings suggest that the antagonistic effect of Nrg4 on ferroptosis-mediated amelioration of high glucose-induced cardiomyocyte injury is dependent on the AMPK/NRF2 signalling pathway. In conclusion, our results confirm that Nrg4 antagonizes ferroptosis through the AMPK/NRF2 signalling cascade.
Overall, the results of our study provide a new perspective on the alleviation of diabetic cardiomyopathy by antagonizing ferroptosis through Nrg4. However, whether Nrg4 can be a clinical agent for the treatment of diabetic cardiomyopathy needs to be further explored.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- DCM:
-
Diabetic cardiomyopathy
- Nrg4:
-
Neuregulin-4
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- NG:
-
Normal glucose
- HG:
-
High glucose
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular shortening fraction
- LVEDV:
-
Left ventricular end-diastolic volume
- HE:
-
Haematoxylin and eosin
- TEM:
-
Transmission electron microscopy
- Comp C:
-
Compound C
References
Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. 2020;126(11):1501–25.
Gao P, Cao M, Jiang X, Wang X, Zhang G, Tang X, Yang C, Komuro I, Ge J, Li L, et al. Cannabinoid receptor 2-centric molecular feedback loop drives necroptosis in diabetic heart injuries. Circulation. 2023;147(2):158–74.
Qiu Y, Buffonge S, Ramnath R, Jenner S, Fawaz S, Arkill KP, Neal C, Verkade P, White SJ, Hezzell M, et al. Endothelial glycocalyx is damaged in diabetic cardiomyopathy: angiopoietin 1 restores glycocalyx and improves diastolic function in mice. Diabetologia. 2022;65(5):879–94.
Jia G, DeMarco VG, Sowers JR. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol. 2016;12(3):144–53.
Jiang X, Stockwell BR, Conrad M. Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol. 2021;22(4):266–82.
Yang J, Zhou Y, Xie S, Wang J, Li Z, Chen L, Mao M, Chen C, Huang A, Chen Y, et al. Metformin induces Ferroptosis by inhibiting UFMylation of SLC7A11 in breast cancer. J Exp Clin Cancer Res. 2021;40(1):206.
Fang X, Ardehali H, Min J, Wang F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat Rev Cardiol. 2023;20(1):7–23.
Wang X, Chen X, Zhou W, Men H, Bao T, Sun Y, Wang Q, Tan Y, Keller BB, Tong Q, et al. Ferroptosis is essential for diabetic cardiomyopathy and is prevented by sulforaphane via AMPK/NRF2 pathways. Acta Pharm Sin B. 2022;12(2):708–22.
Zhao Y, Pan B, Lv X, Chen C, Li K, Wang Y, Liu J. Ferroptosis: roles and molecular mechanisms in diabetic cardiomyopathy. Front Endocrinol (Lausanne). 2023;14:1140644.
Lou X, Zhang Y, Guo J, Gao L, Ding Y, Zhuo X, Lei Q, Bian J, Lei R, Gong W, et al. What is the impact of ferroptosis on diabetic cardiomyopathy: a systematic review. Heart Fail Rev. 2023;29:1.
Gavaldà-Navarro A, Villarroya J, Cereijo R, Giralt M, Villarroya F. The endocrine role of brown adipose tissue: an update on actors and actions. Rev Endocr Metab Disord. 2022;23(1):31–41.
Chen LL, Peng MM, Zhang JY, Hu X, Min J, Huang QL, Wan LM. Elevated circulating Neuregulin4 level in patients with diabetes. Diabetes Metab Res Rev. 2017;33(4):e2870.
Liu Y, Chen M. Neuregulin 4 as a novel adipokine in energy metabolism. Front Physiol. 2022;13:1106380.
Díaz-Sáez F, Blanco-Sinfreu C, Archilla-Ortega A, Sebastian D, Romero M, Hernández-Alvarez MI, Mora S, Testar X, Ricart W, Fernández-Real JM, et al. Neuregulin 4 downregulation induces insulin resistance in 3T3-L1 adipocytes through inflammation and autophagic degradation of GLUT4 vesicles. Int J Mol Sci. 2021;22(23):12960.
Wang Y, Huang S, Yu P. Association between circulating neuregulin4 levels and diabetes mellitus: a meta-analysis of observational studies. PLoS ONE. 2019;14(12): e0225705.
Shi L, Li Y, Xu X, Cheng Y, Meng B, Xu J, Xiang L, Zhang J, He K, Tong J, et al. Brown adipose tissue-derived Nrg4 alleviates endothelial inflammation and atherosclerosis in male mice. Nat Metab. 2022;4(11):1573–90.
Haye A, Ansari MA, Rahman SO, Shamsi Y, Ahmed D, Sharma M. Role of AMP-activated protein kinase on cardio-metabolic abnormalities in the development of diabetic cardiomyopathy: a molecular landscape. Eur J Pharmacol. 2020;888: 173376.
Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z, Zhang Z, Tan Y, Keller BB, Zhou H, et al. Metallothionein is downstream of Nrf2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. 2017;66(2):529–42.
Wang H, Wang L, Hu F, Wang P, Xie Y, Li F, Guo B. Neuregulin-4 attenuates diabetic cardiomyopathy by regulating autophagy via the AMPK/mTOR signalling pathway. Cardiovasc Diabetol. 2022;21(1):205.
Han Z, Zhao D, Han M, Zhang R, Hao Y. Knockdown of miR-372-3p inhibits the development of diabetic cardiomyopathy by accelerating angiogenesis via activating the PI3K/AKT/mTOR/HIF-1α signaling pathway and suppressing oxidative stress. Oxid Med Cell Longev. 2022;2022:4342755.
Zhu B, Mei W, Jiao T, Yang S, Xu X, Yu H, Ding Y, Guo S, Meng B, Zhao L, et al. Neuregulin 4 alleviates hepatic steatosis via activating AMPK/mTOR-mediated autophagy in aged mice fed a high fat diet. Eur J Pharmacol. 2020;884: 173350.
Yang F, Qin Y, Wang Y, Meng S, Xian H, Che H, Lv J, Li Y, Yu Y, Bai Y, et al. Metformin inhibits the NLRP3 inflammasome via AMPK/mTOR-dependent effects in diabetic cardiomyopathy. Int J Biol Sci. 2019;15(5):1010–9.
Li W, Li W, Leng Y, Xiong Y, Xia Z. Ferroptosis is involved in diabetes myocardial ischemia/reperfusion injury through endoplasmic reticulum stress. DNA Cell Biol. 2020;39(2):210–25.
Guo S, Meng XW, Yang XS, Liu XF, Ou-Yang CH, Liu C. Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. Acta Pharmacol Sin. 2018;39(2):195–204.
Wang X, Chen XX, Yu HT, Tan Y, Lin Q, Keller BB, Zheng Y, Cai L. Engineered cardiac tissues: a novel in vitro model to investigate the pathophysiology of mouse diabetic cardiomyopathy. Acta Pharmacol Sin. 2021;42(6):932–41.
Li M, Chen Y, Jiang J, Lu Y, Song Z, Zhang S, Sun C, Ying H, Fan X, Song Y, et al. Elevated serum neuregulin 4 levels in patients with hyperthyroidism. Endocr Connect. 2019;8(6):728–35.
Tutunchi H, Ostadrahimi A, Hosseinzadeh-Attar MJ, Miryan M, Mobasseri M, Ebrahimi-Mameghani M. A systematic review of the association of neuregulin 4, a brown fat-enriched secreted factor, with obesity and related metabolic disturbances. Obes Rev. 2020;21(2): e12952.
Wang GX, Zhao XY, Meng ZX, Kern M, Dietrich A, Chen Z, Cozacov Z, Zhou D, Okunade AL, Su X, et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat Med. 2014;20(12):1436–43.
Pfeifer A. NRG4: an endocrine link between brown adipose tissue and liver. Cell Metab. 2015;21(1):13–4.
Shi J, Xu W, Zheng R, Miao H, Hu Q. Neuregulin 4 attenuate tubulointerstitial fibrosis and advanced glycosylation end products accumulation in diabetic nephropathy rats via regulating TNF-R1 signaling. Am J Transl Res. 2019;11(9):5501–13.
Zhang L, Bai M, Tang H, Zhou F, Zhu Q, Wang S, Zhu K, Liu Q, Liu Y, Wang X, et al. Role of hepatic neuregulin 4 in the regulation of gluconeogenesis in mice. Life Sci. 2019;217:185–92.
Duan JY, Lin X, Xu F, Shan SK, Guo B, Li FX, Wang Y, Zheng MH, Xu QS, Lei LM, et al. Ferroptosis and its potential role in metabolic diseases: A curse or revitalization? Front Cell Dev Biol. 2021;9: 701788.
Du S, Shi H, Xiong L, Wang P, Shi Y. Canagliflozin mitigates ferroptosis and improves myocardial oxidative stress in mice with diabetic cardiomyopathy. Front Endocrinol (Lausanne). 2022;13:1011669.
Zhang W, Lu J, Wang Y, Sun P, Gao T, Xu N, Zhang Y, Xie W. Canagliflozin attenuates lipotoxicity in cardiomyocytes by inhibiting inflammation and ferroptosis through activating AMPK pathway. Int J Mol Sci. 2023;24(1):858.
Wei Z, Shaohuan Q, Pinfang K, Chao S. Curcumin attenuates ferroptosis-induced myocardial injury in diabetic cardiomyopathy through the Nrf2 pathway. Cardiovasc Ther. 2022;2022:3159717.
Sun Y, Zhou S, Guo H, Zhang J, Ma T, Zheng Y, Zhang Z, Cai L. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism. 2020;102: 154002.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (81400217), Natural Science Foundation of Hebei Province (H202006504), and the Central Guidance for Local Science and Technology Development Funds Project (236Z7738G).
Author information
Authors and Affiliations
Contributions
PW and BG designed the study. PW, XG and HW carried out the experiments. PW, LW and MM collected and analysed the data. PW and XG wrote the manuscript. BG supervised the experiments and revised the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Second Hospital of Hebei Medical University (permit number 2021-AE059).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Fig. S1
Changes in the metabolic indicators in each group of mice during the experiment. (A) The blood glucose level of diabetic mice increased significantly after modelling. It then showed a downwards trend in the later stage of the experiment but remained higher than 16.7 mmol/L (random blood glucose). (B) Changes in the body weights of mice. (C) Changes in the food intake of mice. (D) Changes in the water intake of mice.
Additional file 2. Fig. S2
Specific molecular markers of glucose matabolism GLUT4 protein levels. The protein levels of GLUT4 are decreased in DCM in vitro, and Nrg4 treament can restore protein expression. (A–B) Western blot and quantitative analysis of the expression of GLUG4 in vitro.
Additional file 3. Fig. S3
Nrg4 inhibits inflammation in vitro. The levels of the inflammatory factors IL-1β, IL-6, and TNF-α are increased in DCM in vitro, and Nrg4 treatment can reduce these changes. (A) The content of IL-β in vitro. (B) The content of IL-6 in vitro. (C) The content of TNF-α in vitro.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wang, P., Guo, X., Wang, H. et al. Neuregulin-4 protects cardiomyocytes against high-glucose-induced ferroptosis via the AMPK/NRF2 signalling pathway. Biol Direct 19, 62 (2024). https://doi.org/10.1186/s13062-024-00505-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13062-024-00505-x