Abstract
Background
Malaria parasites such as Plasmodium knowlesi, P. inui, and P. cynomolgi are spread from macaques to humans through the Leucosphyrus Group of Anopheles mosquitoes. It is crucial to know the distribution of these vectors to implement effective control measures for malaria elimination. Plasmodium knowlesi is the most predominant zoonotic malaria parasite infecting humans in Malaysia.
Methods
Vector data from various sources were used to create distribution maps from 1957 to 2021. A predictive statistical model utilizing logistic regression was developed using significant environmental factors. Interpolation maps were created using the inverse distance weighted (IDW) method and overlaid with the corresponding environmental variables.
Results
Based on the IDW analysis, high vector abundances were found in the southwestern part of Sarawak, the northern region of Pahang and the northwestern part of Sabah. However, most parts of Johor, Sabah, Perlis, Penang, Kelantan and Terengganu had low vector abundance. The accuracy test indicated that the model predicted sampling and non-sampling areas with 75.3% overall accuracy. The selected environmental variables were entered into the regression model based on their significant values. In addition to the presence of water bodies, elevation, temperature, forest loss and forest cover were included in the final model since these were significantly correlated. Anopheles mosquitoes were mainly distributed in Peninsular Malaysia (Titiwangsa range, central and northern parts), Sabah (Kudat, West Coast, Interior and Tawau division) and Sarawak (Kapit, Miri, and Limbang). The predicted Anopheles mosquito density was lower in the southern part of Peninsular Malaysia, the Sandakan Division of Sabah and the western region of Sarawak.
Conclusion
The study offers insight into the distribution of the Leucosphyrus Group of Anopheles mosquitoes in Malaysia. Additionally, the accompanying predictive vector map correlates well with cases of P. knowlesi malaria. This research is crucial in informing and supporting future efforts by healthcare professionals to develop effective malaria control interventions.
Graphical Abstract

Similar content being viewed by others
Background
Malaria is a significant global health concern that continues to cause fatalities and illnesses, especially in tropical areas [1]. The abundance of mosquito vectors, which thrive in suitable environments, such as those characterized by high humidity, precipitation, temperature and climate seasonality, contributes to the high incidence rate of malaria [2]. Although synthetic insecticides have been utilized to control these vectors, the growing resistance of malaria vectors to chemicals poses a threat to malaria prevention [2, 3]. Therefore, there is a need for more effective control measures to address this issue.
Although Plasmodium knowlesi has become more prevalent in recent years, the emergence of Plasmodium cynomolgi and Plasmodium inui as zoonotic malaria in Southeast Asia has complicated measures aimed at malaria elimination [4,5,6]. The WHO reports that countries will not achieve malaria elimination status if the number of P. knowlesi cases remains high [7]. Studies have identified several mosquito species from the Leucosphyrus Group of the genus Anopheles, including An. cracens [8, 9], An. latens [10], An. balabacensis [11, 12], and An. introlatus [13], that can transmit P. knowlesi to humans.
Most strategies for controlling malaria have focused on eliminating the disease in humans. This is understandable since humans account for the majority of malaria cases worldwide. However, the idea that simian malaria would rarely spill over into humans, proposed in the 1960s, is now outdated [14]. Recent developments have shown that zoonotic malaria is now a significant public health concern in Southeast Asia. Therefore, before declaring malaria eradication status, it is crucial to reconsider the threat of simian malaria and establish monitoring and control strategies [15,16,17,18]. Hence, mapping the distribution of simian malaria vectors in Southeast Asia is essential to the implementation of effective surveillance and control measures to eliminate the disease.
Comprehending the spatial and temporal pattern of simian malaria vectors is crucial, as it helps identify hotspot areas for vector abundance and allocating resources. Many studies have effectively used global geospatial techniques in mosquito environmental research [19,20,21,22]. These tools have also helped predict habitat suitability, which aids in designing optimal mosquito vector control strategies based on precise spatial and temporal information databases [23,24,25]. Geospatial mapping has the potential to identify larval habitats covering a large geographic area, which may be difficult or impossible to obtain through field surveys [20, 26].
Due to deforestation and changes in land use, certain Anopheles species from the Leucosphyrus Group have become more prevalent in farms and villages [27, 28]. However, there is still a need for high-quality knowledge on the distribution of these vectors throughout Malaysia. The spatial distribution of simian malaria vectors is crucial to determining effective vector control strategies but, unfortunately, there is currently a lack of information on their distribution throughout the country. Understanding the transmission patterns and geographical distribution of simian malaria parasites in Malaysia is essential for developing efficient disease control strategies and identifying how ecologies affect the risks of simian malaria. Therefore, this study aims to create a geographical distribution map and a predictive risk map based on the ecology of specific vectors of P. knowlesi. This information will enable possible interventions that can be used for vector control.
Methods
Data search
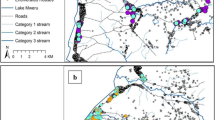
Relevant information on the Leucosphyrus Group of Anopheles mosquitoes was obtained through a combination of: (i) an extensive search of published articles on the Anopheles leucosphyrus sensu lato (A. leucosphyrus s.l.) mosquitoes between 1957 and 2021; (ii) mosquito sample collections carried out in the course of the present study from June 2019 until January 2021 in Malaysia (Fig. 1), the details of which regarding the sample collection are described in a previous study [29]; and (iii) direct contacts with district officers for Anopheles collection sites coordinates or unpublished research data were used for the spatial analysis. Online platform databases, such as PubMed, Medline and Google Scholar, were searched used to identify relevant studies on distribution of the simian malaria vector. We also compiled information from gray literature, such as hard copies of old publications, reports, thesis and dissertations pertinent to the research.
Geo-positioning procedures
The surveyed geographic coordinates were determined using electronic resources, including GeoNet Names Server (http://earth-info.nga.mil), Wikimapia (http://www.wikimapia.org) and Google Earth (http://www.earth.google.com), which are freely available online. The identified location from one source was cross-checked against the other sources to confirm the consistency of the coordinates. Each collection area's coordinates were recorded using the Google Maps coordinate system. The recorded coordinates were then transferred to Microsoft Excel 2016 (Microsoft Corp., Redmond, WA, USA) to compile all the data for further analysis. All digital data and geographical coordinate were synchronized using the World Geodetic System (WGS1984; https://gisgeography.com/wgs84-world-geodetic-system/), which served as the x- (longitude or east–west) and y-coordinates (latitude or north–south), thereby allowing geographic positions to be expressed anywhere worldwide. The base map and all the environmental data were generated from freely available online sources reported in Table 1. The data were then exported and stored in ArcGIS 10.4.1 software (ESRI, Redlands, CA, USA) for further analysis.
Spatial cluster analysis
The distribution of the Leucosphyrus Group of Anopheles mosquitoes was mapped at the district and sub-district levels. Each coordinate was plotted as point features, creating a new geographic information system (GIS) layer representing the point locations of the mosquitoes. Distribution data on some Leucosphyrus Group of Anopheles mosquitoes from 1957 to 2021 were included in this map separately from the mosquito samplings conducted from 2019 to 2021 for the present study [8, 9, 11,12,13,14, 26, 27, 30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58].
The distribution of the Leucosphyrus Group of Anopheles mosquitoes was determined using the average nearest neighbor (ANN) analysis to calculate the ANN ratio (R) (Table 2) based on the observed average distance between the nearest mosquito location to determine the distribution pattern of the Anopheles mosquitoes.
The ANN analysis provides statistical values, such as R, Z-scores and P values. The distribution pattern of mosquitoes was used to determine the R value. When R < 1, the distribution of the Anopheles mosquitoes indicates clustering; when R > 1, the distribution pattern of the Anopheles mosquitoes is dispersing. The Z-scores were used to validate the calculated R-value to determine the significance of rejecting the null hypothesis.
Inverse distance weighted interpolation method
An interpolation map was created to predict the risk area based on the Leucosphyrus Group of Anopheles mosquitoes’ coordinates by the inverse distance weighted (IDW) interpolation method. The IDW map was created from mosquito distribution data from 1957 up to 2021 and overlaid with the environmental variables, thus allowing the correlation of risk areas and the environmental factors to be observed. ArcGIS 10.4.1 (ESRI) software was used to perform all of the interpolation calculations. The IDW analysis formula was:
where, \(\widehat{v}\) = estimated value; Vi = known value; and di…, dn = distances between the n data points and the estimated n.
Statistical analysis
The logistic regression test was performed using SPSS software (Statistics 23; SPSS IBM Corp., Armonk, NY, USA) to identify the significant environmental variables linked to Anopheles mosquito abundance and develop a statistical risk model. The environmental variables were selected and entered into the regression model based on their significant values. The accuracy of the model was determined using regression analysis, which was divided into two groups: (i) sampling sites (218 locations); and (ii) random sampling sites (220 locations). The random sampling sites were created by ArcGIS 10.4.1 software (ESRI). Hosmer and Lemeshow goodness-of-fit tests were used to assess whether the model fits the observed data. The spatial autocorrelation test (Moran’s I-test) was applied to determine whether mosquito distribution patterns were clustered, scattered or randomly distributed. The I-test calculates the mean of each value at each site and compares it to the mean value of all locations. Moran’s I-test values range from − 1, which reflects a strong negative correlation, to + 1 which depicts a strong positive correlation. A Moran’s I-test value of 0 denotes a spatially random pattern.
Moran’s I-test was performed using ArcGIS 10.4.1 software (ESRI) [59]. The best fit logistic regression model was used to produce a predictive risk map based on the abundance of mosquitoes. The equation of the logistic regression model is denoted by:
where βi is the regression coefficient for variable xi.
This equation can be re-written as:
.
(p) can be calculated by rearranging this equation as follows:
Results
Distribution of Leucosphyrus Group of Anopheles mosquitoes from 1957 to 2021
This database contains information on the whereabouts of the Leucosphyrus Group of Anopheles mosquitoes in Malaysia, and all locations have been successfully geopositioned (Additional file 1: Table S1). Figures 2 and 3 depict the overall geographical distribution of Anopheles mosquitoes and their distribution every 10 years. The ANN analysis reveals a consistent clustering pattern of Anopheles mosquitoes, with a nearest neighbor ratio (R) < 1 for overall distribution and every 10 years (Table 3). However, for the years 1988–1997, a dispersed pattern was observed (R > 1). The negative Z-score values indicate that clustering occurred randomly for almost every 10 years, except for the 1988–1997 period.
Spatial interpolation
The IDW interpolation method was utilized to analyze spatial interpolation based on vector data collected between 1957 and 2021. Predictions for Anopheles mosquito abundance were categorized by color, ranging from low (yellow) to moderate (orange) to high (red). To enhance the visual representation, maps displaying environmental factors, such as forest loss, forest cover, elevation, temperature and water bodies, were overlaid onto the prediction map. The IDW results revealed that a high number of vectors could be observed in the southwest part of Sarawak (Betong, Kapit, Bintulu, Miri, Sibu, Mukah, Sri Aman, Song and Sarikei), Kedah (Baling), Perak (Hulu Perak and Kerian), Pahang (Kuala Lipis, Jerantut, Bentong, Temerloh, Bera, Maran and Kuantan), Negeri Sembilan (Jelebu, Tampin and Jempul), Kelantan (Gua Musang, Kota Baharu, Jeli, Pasir Mas and Tumpat), Terengganu (Hulu Terengganu, Dungun and Kemaman), Selangor (Kuala Langat, Kuala Selangor and Hulu Selangor), Johor (Mersing), Melaka (Alor Gajah and Jasin) and the northwest part of Sabah (Kudat, Ranau, Penampang, Papar, Tuaran and Beaufort). In contrast, most parts of Johor, Sabah, Perlis, Penang, Kelantan, and Terengganu had low vector abundance (Fig. 4).
High elevations in Pahang (Titiwangsa range), Sarawak (Sarawak-Kalimantan border range and northeast part) and Sabah (northwest and southwest part) were locations with moderate and low mosquito abundance (Fig. 4a). The orange and red zones of Sabah, Sarawak, Pahang, Perak, Kelantan, Terengganu and Kedah are associated with high forest cover (Fig. 4c). Areas with high and moderate mosquito abundance exhibit noticeable tree loss across Malaysia (Fig. 4d). Low temperature (Fig. 4e) was linked with high elevations in Malaysia (Fig. 4b). Water bodies were also present near zones with moderate and high mosquito abundance (Fig. 4f). Maps of the environmental factors are shown in Additional file 2: Figure S1.
Statistical and spatial analysis
Based on ecological factors, this study used logistic regression analysis to predict the abundance of Anopheles mosquitoes in unsampled areas. The results showed that elevation, forest cover, forest loss and temperature were significant factors affecting the distribution of the Leucosphyrus Group of Anopheles mosquitoes (Table 4). However, water bodies were excluded from the final model as they were not statistically significant. The model's accuracy in predicting sampled and non-sampled areas was 75.3%, with 73% accuracy for sampled areas and 77.6% accuracy for non-sampled areas. The model's accuracy was reliable, as most non-sampled areas were predicted with P < 0.5, and sampled areas were predicted at P > 0.5. The Hosmer and Lemeshow goodness-of-fit value was statistically significant, indicating that the model's estimates fit well with the data. The spatial distribution of mosquitoes was evaluated using Moran’s I-index, which showed a significantly positive spatial autocorrelation for the distribution of the Leucosphyrus Group of Anopheles within districts, indicating that mosquito distribution was more spatially clustered.
Hence, our logistic regression model is:
Which can be arranged as follows:
Modeled distribution of Leucosphyrus Group of Anopheles
A logistic regression model was used to predict the distribution of the Leucosphyrus Group of Anopheles mosquitoes based on environmental factors such as elevation, forest cover, forest loss and temperature. The results showed a higher predicted distribution of Anopheles mosquitoes (indicated by red–orange coloration) in certain areas, including the Titiwangsa range, central and northern parts of Peninsular Malaysia, Kudat Division, West Coast Division, Interior Division and Tawau Division of Sabah, as well as Kapit, Lawas, Marudi, Belaga, Song and Sri Aman of Sarawak. Meanwhile, the predicted distribution of Anopheles mosquitoes was lower (indicated by shades of green coloration) in the southern part of Peninsular Malaysia (mainly in Johor, Perlis, Melaka, Selangor, Kuala Lumpur and Penang), the eastern part of Sabah (mainly in Pitas, Sandakan, Sempurna, Beluran and Kuala Penyu), and the western region of Sarawak (primarily Kuching, Serian, Betong and Mukah) (Fig. 5).
Discussion
Using GIS, in this study we have outlined the distribution of the Anopheles Leucosphyrus Group of mosquitoes in Malaysia. The information gathered from previous published studies and from current field data is crucial for identifying and managing malaria vectors. The study also suggests that this approach could facilitate the planning and advancement of vector management strategies. The results show significant geographic variation in the distribution of Anopheles throughout Malaysia, which opens the door to an opportunity to effectively target prevention efforts where they are needed most, particularly when resources are limited.
We found that the distribution of malaria vectors, particularly in Malaysian Borneo, has remained largely unchanged from 1957 to 2021. The primary simian malaria vectors vary by region, with An. balabacensis prevalent in Sabah and Sarawak [11, 12, 60], An. latens in Sarawak [10, 53], An. cracens in Pahang [8, 9] and An. introlatus occurring in Peninsular Malaysia and also observed in Sarawak [12]. However, An. introlatus was only confirmed as a vector in Selangor [13]. Our distribution maps illustrate these findings. Recent studies have also identified An. introlatus as a vector of simian malaria in Peninsular Malaysia [61].
Analysis of the geospatial data from 1957 to 2021 revealed that Anopheles mosquitoes tended to cluster in certain areas. This was shown in the ANN analysis, which also revealed clustering patterns among different Anopheles spp. of the Leucosphyrus Group. There were clusters of > 2 species in Selangor from 1957 to 1967, in Negeri Sembilan from 1968 to 1977) and in Sarawak from 1998 to 2007. Anopheles hackeri, Anopheles pujutensis, An. introlatus, and An. latens were found in Selangor, while An. pujutensis, Anopheles macarthuri, An. introlatus and An. latens were found in Negeri Sembilan and Sarawak. These species thrived in these areas due to the presence of their ideal habitat conditions, such as optimal temperature for larval and adult mosquito development [62], clean water bodies for breeding sites [63] and proximity to forested or deforested areas for easy access to humans and animals for blood meals [64, 65].
Developing an interpolation map based on species distribution datasets can help identify vector-prone areas and plan effective vector control programs. Some entomological and vector studies have used interpolation techniques, such as the IDW method, to predict mosquito species abundance in non-sampled areas [66,67,68,69,70]. Despite limited data in some parts of the country, the interpolation map estimates vector abundance. In the present study, high vector abundances were observed in Sarawak's southwestern region, Pahang’s northern region and Sabah's northwestern region. Environmental variables, such as forest cover, deforestation, elevation, water bodies and temperature, can significantly influence vector abundance [62,63,64,65, 71, 72].
The Anopheles Leucosphyrus Group of mosquitoes are forest-dwelling, typically found in forested and agricultural settings [13]. Furthermore, Anopheles larval habitats are found in highly shaded, clean, natural water pockets or puddles near rivers [32]. Thus, the predicted high vector abundance is near forested and water bodies areas. Since Anopheles lives in a shaded, humid and moist environment [73], this environment offers clean and suitable water bodies for Anopheles mosquito breeding sites. With deforestation, long-tailed macaques (Macaca fascicularis) and pig-tailed macaques (Macaca nemestrina) [74], the natural host of simian malaria in Malaysia, have migrated to the forest fringes, and these mosquitoes may have trailed them there and subsequently colonized forest fringes. Therefore, vector abundance is high where deforestation is noted since the vector is localized in the disturbed natural environment where it is observed to have high biting rates in agricultural areas and forest fringes [9, 53, 75]. It is possible that the Leucosphyrus Group of mosquitoes were not prevalent during human malaria entomological studies [26, 76] conducted in Peninsular Malaysia because they may have been residing in the densely forested regions during that time.
The presence of vectors in a particular habitat is affected by elevation and temperature. Elevated areas typically have lower temperatures, which can limit the reproduction and growth of Anopheles mosquitoes, resulting in fewer of them being observed [77, 78]. As a result, this study found that vector abundance was lower in areas with both high elevation and low temperatures. The high number of vectors noted in the IDW interpolation map was thus influenced by factors such as forest loss, forest cover, elevation, and water bodies and temperature.
The logistic regression model for predicting the knowlesi malaria vector performed well when analyzing the covariate data. The resulting predictive map also aligns well with the actual knowlesi malaria cases recorded in a previous study [79]. The risk of contracting knowlesi malaria is higher in forested and deforested areas, with the highest vector population. This was determined through an interpolation map that overlaid forest cover and forest loss covariates. Additionally, the predicted high-risk zones for knowlesi malaria are mainly around the Titiwangsa range and central-northern region of Peninsular Malaysia [79]; the same pattern was noticed from the predictive vector map from this study. The association between the predictive vector and human case maps would further support the model's accuracy and reliability.
Furthermore, most of these published data were published more than a few years back and applying that to the given day is doubtful. Despite these drawbacks, the existing data provide a reasonably estimated distribution of simian malaria vectors in Malaysia. Although the predictive model can be used to visualize the entire distribution of vectors based on environmental parameters, some variables also give valuable information for the prediction, such as the distribution of simian malaria infection and case characteristics like gender, age, and occupation. Indeed, such details were not included in the present mapping survey, but they could be a focus for future research. Consequently, we must know that this data is difficult to obtain on a broad spatial scale. Apart from such limitations, the information in the current database can assist in identifying and emphasizing where such information is lacking and, therefore, may collect the necessary data for further research on the geographical distribution of vectors in the country. In addition, the study used freely available databases to create a predictive map, a cost-effective way to support entomological surveillance efforts.
This map can help identify areas or populations with the greatest needs. It considers factors such as forest cover and loss, which can affect the abundance of disease-carrying insects. The map predicts that Kedah, Pahang, Kelantan, Terengganu, Selangor, Negeri Sembilan, Sabah, and Sarawak likely have the highest vector distribution. These areas have increased forest cover and forest loss, more water bodies, low elevation, and moderate temperatures.
Forest cover effects on anopheline abundances during the dry and wet seasons can be linked to the behavior of adults and larvae formations [80]. Water quality is influenced by forest cover through shading, organic matter inputs, and erosion processes [81]. These factors impact water quality and facilitate vector breeding sites [82]. Vectors and their hosts correlate with the forest. Some mosquitos are zoophilic and feed on animals [83], commonly more abundant in forested areas. When forested areas are replaced by agricultural land, the plants can still offer the bushy cover that some Anopheles mosquito species or larval development stages require. Thus, it increases the rate of mosquito densities [84,85,86]. Anopheles balabacensis, the primary simian malaria vector in Sabah [11], is a forest-dwelling species with larval development that prefers humid, shaded water conditions [87]. Anopheles balabacensis abundances in Sabah have recently been higher in the disturbed, cleared forest, plantations, and farms than in undisturbed primary and secondary forested areas. An example of how land use can affect the ecology of a vector can be seen in Sabah [11, 36, 39].
The man biting rate of An. donaldi and An. letifer was higher in forested areas of Sarawak than in villages [37]. However, when deforestation occurred and palm oil plantations were established over four years, the vector population declined [88]. On the other hand, another study in Peninsular Malaysia found that the man-biting rate of the Anopheles Leucospyrus Group of mosquitoes was higher in forested areas and remained relatively similar in agricultural areas in Sungai Dara (Perak), Kem Sri Gading, (Pahang), Kampung Lalang (Kelantan) and Bukit Tinggi and Gunung Panti (Johor)[61]. These findings suggest that forest clearing disrupts habitats and brings different ecosystems closer together, creating new environments at the forest edge [89]. Deforestation can also alter the microclimate, vegetation, and soil composition, bringing a new environment for vectors [90, 91] and adapting the new ecology. Therefore, the abundance of vectors tends to be higher in areas where there has been forest loss.
The immature stages of malaria vector mosquitoes, such as eggs, larvae, and pupae, can be found in surface water [92]. Using satellite data to monitor these water bodies is valuable in identifying the source of these disease-carrying mosquitoes. However, it's important to note that the assumption that vector abundance is negatively correlated with water bodies may not be accurate. This is because most of the sampled locations were near water bodies but not within them, which failed to obtain the necessary data for analysis. To improve future studies, conducting Anopheles larval surveys and including them in predictive maps is crucial, as larval habitat presence is a direct approach to predicting vector density. It is essential for researchers to carefully evaluate the study's outcomes, as one study found similar limitations and discussed ways to improve their analysis [92]. This limitation highlights the importance of identifying Anopheles larval sites near sampling areas, considering the prevalence of water body characteristics, as female Anopheles mosquitoes require water to complete their life cycle [93].
The density of vectors is generally lower in the highlands compared to the nearby lowlands, as observed in this study [94,95,96]. The elevation and temperature are linked, and as the temperature decreases with increasing elevation, malaria vectors' density and species diversity may also vary [96]. Temperature also affects the development rates of juveniles, the duration of the gonotrophic cycle, and the survival of both juvenile and adult phases at an optimal temperature [71]. Anopheles mosquitoes’ survival is directly influenced by environmental temperature during juvenile and adult stages. A warmer atmosphere promotes rapid growth and smaller adults [98]. High temperatures speed up the evaporation rate of water pools and reduce pool lifetime, thus, mosquito immatures have limited time to reach the adult phase. Studies have shown that Anopheles larvae cannot survive at temperatures higher than 35 °C [62, 97]. Higher temperatures can speed up blood meal digestion, shorten gonotrophic cycles, and alter mosquitoes' reproduction ability [98]. At temperatures below 17 °C, malaria vectors fail to survive [99]. Therefore, vector abundance is low at both high and low temperatures as they require an ideal temperature for their life history.
Conclusion
This study provides important information about the distribution of simian malaria vectors in Malaysia from 1957 to 2021. The study also includes a predictive map of vector abundance based on environmental factors. This information can help reduce malaria by allowing authorities to focus on areas with high transmission rates. Understanding the environment is crucial because it can increase or decrease the number of vector breeding sites. According to the study, water bodies, areas with ideal temperatures, low-lying areas, deep forests, and deforested zones have high vector density. The study used freely available databases to create a predictive map, a cost-effective way to support entomological surveillance efforts. Health professionals in areas with "very high" and "high" vector abundance should take targeted measures to reduce vector populations and human cases. In doing so, they can maximize the benefits of their efforts and optimize the impact of vector control interventions.
Availability of data and materials
The datasets generated from the journals during the current study are available in online.
Abbreviations
- ANN:
-
Average nearest neighbor
- GIS:
-
Geographic information system
- IDW:
-
Inverse distance weighted
- RS:
-
Remote sensing
References
Ceccato PN, Ghebremeskel T, Jaiteh MS, Graves PM, Levy MA, Ghebreselassie S, et al. Malaria stratification, climate, and epidemic early warning in Eritrea. Am J Trop Med Hyg. 2007;77:61–8.
Munywoki DN, Kokwaro ED, Mwangangi JM, Muturi EJ, Mbogo CM. Insecticide resistance status in Anopheles gambiae (s.l.) in coastal Kenya. Parasit Vectors. 2021;14:207.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: A worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Jeyaprakasam NK, Liew JWK, Low VL, Wan-Sulaiman W-Y, Vythilingam I. Plasmodium knowlesi infecting humans in Southeast Asia: What’s next? PLoS Negl Trop Dis. 2021;14:e0008900.
Liew JWK, Bukhari FDM, Jeyaprakasam NK, Phang WK, Vythilingam I, Lau YL. Natural Plasmodium inui infections in humans and Anopheles cracens mosquito, Malaysia. Emerg Infect Dis. 2021;27:2700–3.
Yap NJ, Hossain H, Nada-Raja T, Ngui R, Muslim A, Hoh B-P, et al. Natural human infections with Plasmodium cynomolgi, P. inui, and 4 other simian malaria parasites, Malaysia. Emerg Infect Dis. 2021;27:2187–91.
WHO. World malaria report 2022. 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022. Accessed 27 Apr 2023.
Jiram AI, Vythilingam I, NoorAzian YM, Yusof YM, Azahari AH, Fong M-Y. Entomologic investigation of Plasmodium knowlesi vectors in Kuala Lipis, Pahang, Malaysia. Malar J. 2012;11:213.
Vythilingam I, NoorAzian YM, Huat TC, Jiram AI, Yusri YM, Azahari AH, et al. Plasmodium knowlesi in humans, macaques and mosquitoes in peninsular Malaysia. Parasit Vectors. 2008;1:26.
Vythilingam I, Tan CH, Asmad M, Chan ST, Lee KS, Singh B. Natural transmission of Plasmodium knowlesi to humans by Anopheles latens in Sarawak, Malaysia. Trans R Soc Trop Med Hyg. 2006;100:1087–8.
Wong ML, Chua TH, Leong CS, Khaw LT, Fornace K, Wan-Sulaiman W-Y, et al. Seasonal and spatial dynamics of the primary vector of Plasmodium knowlesi within a major transmission focus in Sabah, Malaysia. PLoS Negl Trop Dis. 2015;9:e0004135.
Ang JXD, Kadir KA, Mohamad DSA, Matusop A, Divis PCS, Yaman K, et al. New vectors in northern Sarawak, Malaysian Borneo, for the zoonotic malaria parasite, Plasmodium knowlesi. Parasit Vectors. 2020;13:472.
Vythilingam I, Lim YAL, Venugopalan B, Ngui R, Leong CS, Wong ML, et al. Plasmodium knowlesi malaria an emerging public health problem in Hulu Selangor, Selangor, Malaysia (2009–2013): epidemiologic and entomologic analysis. Parasit Vectors. 2014;7:436.
Warren M, Cheong W, Fredericks H, Coatney GR. Cycles of jungle malaria in West Malaysia. Am J Trop Med Hyg. 1970;19:383–93.
Anstey NM, Grigg MJ. Zoonotic malaria: the better you look, the more you find. J Infect Dis. 2019;219:679–81.
Baird JK. Malaria in the Asia-Pacific region. Asia Pac J Jpn Focus. 2015;13:4395.
Ekawati LL, Johnson KC, Jacobson JO, Cueto CA, Zarlinda I, Elyazar IRF, et al. Defining malaria risks among forest workers in Aceh, Indonesia: a formative assessment. Malar J. 2020;19:441.
Verhulst NO, Smallegange RC, Takken W. Mosquitoes as potential bridge vectors of malaria parasites from non-human primates to humans. Front Physiol. 2012;3:197.
Hayes RO, Maxwell EL, Mitchell CJ, Woodzick TL. Detection, identification, and classification of mosquito larval habitats using remote sensing scanners in earth-orbiting satellites. Bull World Health Organ. 1985;63:361.
Jiang A-L, Lee M-C, Zhou G, Zhong D, Hawaria D, Kibret S, et al. Predicting distribution of malaria vector larval habitats in Ethiopia by integrating distributed hydrologic modeling with remotely sensed data. Sci Rep. 2021;11:10150.
McMahon A, Mihretie A, Ahmed AA, Lake M, Awoke W, Wimberly MC. Remote sensing of environmental risk factors for malaria in different geographic contexts. Int J Health Geogr. 2021;20:28.
Srivastava A, Nagpal B, Saxena R, Subbarao S. Predictive habitat modelling for forest malaria vector species An. dirus in India—a GIS-based approach. Curr Sci. 2001;80:1129–34.
Agarwal SA, Sikarwar SS, Sukumaran D. Application of RS & GIS in risk area assessment for mosquito borne diseases—a case study in a part of Gwalior City (MP). Int J Adv Technol Eng Res. 2012;2:1–4.
Chockalingam J, Khan S, Chandra R, Singh H, Srivastava V, Raju P. Characterisation of malaria vector habitats using remote sensing and GIS. J Indian Soc Remote Sens. 2001;29:31–6.
Gwitira I, Murwira A, Zengeya FM, Shekede MD. Application of GIS to predict malaria hotspots based on Anopheles arabiensis habitat suitability in Southern Africa. Int J Appl Earth Obs Geoinf. 2018;64:12–21.
Ahmad R, Ali WNWM, Nor ZM, Ismail Z, Hadi AA, Ibrahim MN, et al. Mapping of mosquito breeding sites in malaria endemic areas in Pos Lenjang, Kuala Lipis, Pahang, Malaysia. Malar J. 2011;10:361.
Hawkes FM, Manin BO, Cooper A, Daim S, Homathevi R, Jelip J, et al. Vector compositions change across forested to deforested ecotones in emerging areas of zoonotic malaria transmission in Malaysia. Sci Rep. 2019;9:13312.
Vythilingam I, Wong ML, Wan-Yussof WS. Current status of Plasmodium knowlesi vectors: a public health concern? Parasitol. 2018;145:32–40.
Pramasivan S, Van Lun LL, Jeyaprakasm N, Wee J, Ngui R, Vythilingam I. Cryptic diversity and demographic expansion of Plasmodium knowlesi malaria vectors in Malaysia. Genes. 2023;14:1369.
Ahmad R. Binomics of Anopheles balabacensis baisas, the principal malaria vector, in Ranau, Sabah. Trop Biomed. 1999;16:31–8.
Ahmad R, Chan S, Abdullah AG, Tanrang H, Hanlim L. Species composition of mosquito fauna in Ranau, Sabah, Malaysia. Trop Biomed. 2009;25:232–6.
Ahmad R, Ali W, Ali W, Omar M, Azahary A, Rahman A, et al. Characterization of the larval breeding sites of Anopheles balabacensis (Baisas), in Kudat, Sabah, Malaysia. Southeast Asian J Trop Med Public Health. 2018;49:566–79.
Ahmad R, Fakhriy HA, Suzilah I, Zurainee MN, Najdah W, Ariffin MM, et al. Indoor and outdoor residual spraying of a novel formulation of deltamethrin K-Othrine® (Polyzone) for the control of simian malaria in Sabah, Malaysia. PLoS ONE. 2020;15:e0230860.
Ahmad R, Nor Z, Ali W, Ahmad H, David L, Mohd M, et al. Outdoor residual spray for the control of monkey malaria vectors in Sarawak, Malaysia. Int J Mosq Res. 2021;8:54–62.
Ang JX, Yaman K, Kadir KA, Matusop A, Singh B. New vectors that are early feeders for Plasmodium knowlesi and other simian malaria parasites in Sarawak, Malaysian Borneo. Sci Rep. 2021;11:7739.
Brant HL, Ewers RM, Vythilingam I, Drakeley C, Benedick S, Mumford JD. Vertical stratification of adult mosquitoes (Diptera: Culicidae) within a tropical rainforest in Sabah, Malaysia. Malar J. 2016;15:370.
Chang M, Doraisingam P, Hardin S, Nagum N. Malaria and filariasis transmission in a village/forest setting in Baram District, Sarawak, Malaysia. J Trop Med Hyg. 1995;98:192–8.
Cheong W, Loong K, Mahadevan S, Mak JW, Kan SK. Mosquito fauna of the Bengkoka peninsula, Sabah, Malaysia. Southeast Asian J Trop Med Public Health. 1984;15:19–26.
Chua TH, Manin BO, Vythilingam I, Fornace K, Drakeley CJ. Effect of different habitat types on abundance and biting times of Anopheles balabacensis Baisas (Diptera: Culicidae) in Kudat district of Sabah, Malaysia. Parasit Vectors. 2019;12:364.
Hawkes F, Manin BO, Ng SH, Torr SJ, Drakeley C, Chua TH, et al. Evaluation of electric nets as means to sample mosquito vectors host-seeking on humans and primates. Parasit Vectors. 2017;10:338.
Hii J. Evidence for the existence of genetic variability in the tendency of Anopheles balabacensis to rest in houses and to bite man. Southeast Asian J Trop Med Public Health. 1985;16:173–82.
Hii JL, Kan S, Vun YS, Chin KF, Tambakau S, Chan MK, et al. Transmission dynamics and estimates of malaria vectorial capacity for Anopheles balabacensis and An flavirostris (Diptera: Culicidae) on Banggi island, Sabah, Malaysia. Ann Trop Med Parasitol. 1988;82:91–101.
Hii J, Birley MH, Sang VY. Estimation of survival rate and oviposition interval of Anopheles balabacensis mosquitoes from mark-recapture experiments in Sabah, Malaysia. Malaysia Med Vet Entomol. 1990;4:135–40.
Hii JLK, Chew MK, Sang VY, Munstermann LE, Tan SG, Panyim S, et al. Population genetic analysis of host seeking and resting behaviors in the malaria vector, Anopheles balabacensis (Diptera: Culicidae). J Med Entomol. 1991;28:675–84.
Hii JLK. Entomological evaluation of sumithion, DDT and malathion for the control of Anopheles balabacensis balabacensis Baisas in Kuala Penyu district, Sabah, Malaysia. J Med Entomol Zool. 1979;30:319–28.
Hii JLK. Responses of Anopheles balabacensis balabacensis Baisas to ultra-low-volume aerosols of sumithion in Sabah, Malaysia. J Community Dis. 1980;12:14–21.
Hii JLK. Insecticide susceptibility studies of three cryptic species of the Anopheles balabacensis complex. Southeast Asian J Trop Med Public Health. 1984;15:104–11.
Hii JLK. The influence of a heterogeneous environment on host feeding behaviour of Anopheles balabacensis (Diptera: Culicidae). Trop Biomed. 1987;4:67–70.
Leake DW Jr, Hii JL. Giving bednets" fair" tests in field trials against malaria: a case from Sabah, East Malaysia. Southeast Asian J Trop Med Public Health. 1989;20:379–84.
Manin BO, Drakeley CJ, Chua TH. Mitochondrial variation in subpopulations of Anopheles balabacensis Baisas in Sabah, Malaysia (Diptera: Culicidae). PLoS ONE. 2018;13:e0202905.
Manin BO, Ferguson HM, Vythilingam I, Fornace K, William T, Torr SJ, et al. Investigating the contribution of peri-domestic transmission to risk of zoonotic malaria infection in humans. PLOS Negl Trop Dis. 2016;10:e0005064.
Rogozi E, Ahmad R, Ismail Z. Biting activity cycles of some antropophilic mosquito species in Malaysia. J Int Environ Appl Sci. 2012;7:5.
Tan CH, Vythilingam I, Matusop A, Chan ST, Singh B. Bionomics of Anopheles latens in Kapit, Sarawak, Malaysian Borneo in relation to the transmission of zoonotic simian malaria parasite Plasmodium knowlesi. Malar J. 2008;7:52.
Vythilingam I, Chan ST, Shanmugratnam C, Tanrang H, Chooi KH. The impact of development and malaria control activities on its vectors in the Kinabatangan area of Sabah, East Malaysia. Acta Trop. 2005;96:24–30.
Vythilingam I. Plasmodium knowlesi in humans: a review on the role of its vectors in Malaysia. Trop Biomed. 2010;27:1–12.
Wharton R, Eyles DE, Warren M, Cheong WH. Studies to determine the vectors of monkey malaria in Malaya. Ann Trop Med Parasitol. 1964;58:56–77.
Wharton R, Eyles DE, Warren M. The development of methods for trapping the vectors of monkey malaria. Ann Trop Med Parasitol. 1963;57:32–46.
Wharton R, Laing A, Cheong W. Studies on the distribution and transmission of malaria and filariasis among aborigines in Malaya. Ann Trop Med Parasitol. 1963;57:235–54.
Moran PA. Notes on continuous stochastic phenomena. Biometrika. 1950;37:17–23.
Chua TH, Manin BO, Daim S, Vythilingam I, Drakeley C. Phylogenetic analysis of simian Plasmodium spp. infecting Anopheles balabacensis Baisas in Sabah, Malaysia. PLoS Negl Trop Dis. 2017;11:e0005991.
Jeyaprakasam NK, Low VL, Pramasivan S, Liew JW, Wan-Sulaiman WY, Vythilingam I. High transmission efficiency of the simian malaria vectors and population expansion of their parasites Plasmodium cynomolgi and Plasmodium inui. PLoS Negl Trop Dis. 2023;17:e0011438.
Christiansen-Jucht C, Parham PE, Saddler A, Koella JC, Basáñez M-G. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae s.s. Parasit Vectors. 2014;7:489.
Akpodiete NO, Diabate A, Tripet F. Effect of water source and feed regime on development and phenotypic quality in Anopheles gambiae (s.l.): prospects for improved mass-rearing techniques towards release programmes. Parasit Vectors. 2019;12:210.
Singhasivanon P, Thimasarn K, Yimsamran S, Linthicum K, Nualchawee K, Dawreang D, et al. Malaria in tree crop plantations in south-eastern and western provinces of Thailand. Southeast Asian J Trop Med Public Health. 1999;30:399–404.
Wang X, Zhou G, Zhong D, Wang X, Wang Y, Yang Z, et al. Life-table studies revealed significant effects of deforestation on the development and survivorship of Anopheles minimus larvae. Parasit Vectors. 2016;9:323.
Dunphy BM, Kovach KB, Gehrke EJ, Field EN, Rowley WA, Bartholomay LC, et al. Long-term surveillance defines spatial and temporal patterns implicating Culex tarsalis as the primary vector of West Nile virus. Sci Rep. 2019;9:6637.
Gouzile A, Bama M, Zamina B, Yapi E, Soro G, Goula B, et al. Mapping of malaria risk related to climatic and environmental factors by multicriteria analysis in the Marahoué Region of Côte d’Ivoire. J Geosci Environ Prot. 2022;10:234–52.
Murindahabi MM, Hoseni A, Corné Vreugdenhil LC, van Vliet AJH, Umupfasoni J, Mutabazi A, et al. Citizen science for monitoring the spatial and temporal dynamics of malaria vectors in relation to environmental risk factors in Ruhuha, Rwanda. Malar J. 2021;20:453.
Saffawati T, Ismail T, Kassim N, Rahman AA, Hamid SA, Yahya K, et al. The application of geographic information system (GIS) to assess the population abundance of Aedes albopictus (Skuse) in mangrove forests of Penang, Malaysia. Int J Mosq Res. 2019;6:50–4.
Suganthi P, Govindaraju M, Devi BS, Kangabam RD, Suganthi K, Thenmozhi V, et al. Mapping of spatio-temporal distribution of mosquito vector density in Sitheri Hills using GIS technology. Int J Adv Remote Sens GIS. 2015;4:873–82.
Ceccato P, Connor S, Jeanne I, Thomson M. Application of geographical information systems and remote sensing technologies for assessing and monitoring malaria risk. Parassitologia. 2005;47:81–96.
Lyons CL, Coetzee M, Chown SL. Stable and fluctuating temperature effects on the development rate and survival of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Parasit Vector. 2013;6:104.
Kar NP, Kumar A, Singh OP, Carlton JM, Nanda N. A review of malaria transmission dynamics in forest ecosystems. Parasit Vector. 2014;7:265.
Sam J, Shamsusah NA, Ali AH, Hod R, Hassan MR, Agustar HK. Prevalence of simian malaria among macaques in Malaysia (2000–2021): a systematic review. PLOS Negl Trop Dis. 2022;16:e0010527.
Hii J, Rueda LM. Malaria vectors in the Greater Mekong Subregion: overview of malaria vectors and remaining challenges. Southeast Asian J Trop Med Public Health. 2013;44:73–165.
Vythilingam I, Foo LC, Chiang GL, Chan ST, Eng KL, Mahadevan S, et al. The impact of permethrin impregnated bednets on the malaria vector Anopheles maculatus (Diptera: Culicidae) in aboriginal villages of Pos Betau Pahang, Malaysia. Southeast Asian J Trop Med Public Health. 1995;26:354–8.
Lindblade KA, Walker ED, Wilson ML. Early warning of malaria epidemics in African highlands using Anopheles (Diptera: Culicidae) indoor resting density. J Med Entomol. 2000;37:664–74.
Lindsay S, Martens WJ. Malaria in the African highlands: past, present and future. Bull World Health Organ. 1998;76:33.
Phang WK, Hamid MHA, Jelip J, Mudin RN, Chuang T-W, Lau YL, et al. Predicting Plasmodium knowlesi transmission risk across Peninsular Malaysia using machine learning-based ecological niche modeling approaches. J Front Microbiol. 2023;14:357.
Arcos AN, Valente-Neto F, da Silva Ferreira FA, Bolzan FP, da Cunha HB, Tadei WP, et al. Seasonality modulates the direct and indirect influences of forest cover on larval anopheline assemblages in western Amazônia. Sci Rep. 2021;11:12721.
Birkinshaw SJ, Bathurst JC, Iroumé A, Palacios H. The effect of forest cover on peak flow and sediment discharge—an integrated field and modelling study in central–southern Chile. J Hydrol Process. 2011;25:1284–97.
Emidi B, Kisinza WN, Mmbando BP, Malima R, Mosha FW. Effect of physicochemical parameters on Anopheles and Culex mosquito larvae abundance in different breeding sites in a rural setting of Muheza, Tanzania. Parasit Vector. 2017;10:304.
Vinod S. Deforestation and water pollution impact on mosquitoes related epidemic diseases in nanded region. J Biosci Discov. 2011;2:309–16.
Norris DE. Mosquito-borne diseases as a consequence of land use change. EcoHealth. 2004;1:19–24.
Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11.
Vittor AY, Pan W, Gilman RH, Tielsch J, Glass G, Shields T, et al. Linking deforestation to malaria in the Amazon: characterization of the breeding habitat of the principal malaria vector, Anopheles darlingi. Am J Trop Med Hyg. 2009;81:5–12.
Vythilingam I, Hii J. Simian malaria parasites: special emphasis on Plasmodium knowlesi and their Anopheles vectors in Southeast Asia. In: Manguin S, editor. Anopheles mosquitoes—new insights into malaria vectors. IntechOpen: London; 2013. https://doi.org/10.5772/54491.
Chang M, Hii J, Buttner P, Mansoor F. Changes in abundance and behaviour of vector mosquitoes induced by land use during the development of an oil palm plantation in Sarawak. Trans R Soc Trop Med Hyg. 1997;91:382–6.
Loh E, Murray K, Nava A, Aguirre AA, Daszak P. Evaluating the links between biodiversity, land-use change, and infectious disease emergence. In: Aguirre A, Sukumar R, editors. Tropical conservation, 1st ed. Oxford University Press: Oxford; 2016. p. 79–89.
Fornace KM, Abidin TR, Alexander N, Brock P, Grigg MJ, Murphy A, et al. Association between landscape factors and spatial patterns of Plasmodium knowlesi infections in Sabah, Malaysia. Emerg Infect Dis. 2016;22:201.
Yasuoka J, Levins R. Impact of deforestation and agricultural development on anopheline ecology and malaria epidemiology. Am J Trop Med Hyg. 2007;76:450–60.
Valle D, Zaitchik B, Feingold B, Spangler K, Pan W. Abundance of water bodies is critical to guide mosquito larval control interventions and predict risk of mosquito-borne diseases. Parasit Vectors. 2013;6:179.
Menach AL, McKenzie FE, Flahault A, Smith DL. The unexpected importance of mosquito oviposition behaviour for malaria: non-productive larval habitats can be sources for malaria transmission. Malar J. 2005;4:23.
Gone T, Balkew M, Gebre-Michael T. Comparative entomological study on ecology and behaviour of Anopheles mosquitoes in highland and lowland localities of Derashe District, southern Ethiopia. Parasit Vectors. 2014;7:483.
Lindblade KA, Walker ED, Onapa AW, Katungu J, Wilson ML. Land use change alters malaria transmission parameters by modifying temperature in a highland area of Uganda. Trop Med Int Health. 2000;54:263–74.
Minakawa N, Sonye G, Mogi M, Githeko A, Yan G. The effects of climatic factors on the distribution and abundance of malaria vectors in Kenya. J Med Entomol. 2002;39:833–41.
Christiansen-Jucht CD, Parham PE, Saddler A, Koella JC, Basáñez M-G. Larval and adult environmental temperatures influence the adult reproductive traits of Anopheles gambiae ss. Parasit Vector. 2015;8:456.
Afrane YA, Zhou G, Lawson BW, Githeko AK, Yan G. Effects of microclimatic changes caused by deforestation on the survivorship and reproductive fitness of Anopheles gambiae in western Kenya highlands. Am J Trop Med Hyg. 2006;74:772–8.
Agyekum TP, Botwe PK, Arko-Mensah J, Issah I, Acquah AA, Hogarh JN, et al. A Systematic review of the effects of temperature on Anopheles mosquito development and survival: implications for malaria control in a future warmer climate. Int J Environ Res Public Health. 2021;18:14.
Acknowledgements
The authors like to thank the staff of vector team Kluang, Mersing, Kota Tinggi, Jengka, Perak and Negeri Sembilan for all their help in collection of mosquitoes. Additionally, we would like to thank Universiti Malaya (UM) and Universiti Malaysia Sarawak (UNIMAS) for their tremendous support in this research.
Funding
Open Access funding provided by Universiti Malaysia Sarawak. This research was funded by Ministry of Higher Education of Malaysia Long Term Research Grant Scheme (LRGS), grant no. LRGS 1/2018/UM/01/1/3.
Author information
Authors and Affiliations
Contributions
IV and RN conceptualized and designed the work. SP, NKJ, VLL, JWKL and IV did mosquito collections. SP conducted molecular laboratory work, analyzed the data and wrote the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate.
This study was approved by Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-19-962-47606).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Vectors throughout Malaysia and the sites recorded from 1957 to 2022.
Additional file 2: Figure S1.
Maps showing the elevation, forest cover, forest loss, temperature and water bodies of Malaysia.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Pramasivan, S., Ngui, R., Jeyaprakasam, N.K. et al. Spatial analyses of Plasmodium knowlesi vectors with reference to control interventions in Malaysia. Parasites Vectors 16, 355 (2023). https://doi.org/10.1186/s13071-023-05984-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-023-05984-x