Abstract
Background
Microbial larvicides containing both LysiniBacillus sphaericus and Bacillus thuringiensis svar. israelensis (Bti) insecticidal crystals can display advantages for mosquito control. This includes a broader action against larvae that are refractory to the Binary (Bin) toxin from L. sphaericus, as Bin-resistant Culex quinquefasciatus and Aedes aegypti naturally refractory larvae, which often co-habit urban areas of endemic countries for arboviruses. Our principal goal was to assess the toxicity of a combined L. sphaericus/Bti larvicide (Vectomax FG™) to Cx. quinquefasciatus (susceptible CqS and Bin-resistant CqR) and Ae. aegypti (Rocke) and to determine its persistence in the breeding sites with those larvae.
Methods
The toxicity of a combined L. sphaericus/Bti product (VectoMax FG™) to larvae was performed using bioassays, and persistence was evaluated in simulate field trials carried out under the shade, testing two label concentrations during 12 weeks. A laboratory strain SREC, established with CqS and CqR larvae, was kept during four generations to evaluate the ability of the L. sphaericus/Bti to eliminate resistant larvae.
Results
The L. sphaericus/Bti showed toxicity (mg/L) to larvae from all strains with a decreasing pattern for CqS (LC50 = 0.006, LC90 = 0.030), CqR (LC50 = 0.009, LC90 = 0.069), and Rocke (LC50 = 0.042, LC90 = 0.086). In a simulated field trial, the larvicide showed a persistence of 6 weeks and 8 weeks, controlling larvae from all strains in containers with 100 L of water, using 2 g or 4 g per container (100 L), respectively. The treatment of SREC larvae with L. sphaericus/Bti showed its capacity to eliminate the Bin-resistant individuals using suitable concentrations to target those larvae.
Conclusions
Our results showed the high efficacy and persistence of the L. sphaericus/Bti larvicide to control Cx. quinquefasciatus and Ae. aegypti that might cohabit breeding sites. These findings demonstrated that such larvicides can be an effective tool for controlling those species in urban areas with a low potential for selecting resistance.
Graphical Abstract

Similar content being viewed by others
Background
The microbial larvicides based on LysiniBacillus sphaericus or Bacillus thuringiensis svar. israelensis (Bti) can be used to control mosquito species of medical importance within integrated programs [1], and recently, products based on both bacteria have been developed. The active ingredient produced by those bacteria are insecticidal crystals with protoxins that, upon ingestion by mosquito larvae, are activated into toxins and bind to specific receptors on the midgut epithelial cells [2, 3]. The insecticidal L. sphaericus crystals have a single protoxin called binary (Bin), that have been mostly used for controlling Culex and Anopheles species. The Bin toxin action on the major target species, Culex quinquefasciatus and Culex pipiens, depends on its binding to specific receptors that are the α-glucosidases, named Cqm1/Cpm1, which are attached to the midgut epithelial cells by a glycosylphosphatidylinositol (GPI) anchor, as reviewed by Silva-Filha et al. [4]. The action of the Bin depends on this membrane-bound receptor, and resistance often is provoked by the lack of those receptors in the midgut [5, 6]. Ae. aegypti larvae are naturally refractory to the Bin toxin due to the absence of such specific midgut receptors [5, 7]. The Bti insecticidal crystal contains four major protoxins (Cry11Aa, Cry4Ba, Cry4Aa, and Cyt1Aa) that act in synergy and are toxic to Culicidae, as well as Simuliidae, larvae [8, 9]. The Cyt1Aa plays a central role for the synergy of these toxins because it promotes the binding of the Cry toxins with high affinity to receptors in the epithelium [10, 11]. The Cry toxins from Bti can specifically bind to different receptors, which are GPI-anchored proteins in the midgut of larvae such as aminopeptidases, cadherins, alkaline phosphatases, and α-amylases [12], and these interactions can also be modulated by other molecules such as lectins [13]
L. sphaericus- and Bti-based larvicides are effective for mosquito control, but they display some limitations. For L. sphaericus, a major issue can be the resistance to the Bin toxin already reported for Cx. quinquefasciatus or Cx. pipiens field-treated populations [14,15,16,17,18,19,20,21] or strains artificially selected in laboratory [6, 22,23,24,25]. Resistance to the Bin toxin can be due to mutations in the cqm1/cpm1 gene encoding the toxin receptor, which prevent the expression of Cqm1/Cpm1 proteins that are bound on the midgut epithelium by its GPI anchor [26,27,28,29,30,31]. The cqm1REC allele was identified in a Bin-resistant Cx. quinquefasciatus strain (REC) derived from Recife city (Brazil) that was subjected to artificial selection in our insectary, and it is the major gene that confers the resistance of this strain [23]. This allele has a 19-nucleotide (nt) deletion that encodes a truncated protein without the GPI anchor; therefore, the Cqm1 receptor is no longer available bound to the cell membrane [27, 31]. DNA-screening in field population detected the cqm1REC allele in six nontreated areas, with frequency between 1 and 6 × 10–3, and in one L. sphaericus-treated area at a higher frequency (5 × 10–2) [26, 32]. The cqm1REC is recessively inherited and only the homozygous individuals are resistant to the Bin toxin [6, 27]. The Bti crystal has a complex action on multiple receptors, and to date, there are no reports of resistance to the Bti crystal in field populations subjected to treatments [4, 33] or strains submitted to artificial selection [34,35,36]. The complex action of the set of toxins from the Bti crystal is a determining factor to prevent the onset of resistance. The selection in laboratory can lead to resistance but only when single Bti toxins were used, that is, not when using the whole crystal [36, 37]. The major limitation of Bti crystal is its vulnerability to biotic and abiotic factors, which makes its field persistence shorter, in particular under solar radiation [1, 38].
Thus, combined larvicides containing crystals with both protoxins from L. sphaericus and Bti, here named L. sphaericus/Bti, and also referred as long-lasting microbial larvicides [39,40,41], were developed to offer advantages as a broader spectrum of action for controlling different species occurring in the same breeding sites, improved field persistence, and low risk for selecting resistance. The utilization of L. sphaericus/Bti larvicides has been evaluated under different scenarios, in particular, for Anopheles in peri-urban [42,43,44] and rural areas that are endemic for malaria in Africa [39,40,41, 45, 46]. Their effectiveness for controlling anophelines in fish farming ponds and other breeding sites from malaria-endemic areas in Brazil has also been demonstrated [47]. In view of their environmentally safe profile, L. sphaericus/Bti larvicides can be used to control mosquitoes in sylvatic ecosystems and sensitive areas [48]. L. sphaericus/Bti larvicides have been used to fight Culex and Aedes, but most reports come from areas with relatively low proliferation of mosquitoes and pathogens [49,50,51,52,53]. The evaluation of L. sphaericus/Bti larvicides for controlling species such as Cx. quinquefasciatus and Ae. aegypti in areas characterized by their simultaneous and permanent proliferation in territories under high pathogen transmission is still scarce. Furthermore, few studies have compared the susceptibility of these target species with these compounds. Our hypothesis is that the association of mosquito-active protoxins in L. sphaericus/Bti-based larvicides can display high efficacy against Cx. quinquefasciatus, including the Bin-resistant larvae, and to Ae. aegypti that can be found in the same territory of urban areas. Therefore, this study aimed to determine the susceptibility of these species to a L. sphaericus/Bti larvicide under laboratory conditions and to determine its persistence for controlling these larvae in the same breeding site under simulated field trials. In addition, we evaluated whether the L. sphaericus/Bti larvicide could reduce the frequency of Cx. quinquefasciatus Bin-resistant larvae, using a laboratory colony established with a known frequency of resistant genotypes.
Methods
Mosquito colonies
Three colonies kept in the insectary of Instituto Aggeu Magalhães (IAM-Fiocruz) were used. The Cx. quinquefasciatus CqSLab, here named CqS, is a susceptible reference colony established with eggs collected in Recife city (Brazil) whose individuals are homozygous for the cqm1 allele that encodes the receptor of the Bin toxin [31, 54]. The Cx. quinquefasciatus REC, here named CqR, displays high resistance ratio (RR > 1000-fold) to the Bin toxin from L. sphaericus strain 2362 and is composed of homozygous individuals for the cqm1REC, the major recessive allele that confers Bin-resistance [27, 55]. The Cx. quinquefasciatus SREC colony was established for this study, using CqS and CqR individuals to evaluate the genotypes for the cqm1 and cqm1REC alleles, after larvae were subjected to larvicides treatments for four generations, as further described in the section Establishment, maintenance, and treatment of the SREC colony. The Ae. aegypti Rockefeller colony, here named Rocke, is an international reference for insecticide susceptibility and was used in this study. All colonies were maintained under controlled insectary conditions of temperature (26 ± 1 °C), relative humidity (70%), and photoperiod (14 h light:10 h dark). Larvae were reared in tap water from the public supply system and fed cat food (Friskies®). The adults fed sucrose solution 10% ad libitum, and females also fed, once per week, on defibrinated rabbit blood provided by the Institute of Science and Technology in Biomodels- Fiocruz (Rio de Janeiro, RJ, Brazil).
Larvicides
Two microbial larvicides from Sumitomo Chemical/Valent Biosciences (www.valentbiosciences.com/publichealth/products) were used. The combined L. sphaericus/Bti based-larvicide was VectoMax FG™ (batch 313–533-N830) containing 2.7% of crystals/spores from L. sphaericus 2362 (strain ABTS 1743) and 4.95% of crystals/spores from Bti (strain AM65-52) as active ingredients, with a potency of 50 L. sphaericus international toxic units (ITU)/mg against Cx. quinquefasciatus larvae. The doses of VectoMax FG™ recommended by the manufacturer are the following: for Culex spp (open areas 5–20 kg/ha, cesspits 5–10 g/m2, polluted water 10–20 kg/ha); for Anopheles spp (open areas 5–10 kg/ha); and for Aedes spp (open areas 5–10 kg/ha, water reservoirs 2–4 g/100 L, polluted water 20 kg/ha). The larvicide VectoLex WG® (batch 285–416-PG30), containing only 51.2% of crystals/spores from L. sphaericus 2362 (strain ABTS 1743) with a potency of 650 L. sphaericus ITU/mg against Cx. quinquefasciatus larvae, was used for the selection of Bin-resistant Cx. quinquefasciatus larvae from the SREC colony, or for comparative purposes, when needed. Both larvicides are presented as slow-release granules and were stored at room temperature (RT) according to the manufacturer’s instructions. Additional technical information is available at sumitomochemical.com/ehd-public-health-products.
Dose response bioassays
These assays were done to assess larvae susceptibility and to determine the lethal concentrations of L. sphaericus/Bti larvicide (Vectomax FG™) for 50% (LC50) and 90% (LC90) to groups of 20 third instar larvae in 100 ml of water, after 48 h exposure, which were performed on the basis of the standard protocol [55]. For these bioassays, stock aqueous suspensions using each larvicide were prepared on the basis of an adapted protocol at 5 g/L considering the content of the active ingredient (L. sphaericus/Bti 7.65%). Then, the stock suspension at 5 g/L was incubated at RT for 72 h under gentle agitation to release the active ingredients. After, the samples were subjected to agitation (Vortex) for 3 min at RT. Immediately after this step, the liquid phase was collected to prepare aliquots that were stored at −20 °C, until use. The solid phase was discarded. The dose–response bioassays were performed using experimental sets of 20 late third instar larvae in cups with 100 mL of distilled water, in three biological replicas, and food was not provided. In each bioassay, three technical replicas of larvae were exposed to each of six concentrations of the bacterial suspensions (0.0025–0.1 mg/L) diluted from the stock suspension (5 g/L). An untreated control group, three technical replicas of larvae, was run at each bioassay. The mortality was recorded after 48 h and the maximum mortality allowed in the untreated control was 10%. A total of three or four biological replicas of each bioassay were done to determine the LC50 and LC90 values and their respective 95% confidence interval (CI) on the basis of probit analysis using SPSS v.16.0 for Windows. The LCs whose 95% confidence intervals (CI) overlap are not considered different.
Diagnostic concentrations bioassays
Bioassays were also done to empirically determine the concentrations of L. sphaericus/Bti (Vectomax FG™) and L. sphaericus (VectoLex WG®) that could be lethal to around 80–90% of large pools of third instar larvae (n = 100–300) set in rearing trays filled with tap water (1 L) and a small amount of food (0.05–0.1 g/tray). Pools of larvae from the CqS, CqR, and Rocke strains were tested. The stock suspension at 5 g/L were prepared as described in the section Dose response bioassays, considering the content of the active ingredient of each larvicide tested (L. sphaericus/Bti 7.65%, L. sphaericus 52.1%). Single concentrations between 0.005 mg/L and 0.2 mg/L were tested against pools of larvae of each strain. At least three technical replicas per concentration tested and per strain were used. The mortality was recorded after 48 h exposure. These assays indicate the concentrations of each larvicide that were used to treat the third instar of the SREC larvae at every generation.
Simulated field trial
A simulated field trial to evaluate the residual activity of the combined L. sphaericus/Bti larvicide against Cx. quinquefasciatus and Ae. aegypti larvae colonizing the same breeding site was run in an experimental area at IAM-FIOCRUZ from October 2022 to January 2023. This facility was covered on top and open on the sides, and containers, which simulated larvae breeding sites, were not exposed to rain or direct sunlight (Fig. 1). The methodology, adapted from previous studies [56, 57], is briefly described here. The breeding sites were 150 L-white plastic containers filled with 100 L of tap water, 0.1 g of food, and colonized with a pool of 150 third instar larvae composed of CqS (n = 70), CqR (n = 30), and Rocke (n = 50). All containers were covered with a fine mesh. The L. sphaericus/Bti larvicide was tested at 2 g or 4 g per container with 100 L of water, using four technical replicas. These doses were based on the manufacturer’s recommendation for Aedes in water reservoirs, as described in the section Larvicides. An untreated control group using four technical replicas was kept during the trial. After the first colonization with larvae, the containers, except the control ones, were treated. The larvae mortality in the experimental containers was evaluated 48 h after the colonization done each week, during 12 weeks. The dead larvae were not removed from the containers. The persistence in the treated groups was considered suitable when the average mortality in the treated containers was ≥ 80%. When the mortality in the untreated containers exceeded 20%, the container was excluded and replaced. The temperature and relative humidity were recorded weekly during the trial.
Experimental area of the trial for evaluating the residual activity of a larvicide to mosquito. Lysinibacillus sphaericus/Bacillus thuringiensis svar. israelensis larvicide (Vectomax™ FG) was tested against third instar larvae of Culex quinquefasciatus and Aedes aegypti (n = 150) in containers with 100 L of water treated with 2 g/container or 4 g/container using four replicas (R). An untreated control group was run during the trial
Establishment, maintenance, and treatment of the SREC colony
The SREC colony was established with CqS and CqR adults with the purpose of evaluating the genotypes for the cqm1 and cqm1REC alleles of individuals, after treatments with the larvicide, along four generations. Briefly, the major procedures carried out in each generation, represented in the Additional file 1 (Fig. S1), were: determination of the genotypes of a larvae sample before the treatment, treatment of larvae, mortality recording, and determination of the genotype in a sample of the surviving adults. The parental generation was composed of 200 adults (1:1 sex ratio) using a frequency of 0.70 of CqS (n = 140, 1:1 sex ratio) and 0.30 of CqR (n = 60, 1:1 sex ratio). Pupae from each sex and each strain were kept separately in individual cages. After emergence, adults from those four cages were pooled simultaneously in a single cage (30 cm × 30 cm × 30 cm) as an effort to promote a random crossing. Adults were kept as previously described. After the first gonadotrophic cycle, each filial generation was established using egg samples, taken from around 76 rafts, and larvae were reared under controlled conditions [54]. Briefly, groups of 500–700 larvae were reared in trays (34 cm × 24 cm × 7 cm, 4 L capacity) filled with 2 L of tap water and 1.6 g of cat food provided during the larval phase. When the larvae achieved the third instar, the genotype of a larvae sample was determined (n ~ 50–100) by polymerase chain reaction (PCR) assays, as described in the next section. After this step, between 1200 and 3600 third instar larvae per generation were treated with a concentration of larvicide that was established in the section Diagnostic concentrations bioassays. After the treatment, the surviving larvae were washed twice with tap water, transferred to trays, and reared under standard conditions until they reached the adult stage. The F1 larvae were treated with L. sphaericus to promote the selection of Bin-resistant individuals, while F2, F3, and F4 larvae were treated with L. sphaericus/Bti larvicide. Total mortality, achieved until the emergence of adults, was recorded and the genotype of those individuals was also assessed (n ~ 50). This assay was performed twice.
PCR assays
The genotype for the cqm1 and cqm1REC alleles of Cx. quinquefasciatus individuals from the SREC colony was determined using a PCR assay [32]. The 19-nt deletion found in the allele cqm1REC was previously reported [31] and the full-length cDNA sequence is deposited in Gene Bank (accession number DQ333335). The individuals were classified for this genotype as: susceptible (cqm1/cqm1 or SS, cqm1/cqm1REC or SR) and resistant (cqm1REC/cqm1REC or RR) according to the amplicon sizes, as described below. The PCR assay was designed to amplify a fragment from the cqm1 gene using two primers that flank the 19-bp deletion found in the cqm1REC allele, producing amplicons of 208 bp or 189 bp from the cqm1 and cqm1REC alleles, respectively [31]. At each generation, larvae and adult samples were collected and individually stored at −80 °C for genotype determination. The DNA extraction from these samples was done using DNAzol™ (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s recommendations, and procedures to prevent DNA contamination were also adopted. The DNA samples were quantified using a NanoDrop 2000c® spectrophotometer (Thermo Fisher Scientific), and concentration was normalized to 12.5 µg/µl. The PCR reactions were carried out as described [32] using forward primer (5'-CGAGAATTCATGCAGGACTTCAAAGAG-3’) and reverse primer (5'-GCACTGCAGGGAAGTGGTGGAAGGTAC-3). The amplified products were separated by electrophoresis in a 2.5% agarose gel, stained with ethidium bromide, and visualized in an ultraviolet transilluminator. The following controls were run during each reaction: a positive control with DNA from a known homozygous-susceptible individual to amplify the 208 bp amplicon; a positive control with DNA from a known resistant individual to amplify the 189 bp-amplicon; and a negative control sample without DNA. To confirm the identity of the amplicons, 17 samples of PCR products were purified using the Qiaquick® PCR & Gel Cleanup Kit (QIAGEN, Hilden, Germany) and then subjected to sequencing with the ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) at the Núcleo de Plataformas Integradas (NPT) from IAM- Fiocruz.
Results
Toxicity to larvae
The combined L. sphaericus/Bti larvicide showed high activity against all three strains tested, which was decreasing for CqS, CqR, and Rocke larvae (Table 1). The LCs values presented in Table 1 represent the average of the LCs obtained in three or four independent bioassays and the 95% confidence intervals represent the range of the intervals found in those assays (Table S1). The average LC50 and LC90 of L. sphaericus/Bti toward CqS larvae were 0.006 mg/L and 0.030 mg/L, while the respective LCs for CqR larvae were 0.009 mg/L and 0.069 mg/L. At the LC90, only a 2.3-fold increase toward the CqR larvae compared with the CqS was found. This larvicide also showed high toxicity to Rocke larvae, which is naturally refractory to L. sphaericus Bin toxin, with an LC50 of 0.042 mg/L and LC90 of 0.086 mg/L. The comparison of the toxicity ratio, taking the most susceptible larvae CqS as the reference, showed that the larvicide acts on the CqR and Rocke larvae with a LC90, which was only threefold greater. The TR at LC90 between CqR and CqS is not considered significant, as the 95% confidence intervals (CI) overlap, while the TR at LC90 between Rocke and CqS was considered significant. A total of 11 bioassays comprising a total of 3900 larvae were used to establish the LCs and the analytical data generated from each bioassay is presented in the Additional file 2: Table S1.
Toxicity of L. sphaericus/Bti and L. sphaericus larvicides was tested against larger pools of larvae from all strains set in rearing trays and results are presented in the Additional file 3: Table S2. Briefly, for CqS larvae, more than 80% mortality was achieved using from 0.02 mg/L of L. sphaericus/Bti and from 0.005 mg/L of L. sphaericus larvicide. For CqR larvae, 0.1 mg/L of the L. sphaericus/Bti provoked more than 90% mortality, while 0.1 mg/L of L. sphaericus did not provoke mortality, as was expected since these are Bin-resistant larvae. For Rocke, a mortality greater than 90% was observed using 0.2 mg/L of L. sphaericus/Bti, while larvae treated with 0.2 mg/L of L. sphaericus showed no mortality, as detected for CqR. This evaluation showed decreasing susceptibility for CqS, CqR, and Rocke larvae to L. sphaericus/Bti, similar to the pattern found in the dose-mortality bioassays. The results also confirmed the susceptibility of CqS to L. sphaericus larvicide, contrasting with the resistant profile of CqR and Rocke larvae to this compound.
Persistence in a simulated field trial
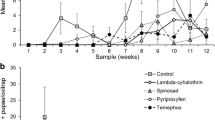
The residual activity of L. sphaericus/Bti-larvicide (Vectomax™ FG) for controlling CqS, CqR, and Rocke larvae cohabiting the same breeding site was evaluated under simulated field conditions. This trial took place for 12 weeks during the warm season of Recife city, with temperatures of 29.1 ± 1.5 °C (27.9 ± 1.0 °C in the water of containers) and relative humidity ranging between 52% and 88%. Treatment with L. sphaericus/Bti-larvicide at 2 g/container or 4 g/container showed initial efficacy, as 100% mortality for all larvae groups was recorded 48 h after the single treatment (Fig. 2). The persistence, considering an average mortality ≥ 80% for all larvae, was 6 weeks and 8 weeks using 2 g or 4 g/container, respectively (Fig. 2). The persistence according to the species was greater for Cx. quinquefasciatus, 7 weeks and 9 weeks at 2 g/container and 4 g/container, respectively, than for Ae. aegypti larvae, 6 weeks and 8 weeks at 2 g/container and 4 g/container, respectively. After 12 weeks, when the trial was finished, 72% and 54% average mortality for Cx. quinquefasciatus and Ae. aegypti larvae, respectively, was still recorded in the set containers treated with 2 g. In the containers treated with 4 g, 80% and 68% of mortality for the respective species mentioned above was detected. Overall, the finest performance for both species was achieved using 4 g/container, which provided an average mortality above 80% for 8 weeks and remained around 65% after 12 weeks. The analysis of the replicate dataset showed an earlier mortality decline in the R1 containers (Additional file 4: Table S3, Additional file 5: Fig. S2), which were exposed to a greater indirect solar incidence compared with the others due to their position in the experimental area (Fig. 1). In the containers treated with 2 g/container, the mortality of both species in the R1 declined below 80% in the third week. For those treated with 4 g, a similar decline was observed in the R1 container after 8 weeks. During the trial, some replicates of the untreated control group showed mortality greater than 20%; when this occurred, the container was replaced.
Residual activity of Lysinibacillus sphaericus/Bacillus thuringiensis svar. israelensis larvicide (Vectomax™ FG) for mosquito larvae. This trial was performed under simulated field conditions using samples of third instar larvae of Culex quinquefasciatus strain CqS (n = 70), Cx. quinquefasciatus resistant to the Binary toxin strain CqR (n = 30), and Aedes aegypti strain/Rockefeller (n = 50). At every week, larvae samples (n = 150) were introduced into each container with 100 L of water, and the average mortality in the four replicates of the treated and untreated groups (control) was recorded 48 h after each colonization. (A) Treatment with 2 g/100 L. (B) Treatment with 4 g/100 L
Genotypes of the SREC individuals treated with larvicides
The genotypes of the SREC individuals for the cqm1 and cqm1REC alleles, subjected to treatments with L. sphaericus or L. sphaericus/Bti larvicides, were determined by PCR (Fig. 3) across four generations. Samples of amplicons with the observed sizes of 208 bp (n = 10) and 189 bp (n = 7) were sequenced, and their identity, as fragments of the cqm1 and cqm1REC alleles, respectively, was confirmed (Additional file 6: Fig. S3). The results are presented in Table 2 and Fig. 4, and the mortality dataset is available in Additional file 7: Table S4. First, the genotypes of samples of CqS (n = 10) and CqR (n = 10) larvae that were used for the parental generation were assessed, and they displayed the expected SS and RR genotypes, respectively. This parental generation (SS 0.7, RR 0.3) produced F1 larvae that displayed a reduction of the frequency of the resistant ones (SS 0.6, SR 0.3, RR 0.1), and this dilution effect was observed at each progeny. The treatment of F1 larvae with L. sphaericus (86% mortality) resulted in the increase of the RR frequency in the surviving adults (0.7). For F2 larvae, the RR genotype decreased as expected (RR 0.4). These larvae were treated using L. sphaericus/Bti with a lethal concentration of around 70% for susceptible larvae (0.01 mg/L); only 47% mortality was achieved and the RR genotype increased for the surviving adults (0.6). This L. sphaericus/Bti concentration was used to treat F3 larvae and similar results were obtained: low mortality and the rise of RR frequency among adults (0.6). Those results showed that sublethal doses of L. sphaericus/Bti to treat larvae samples that exhibit a high frequency of RR genotype have a selective effect. In view of these results, the F4 larvae with a high RR frequency (0.7) were then treated with a concentration of L. sphaericus/Bti lethal for 90% of resistant larvae (0.1 mg/L), and 95% mortality was achieved. In parallel, another sample of F4 larvae treated with L. sphaericus at the same concentration (0.1 mg/L) showed only 45% mortality. These results showed that L. sphaericus/Bti eliminate resistant individuals when suitable concentrations were used. Positive control samples using SS larvae treated with L. sphaericus/Bti or L. sphaericus larvicides at 0.1 mg/L displayed 99% mortality for both larvicides (Additional file 7: Table S4). This assay was performed again using another parental strain named SREC2 established with the same initial frequency of the resistant allele, and produced similar results that were recorded during three generations (Additional file 8: Table S5, Additional file 9: Table S6, Additional file 10: Fig. S4).
Fragments of Culex quinquefasciatus cqm1 (208 bp) and cqm1REC (189 bp) alleles amplified by PCR. The pProfile of amplicons produced are: homozygous-susceptible cqm1/cqm1 (SS), homozygous-resistant cqm1REC/cqm1REC (RR), and heterozygous-susceptible cqm1/cqm1REC (SR) individuals. CS. Positive control for the cqm1 amplicon (208 bp). CR. Positive control for the cqm1REC amplicon (189 bp). CN. Negative control sample without DNA. The DNA used in the test samples was extracted from larvae of the SREC strain (F4). Molecular size marker of 200 bp on the left
Genotypes for cqm1 and cqm1REC alleles in individuals from the Culex quinquefasciatus SREC strain. The parental generation (P) was established with homozygous-susceptible and homozygous-resistant adults. The genotypes of larvae were determined in samples (n ~ 100) at each generation (F) before treatment. Larvae from each generation were treated with Lysinibacillus sphaericus-VectoLex WG® (F1) or L. sphaericus/Bacillus thuringiensis svar. israelensis-VectoMax FG™ (F2-F4). Mortality was recorded and the genotypes of a sample of surviving adults (n ~ 50) was assessed. Full dataset is available in Table 2 and Additional file 7: Table S4
Discussion
The toxicity of combined L. sphaericus/Bti larvicides to different target mosquito species has been scarcely assessed when compared with data available for those individual larvicides. Our study demonstrated that the L. sphaericus/Bti larvicide (Vectomax™ FG) displayed high toxicity to Cx. quinquefasciatus, including Bin-resistant Cx. quinquefasciatus and Ae. aegypti. This larvicide showed action for these individuals lacking functional receptors for the Bin toxin in the midgut [5, 7, 27] whose LC90 value was higher, but it was only threefold compared with that for Cx. quinquefasciatus CqS, considered as the reference strain. Indeed, previous studies showed that Bin-resistant Cx. quinquefasciatus and Ae. aegypti larvae were susceptible to Bti [23, 58,59,60] and to mixtures of Bin and Cyt1Aa toxin [59, 61,62,63,64]. This is likely due to the Cyt1Aa toxin from Bti that enables the Bin toxin to enter the midgut epithelial cells lacking Bin-receptors, whose mechanism is related to the ability of Cyt1Aa to form pores in the cell membrane allowing the entry of Bin [65]. It is worth noting that the LCs found in the present study, and in a previous one, suggest that the Bin action in the midgut cells lacking Bin-receptors might be less efficient compared with that in which the Bin toxin interacts with those cells having Bin-receptors, since the LC values are lower [65]. Nevertheless, the most important fact is that Cyt1Aa synergizes not only the Bti Cry toxins, but also the Bin toxin, improving the in vivo toxicity.
Comparing our toxicity data of L. sphaericus/Bti to Cx. quinquefasciatus/Cx. pipiens and to Ae. aegypti with few reports that are available, our LC90 values were much lower [19, 20, 66, 67]. This may be explained by the origin and rearing conditions of the tested larvae, but also to the methodological procedures to process the stock suspensions for the bioassays. This is particularly relevant when testing commercial products. In our study, for instance, it is likely that the step of incubation for releasing the active ingredient from the Vectomax™ FG granules could be behind the greater toxicity found. The LCs values of Vectomax™ FG to Aedes albopictus [68], previously established, were close to those found for Ae. aegypti in our study, and both were determined using the same methodology. Therefore, the evaluation of the toxicity (LCs) of commercial products, instead of technical powders, must be carefully analyzed to avoid mistaken conclusions. From a qualitative point of view, our dataset is in agreement with in vivo toxicity studies from the literature, as they revealed the same pattern of decreasing susceptibility of Cx. quinquefasciatus and Aedes spp. This is an important parameter for establishing suitable doses for treating different species that can be found in same breeding sites.
The coexistence and high proliferation of Cx. quinquefasciatus and Ae. aegypti larvae in the same areas [69] and even in the same breeding sites [70, 71], might reflect the scenario of several urban areas in endemic countries. In our study, the performance of L. sphaericus/Bti larvicide in containers colonized with both Cx. quinquefasciatus and Ae. aegypti larvae showed promising results with a persistence of 8 weeks, providing at least 80% mortality to both species after a single treatment. The greater persistence observed toward Cx. quinquefasciatus compared with Ae. aegypti corroborates the susceptibility profile found for these species in the bioassays. In addition, it is possible that the lower persistence to Ae. aegypti recorded under simulated field conditions could also be attributed to the faster degradation of Bti crystals, mainly due to temperature and solar radiation compared with the L. sphaericus crystals [56, 72,73,74]. Under such conditions, the lower availability of Cyt1Aa toxin, which is necessary to synergize the action of Bin and Cry toward Ae. aegypti [59, 65], could, for instance, reduce the toxicity to Ae. aegypti larvae. The 8-week persistence period recorded for the L. sphaericus/Bti larvicide (Vectomax™ FG), under the conditions tested, is consistent with other trials in urban and peri-urban for controlling Culex and Aedes, whose persistence ranged from 2 weeks to 5 weeks, according to the breeding sites (for example, catch basis, vegetated ditches), species, and other factors [53, 75,76,77]. The performance found for controlling anophelines, such as Anopheles darlingi, Anopheles funestus, and Anopheles arabiensis, in shaded areas and non-shaded areas was shorter, ranging from 1 week to 4 weeks [47, 78, 79]. The L. sphaericus/Bti product label informs a persistence up to 4 weeks, according to local conditions, and the greater persistence recorded in our trial could be related to the protection from the direct insolation and rain, since these are the main factors that negatively impact the persistence of microbial larvicides [1]. Our dataset revealed an earlier increase of the mortality in containers that were more exposed to indirect sunlight than the others, which corroborates this hypothesis. Another factor to be considered is that, in our trial, the dead larvae were not removed from the containers, and this condition might have allowed for the recycling of L. sphaericus and Bti spores [1, 56], providing an extended action. The removal of dead larvae used in some studies [79] might avoid the beneficial effects of recycling.
The detection of Bin-resistance alleles in Cx. quinquefasciatus populations from several countries remains a threat for the onset of resistance [4]. Our study, using the SREC colony as a model to assess the frequency of resistant genotype of larvae submitted to larvicide treatments, demonstrated that a single L. sphaericus treatment of larvae with a high frequency of that genotype could dramatically raise the frequency. As previously described, the frequency of the cqm1REC, which was the major resistance allele found in nontreated populations from Recife city, was low (10–3) [26, 32, 80]. Therefore, the selection of a resistance allele with a low initial frequency, associated with the recessive inheritance of the cqm1REC [27], can be a gradual process. Further, in treated areas with records of operational failures, the frequency of such alleles in treated populations can be high [28, 30, 32] and their selection can be fast, as demonstrated in this study. In this scope, our results reinforced that the frequency and inheritance of the resistant alleles are key parameters for the resistance selection and can be used to indicate the adoption of proactive measures to avoid the rise of the resistance allele frequency [81]. Our data showed that once a high frequency of the Bin-resistant genotype was achieved, lethal doses of L. sphaericus/Bti larvicide to those individuals have to be used for their elimination, but sublethal doses can increase their frequency. L. sphaericus/Bti-based larvicides can also be employed to counteract the resistance to other insecticidal compounds. This was shown for an Anopheles coluzzii resistant to pyrethroids whose larvae were treated with L. sphaericus/Bti to eliminate resistant individuals and restore the susceptibility [82]. These results highlight the strategic importance of the complex composition of toxins in such larvicides for resistance management in different scenarios.
Conclusions
Our dataset showed that the combined L. sphaericus/Bti larvicide displays high toxicity to Cx. quinquefasciatus, Bin-resistant Cx. quinquefasciatus and Ae. aegypti, and under a simulated trial it displayed a fine persistence compatible with bimonthly schemes of product application. The choice of suitable doses of the larvicide to control different target species considered their susceptibility profiles, which is crucial for its performance, as demonstrated for the control of Cx. quinquefasciatus and Ae. aegypti. These findings demonstrated that L. sphaericus/Bti larvicides can be an effective tool for controlling those species in urban areas with a low risk for selecting resistance.
Availability of data and materials
All data are provided in the manuscript or in the supplementary files.Raw data from all assays are available in the supplementary tables.
Abbreviations
- Bin:
-
Binary toxin
- Bti:
-
Bacillus thuringiensis svar. israelensis
- Cqm1:
-
Culex quinquefasciatus maltase 1
- cqm1 :
-
Gene encoding the Culex quinquefasciatus maltase 1
- cqm1 REC :
-
Resistance allele of the cqm1 gene
- CqR:
-
Culex quinquefasciatus Bin-resistant
- Cqs:
-
Culex quinquefasciatus susceptible
- Fiocruz:
-
Fundação Oswaldo Cruz
- GPI:
-
Glycosylphosphatidylinositol
- IAM:
-
Instituto Aggeu Magalhães
- ITU:
-
International toxic units
- LC:
-
Lethal concentration
- Lsp:
-
Lysinibacillus sphaericus
- P:
-
Parental generation
- PCR:
-
Polymerase chain reaction
- REC:
-
Culex quinquefasciatus strain composed of CqR individuals
- Rocke:
-
Aedes aegypti Rockefeller strain
- RR:
-
Homozygous individuals for the cqm1REC allele
- RR:
-
Resistance ratio
- RT:
-
Room temperature
- S:
-
Culex quinquefasciatus strain composed of CqS individuals
- SD:
-
Standard deviation
- SR:
-
Heterozygous individuals for the cqm1 and cqm1REC alleles
- SREC:
-
Culex quinquefasciatus strain composed of CqS and CqR individuals
- SS:
-
Homozygous individuals for the cqm1 allele
- TR:
-
Toxicity ratio
- WHO:
-
World Health Organization
References
Lacey LA. Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc. 2007;23:133–63. https://doi.org/10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2.
Berry C. The bacterium, LysiniBacillus sphaericus, as an insect pathogen. J Invertebr Pathol. 2012;109:1–10.
Soberón M, Fernández LE, Pérez C, Gill SS, Bravo A. Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon. 2007;49:597–600. https://doi.org/10.1016/j.toxicon.2006.11.008.
Silva-Filha MHNL, Romão TP, Rezende TMT, Carvalho KS, Menezes HSG, Nascimento AL, et al. Bacterial toxins against mosquitoes: mode of action and resistance. Toxins. 2021;13:523. https://doi.org/10.3390/toxins13080523.
Nielsen-Leroux C, Charles JF. Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur J Biochem /FEBS J. 1992;210:585–90.
Nielsen-Leroux C, Charles JF, Thiery I, Georghiou GP. Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur J Biochem /FEBS J. 1995;228:206–10.
Ferreira LM, Romão TP, de-Melo-Neto OP, Silva-Filha MH. The orthologue to the Cpm1/Cqm1 receptor in Aedes aegypti is expressed as a midgut GPI-anchored alpha-glucosidase, which does not bind to the insecticidal binary toxin. Insect Biochem Mol Biol. 2010;408:604–10. https://doi.org/10.1016/j.ibmb.2010.05.007.
Berry C, O’Neil S, Ben-Dov E, Jones AF, Murphy L, Quail MA, et al. Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. App Environ Microbiol. 2002;68:5082–95.
Crickmore N, Bone EJ, Wiliams JA, Ellar DJ. Contribution of the individual components of the delta-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subsp. israelensis. FEMS Microbiol Lett. 1995;131:249–54.
Cantón PE, Zanicthe-Reyes EZ, de Escudero I, Bravo A, Soberón M. Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides. 2011;323:595–600. https://doi.org/10.1016/j.peptides.2010.06.005.
Pérez C, Muñoz-Garay C, Portugal LC, Sánchez J, Gill SS, Soberón M, et al. Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol. 2007;9:2931–7. https://doi.org/10.1111/j.1462-5822.2007.01007.x.
Likitvivatanavong S, Chen J, Evans AM, Bravo A, Soberón M, Gill SS. Multiple receptors as targets of Cry toxins in mosquitoes. J Agricult Food Chem. 2011;59:2829–38. https://doi.org/10.1021/jf1036189.
Alam I, Batool K, Idris AL, Tan W, Guan X, Zhang L. Role of Lectin in the Response of Aedes aegypti Against Bt Toxin. Front Immunol. 2022;13:898198. https://doi.org/10.3389/fimmu.2022.898198.
Mulla MS, Thavara U, Tawatsin A, Chomposri J, Su T. Emergence of resistance and resistance management in field populations of tropical Culex quinquefasciatus to the microbial control agent Bacillus sphaericus. J Am Mosq Control Assoc. 2003;19:39–46.
Nielsen-Leroux C, Pasteur N, Pretre J, Charles JF, Sheikh HB, Chevillon C. High resistance to Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae): the complex situation of West Mediterranean countries. J Med Entomol. 2002;39:729–35.
Rao DR, Mani TR, Rajendran R, Joseph AS, Gajanana A, Reuben R. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi. India J Am Mosq Control Assoc. 1995;11:1–5.
Sinègre G, Babinot M, Vigo G, Jullien JL: First occurrence of Culex pipiens resistance to Bacillus sphaericus in Southern France. In: VIII European Meeting of Society of Vector Ecology 5–8 September 1994 Faculty of Biologia, University of Barcelona Spain1994.
Su T, Mulla MS. Documentation of high-level Bacillus sphaericus 2362 resistance in field populations of Culex quinquefasciatus breeding in polluted water in Thailand. J Am Mosq Control Assoc. 2004;20:405–11.
Su T, Thieme J, Ocegueda C, Ball M, Cheng ML. Resistance to LysiniBacillus sphaericus and Other Commonly Used Pesticides in Culex pipiens (Diptera: Culicidae) from Chico. California J Med Entomol. 2018;55:423–8. https://doi.org/10.1093/jme/tjx235.
Su T, Thieme J, White GS, Lura T, Mayerle N, Faraji A, et al. High Resistance to Bacillus sphaericus and Susceptibility to Other Common Pesticides in Culex pipiens (Diptera: Culicidae) from Salt Lake City. UT J Med Entomol. 2019;56:506–13. https://doi.org/10.1093/jme/tjy193.
Yuan ZM, Zhang YM, Liu EY. High-level field resistance to Bacillus sphaericus C3–41 in Culex quinquefasciatus from Southern China. Biocontrol Sci Technol. 2000;10:43–51.
Amorim LB, Oliveira CMF, Rios EM, Regis L, Silva-Filha MHNL. Development of Culex quinquefasciatus resistance to Bacillus sphaericus strain IAB59 needs long term selection pressure. Biol Control. 2007;42:155–60.
Pei G, Oliveira CM, Yuan Z, Nielsen-LeRoux C, Silva-Filha MH, Yan J, et al. A strain of Bacillus sphaericus causes slower development of resistance in Culex quinquefasciatus. Appl Environ Microbiol. 2002;68:3003–9.
Rodcharoen J, Mulla MS. Resistance development in Culex quinquefasciatus to the microbial agent Bacillus sphaericus. J Econ Entomol. 1994;87:1133–40.
Wirth MC, Georghiou GP, Malik JI, Abro GH. Laboratory selection for resistance to Bacillus sphaericus in Culex quinquefasciatus (Diptera: Culicidae) from California, USA. J Med Entomol. 2000;37:534–40.
Chalegre KD, Romão TP, Tavares DA, Santos EM, Ferreira LM, Oliveira CMF, et al. Novel mutations associated to Bacillus sphaericus resistance are identified in a polymorphic region of the Culex quinquefasciatus cqm1 gene. Appl Environ Microbiol. 2012;7817:6321–6.
Chalegre KD, Tavares DA, Romao TP, Menezes HSG, Nascimento AL, Oliveira CMF, et al. Co-selection and replacement of resistance alleles to LysiniBacillus sphaericus in a Culex quinquefasciatus colony. FEBS J. 2015;282:3592–602. https://doi.org/10.1111/febs.13364.
Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D. Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae). Cell Microbiol. 2007;9:2022–9. https://doi.org/10.1111/j.1462-5822.2007.00934.x.
Darboux I, Pauchet Y, Castella C, Silva-Filha MH, Nielsen-LeRoux C, Charles JF, et al. Loss of the membrane anchor of the target receptor is a mechanism of bioinsecticide resistance. Proc Natl Acad Sci USA. 2002;99:5830–5.
Guo QY, Cai QX, Yan JP, Hu XM, Zheng DS, Yuan ZM. Single nucleotide deletion of cqm1 gene results in the development of resistance to Bacillus sphaericus in Culex quinquefasciatus. J Insect Physiol. 2013;59:967–73. https://doi.org/10.1016/j.jinsphys.2013.07.002.
Romão TP, de Melo-Chalegre KD, Key S, Ayres CF, de Oliveira CM, de Melo-Neto OP, et al. A second independent resistance mechanism to Bacillus sphaericus binary toxin targets its alpha-glucosidase receptor in Culex quinquefasciatus. FEBS J. 2006;2737:1556–68. https://doi.org/10.1111/j.1742-4658.2006.05177.x.
Chalegre KD, Romão TP, Amorim LB, Anastacio DB, de Barros RA, de Oliveira CM, et al. Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol. 2009;75:1044–9. https://doi.org/10.1128/AEM.02032-08.
Becker N, Ludwig M, Su T. Lack of Resistance in Aedes vexans Field Populations After 36 Years of Bacillus thuringiensis subsp israelensis Applications in the Upper Rhine Valley. Germany J Am Mosq Control Assoc. 2018;34:154–7. https://doi.org/10.2987/17-6694.1.
Carvalho KDS, Crespo MM, Araujo AP, da Silva RS, de Melo-Santos MAV, de Oliveira CMF, et al. Long-term exposure of Aedes aegypti to Bacillus thuringiensis svar israelensis did not involve altered susceptibility to this microbial larvicide or to other control agents. Parasit Vectors. 2018;111:673. https://doi.org/10.1186/s13071-018-3246-1.
Goldman IF, Arnold J, Carlton BC. Selection for resistance to Bacillus thuringiensis subspecies israelensis in field and laboratory populations of the mosquito Aedes aegypti. J Invertebr Pathol. 1986;47:317–24.
Stalinski R, Laporte F, Tetreau G, Despres L. Receptors are affected by selection with each Bacillus thuringiensis israelensis Cry toxin but not with the full Bti mixture in Aedes aegypti. Infect Genet Evol. 2016;44:218–27. https://doi.org/10.1016/j.meegid.2016.07.009.
Cadavid-Restrepo G, Sahaza J, Orduz S. Treatment of an Aedes aegypti colony with the Cry11Aa toxin for 54 generations results in the development of resistance. Mem Inst Oswaldo Cruz. 2012;107:74–9.
Ben-Dov E. Bacillus thuringiensis subsp. israelensis and its dipteran-specific toxins. Toxins (Basel). 2014;64:1222–43. https://doi.org/10.3390/toxins6041222.
Afrane YA, Mweresa NG, Wanjala CL, Gilbreath-Iii TM, Zhou G, Lee MC, et al. Evaluation of long-lasting microbial larvicide for malaria vector control in Kenya. Malar J. 2016;151:577. https://doi.org/10.1186/s12936-016-1626-6.
Kahindi SC, Muriu S, Derua YA, Wang X, Zhou G, Lee MC, et al. Efficacy and persistence of long-lasting microbial larvicides against malaria vectors in western Kenya highlands. Parasit Vectors. 2018;111:438. https://doi.org/10.1186/s13071-018-3009-z.
Zhou G, Lo E, Githeko AK, Afrane YA, Yan G. Long-lasting microbial larvicides for controlling insecticide resistant and outdoor transmitting vectors: a cost-effective supplement for malaria interventions. Infect Dis Poverty. 2020;91:162. https://doi.org/10.1186/s40249-020-00767-3.
Antonio-Nkondjio C, Doumbe-Belisse P, Djamouko-Djonkam L, Ngadjeu CS, Talipouo A, Kopya E, et al. High efficacy of microbial larvicides for malaria vectors control in the city of Yaounde Cameroon following a cluster randomized trial. Sci Rep. 2021;111:17101. https://doi.org/10.1038/s41598-021-96362-z.
Mwangangi JM, Kahindi SC, Kibe LW, Nzovu JG, Luethy P, Githure JI, et al. Wide-scale application of Bti/Bs biolarvicide in different aquatic habitat types in urban and peri-urban Malindi. Kenya Parasitol Res. 2011;108:1355–63. https://doi.org/10.1007/s00436-010-2029-1.
Talipouo A, Doumbe-Belisse P, Ngadjeu CS, Djamouko-Djonkam L, Nchoutpouen E, Bamou R, et al. Larviciding intervention targeting malaria vectors also affects Culex mosquito distribution in the city of Yaounde, Cameroon. Curr Res Parasitol Vector Borne Dis. 2023;4:100136. https://doi.org/10.1016/j.crpvbd.2023.100136.
Derua YA, Kweka EJ, Kisinza WN, Githeko AK, Mosha FW. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: review of their effectiveness and operational feasibility. Parasit Vectors. 2019;12:426. https://doi.org/10.1186/s13071-019-3683-5.
Zhou G, Wiseman V, Atieli HE, Lee MC, Githeko AK, Yan G. The impact of long-lasting microbial larvicides in reducing malaria transmission and clinical malaria incidence: study protocol for a cluster randomized controlled trial. Trials. 2016;171:423. https://doi.org/10.1186/s13063-016-1545-4.
Fontoura PS, Silva MF, da Costa AS, Ribeiro FS, Ferreira MS, Ladeia-Andrade S, et al. Monthly biological larviciding associated with a tenfold decrease in larval density in fish farming ponds and reduced community-wide malaria incidence in northwestern Brazil. Parasit Vectors. 2021;14:445. https://doi.org/10.1186/s13071-021-04964-3.
Dritz DA, Lawler SP, Evkhanian C, Graham P, Baracosa V, Dula G. Control of mosquito larvae in seasonal wetlands on a wildlife refuge using VectoMax CG. J Am Mosq Control Assoc. 2011;27:398–403.
Anderson JF, Ferrandino FJ, Dingman DW, Main AJ, Andreadis TG, Becnel JJ. Control of mosquitoes in catch basins in Connecticut with Bacillus thuringiensis israelensis, Bacillus sphaericus, [corrected] and spinosad. J Am Mosq Control Assoc. 2011;27:45–55. https://doi.org/10.2987/10-6079.1.
Baldacchino F, Bussola F, Arnoldi D, Marcantonio M, Montarsi F, Capelli G, et al. An integrated pest control strategy against the Asian tiger mosquito in northern Italy: a case study. Pest Manag Sci. 2017;73:87–93. https://doi.org/10.1002/ps.4417.
Eritja R. Laboratory tests on the efficacy of VBC60035, a combined larvicidal formulation of Bacillus thuringiensis israelensis (strain AM65-52) and Bacillus sphaericus (strain 2362) against Aedes albopictus in simulated catch basins. J Am Mosq Control Assoc. 2013;29:280–3.
Guidi V, Lehner A, Luthy P, Tonolla M. Dynamics of Bacillus thuringiensis var israelensis and LysiniBacillus sphaericus spores in urban catch basins after simultaneous application against mosquito larvae. PLoS One. 2013;82:e55658. https://doi.org/10.1371/journal.pone.0055658.
Nasci RS, Runde AB, Henry M, Harbison JE. Effectiveness of Five Products To Control Culex pipiens Larvae In Urban Stormwater Catch Basins. J Am Mosq Control Assoc. 2017;33:309–17. https://doi.org/10.2987/17-6686.1.
Menezes HSG, Nascimento NA, Paiva-Cavalcanti M, da Costa-Latge SG, Genta FA, Oliveira CM, et al. Molecular and biological features of Culex quinquefasciatus homozygous larvae for two cqm1 alleles that confer resistance to LysiniBacillus sphaericus larvicides. Pest Manag Sci. 2021;77:3135–3144. https://doi.org/10.1002/ps.6349.
WHO: Informal consultation on the development of Bacillus sphaericus as microbial larvicide. In: World Health Organization, vol. Geneve1985: TDR/BCV/SPHAERICUS/85.3.1–24.
Melo-Santos MA, Araújo AP, Rios EM, Regis L. Long lasting persistence of Bacillus thuringiensis serovar israelensis larvicidal activity in Aedes aegypti (Diptera: Culicidae) breeding places is associated to bacteria recycling. Biol Control. 2009;49:186–91.
Nakasawa MM, Araujo AP, Melo-Santos MA, Oliveira CMF, Silva-Filha MH. Persistence of Bacillus thuringiensis svar israelensis (Bti) and pyriproxyfen based products in artificial breeding sites colonized with susceptible or Bti-exposed Aedes aegypti larvae. Biological Control. 2020;151:104400. https://doi.org/10.1016/j.biocontrol.2020.104400.
Nielsen-LeRoux C, Rao DR, Murphy JR, Carron A, Mani TR, Hamon S, et al. Various levels of cross-resistance to Bacillus sphaericus strains in Culex pipiens (Diptera: Culicidae) colonies resistant to B. sphaericus strain 2362. Appl Environ Microbiol. 2001;67:5049–54.
Wirth MC, Jiannino JA, Federici BA, Walton WE. Synergy between toxins of Bacillus thuringiensis subsp. israelensis and Bacillus sphaericus. J Med Entomol. 2004;41:935–41.
Yuan ZM, Pei GF, Regis L, Nielsen-Leroux C, Cai QX. Cross-resistance between strains of Bacillus sphaericus but not B. thuringiensis israelensis in colonies of the mosquito Culex quinquefasciatus. Med Vet Entomol. 2003;17:251–6.
Bideshi DK, Park HW, Hice RH, Wirth MC, Federici BA. Highly effective broad spectrum chimeric larvicide that targets vector mosquitoes using a lipophilic protein. Sci Rep. 2017;7:11282. https://doi.org/10.1038/s41598-017-11717-9.
Lekakarn H, Promdonkoy B, Boonserm P. Interaction of LysiniBacillus sphaericus binary toxin with mosquito larval gut cells: Binding and internalization. J Invertebr Pathol. 2015;132:125–31. https://doi.org/10.1016/j.jip.2015.09.010.
Park HW, Bideshi DK, Wirth MC, Johnson JJ, Walton WE, Federici BA. Recombinant larvicidal bacteria with markedly improved efficacy against Culex vectors of West Nile virus. Am J Trop Med Hyg. 2005;72:732–8.
Wirth MC, Delecluse A, Walton WE. Cyt1Ab1 and Cyt2Ba1 from Bacillus thuringiensis subsp. medellin and B thuringiensis subsp israelensis Synergize Bacillus sphaericus against Aedes aegypti and resistant Culex quinquefasciatus (Diptera: Culicidae). Appl Environ Microbiol. 2001;67:3280–4.
Nascimento NA, Torres-Quintero MC, Molina SL, Pacheco S, Romao TP, Pereira-Neves A, et al. Functional Bacillus thuringiensis Cyt1Aa is necessary to synergize LysiniBacillus sphaericus Binary toxin (Bin) against Bin-resistant and refractory mosquito species. Appl Environ Microbiol. 2020;86:7. https://doi.org/10.1128/AEM.02770-19.
Algamdi AG, Mahyoub JA. Efficacy of certain conventional and non-conventional insecticides against a vector of dengue fever, the Aedes aegypti Mosquito in Saudi Arabia. Entomol Res. 2022;52:345–55. https://doi.org/10.1111/1748-5967.12607.
Su T, Thieme J, Lura T, Cheng ML, Brown MQ. Susceptibility Profile of Aedes aegypti L. (Diptera: Culicidae) from Montclair, California, to Commonly Used Pesticides, With Note on Resistance to Pyriproxyfen. J Med Entomol. 2019;56:1047–54. https://doi.org/10.1093/jme/tjz019.
Suter T, Crespo MM, de Oliveira MF, de Oliveira TSA, de Melo-Santos MAV, de Oliveira CMF, et al. Insecticide susceptibility of Aedes albopictus and Ae. aegypti from Brazil and the Swiss-Italian border region. Parasit Vectors. 2017;10:431. https://doi.org/10.1186/s13071-017-2364-5.
Santos EM, Regis LN, Silva-Filha MHNL, Barbosa RMB, Gomes TCS, Oliveira CMF. The effectiveness of a combined bacterial larvicide for mosquito control in an endemic urban area in Brazil. Biol Control. 2018;121:190–8.
Barrera R, Amador M, Diaz A, Smith J, Munoz-Jordan JL, Rosario Y. Unusual productivity of Aedes aegypti in septic tanks and its implications for dengue control. Med Vet Entomol. 2008;22:62–9. https://doi.org/10.1111/j.1365-2915.2008.00720.x.
Santos SA, Barbosa RM. Immature Aedes mosquitoes colonize Culex quinquefasciatus breeding sites in neighborhoods in the municipality of Olinda, State of Pernambuco. Rev Soc Bras Med Trop. 2014;47:775–7. https://doi.org/10.1590/0037-8682-0113-2014.
Araújo AP, Melo-Santos MAV, Carlos SO, Rios EM, Regis L. Evaluation of an experimental product based on Bacillus thuringiensis sorovar israelensis against Aedes aegypti larvae (Diptera: Culicidae). Biol Control. 2007;41:339–47.
Christiansen JA, McAbee RD, Stanich MA, DeChant P, Boronda D, Cornel AJ. Influence of temperature and concentration of VectoBac on control of the salt-marsh mosquito, Ochlerotatus squamiger, in Monterey County. California J Am Mosq Control Assoc. 2004;20:165–70.
Nicolas L, Regis LN, Rios EM. Role of the exosporium in the stability of the Bacillus sphaericus binary toxin. FEMS Microbiol Lett. 1994;124:271–5.
Harbison JE, Henry M, Xamplas C, Berry R, Bhattacharya D, Dugas LR. A comparison of FourStar Briquets and natular XRT tablets in a North Shore suburb of Chicago. Il J Am Mosq Control Assoc. 2014;30:68–70. https://doi.org/10.2987/13-6355.1.
Ibanez-Justicia A, Teekema S, den Hartog W, Jacobs F, Dik M, Stroo A. The effectiveness of asian bush mosquito (Aedes japonicus japonicus) control actions in colonised peri-urban areas in the Netherlands. J Med Entomol. 2018;55:673–80. https://doi.org/10.1093/jme/tjy002.
Virgillito C, Manica M, Marini G, Rosa R, Della Torre A, Martini S, et al. Evaluation of Bacillus thuringiensis subsp israelensis and Bacillus sphaericus Combination Against Culex pipiens in Highly Vegetated Ditches. J Am Mosq Control Assoc. 2022;38:40–5. https://doi.org/10.2987/21-7024.
Diedhiou SM, Konate L, Doucoure S, Samb B, Niang EA, Sy O, et al. Effectiveness of three biological larvicides and of an insect growth regulator against Anopheles arabiensis in Senegal. Bull Soc Pathol Exot. 2017;110:102–15. https://doi.org/10.1007/s13149-016-0531-4.
Msugupakulya BJ, Ngajuma SK, Ngayambwa AN, Kidwanga BE, Mpasuka IR, Selvaraj P, et al. Influence of larval growth and habitat shading on retreatment frequencies of biolarvicides against malaria vectors. Sci Rep. 2024;14:1002. https://doi.org/10.1038/s41598-024-51152-1.
Menezes HS, Chalegre KD, Romao TP, Oliveira CM, de Melo-Neto OP, Silva-Filha MH. A new allele conferring resistance to LysiniBacillus sphaericus is detected in low frequency in Culex quinquefasciatus field populations. Parasit Vectors. 2016;9:70. https://doi.org/10.1186/s13071-016-1347-2.
Tabashnik BE, Brevault T, Carriere Y. Insect resistance to Bt crops: lessons from the first billion acres. Nat Biotechnol. 2013;31:510–21. https://doi.org/10.1038/nbt.2597.
Nkahe DL, Sonhafouo-Chiana N, Ndjeunia Mbiakop P, Kekeunou S, Mimpfoundi R, Awono-Ambene P, et al. Can the use of larviciding with biological compounds contribute in increasing Anopheles gambiae s.l. susceptibility to pyrethroid in a population expressing high resistance intensity? Pestic Biochem Physiol. 2023;195:105569. https://doi.org/10.1016/j.pestbp.2023.105569.
Acknowledgement
We would like to thank the insectary team and the Reference Service for Culicide Vector Control (SRCVC) from IAM-Fiocruz; the Technology Platforms Center (NPT) from IAM-Fiocruz for sequencing the DNA samples; Tatiany Patrícia Romão for the assistance with the production of figures; and Dr. George Tadeu Diniz from the team of Statistics and Geoprocessing, IAM-Fiocruz for data analysis.
Funding
This study was financed by the Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (grant 400747/2019–7), INOVA-Fiocruz (grant VPPCB-007-FIO-18–2-64), and Fundação de Apoio à Ciência e Tecnologia do Estado de Pernambuco- FACEPE (grant IBPG-0204–2.13/21).
Author information
Authors and Affiliations
Contributions
H.L.R., M.A.V.M.S., and M.H.N.L.S.F. conceived and designed experiments; H.L.R. and H.S.G.M. performed the experiments; H.L.R., H.S.G.M., M.A.V.M.S., and M.H.N.L.S.F. analyzed data; and H.L.R. and M.H.N.L.S.F. wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Representation of the procedures for the maintenance of the Culex quinquefasciatus SREC strain.
Additional file 2: Table S1
. Dataset of the dose–response bioassays of the Lysinibacillus sphaericus/Bacillus thuringiensis svar. israelensis against mosquito larvae.
Additional file 3: Table S2
. Dataset of the diagnostic bioassays of the Lysinibacillus sphaericus and/or Bacillus thuringiensis svar. israelensis against mosquito larvae.
Additional file 4: Table S3
. Dataset of the residual activity of Lysinibacillus sphaericus/Bacillus thuringiensis svar. israelensis larvicide toward mosquito larvae.
Additional file 5: Figure S2
. Dataset of the residual activity of Lysinibacillus sphaericus/Bti to control mosquito larvae.
Additional file 6: Figure S3
. Nucleotide sequences of amplicons of the Culex quinquefasciatus cqm1 gene from SREC individuals.
Additional file 7: Table S4.
Mortality of Culex quinquefasciatus larvae from the SREC strain from four generations (F).
Additional file 8: Table S5.
Frequency of the genotypes for the cqm1 and cqm1REC alleles in Culex quinquefasciatus SREC2 strain.
Additional file 9: Table S6.
Mortality of Culex quinquefasciatus larvae from SREC2 strain from four generations (F).
Additional file 10: Figure S4.
Genotypes for the cqm1 and cqm1REC alleles in individuals from the Culex quinquefasciatus SREC2 strain.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Rique, H.L., Menezes, H.S.G., Melo-Santos, M.A.V. et al. Evaluation of a long-lasting microbial larvicide against Culex quinquefasciatus and Aedes aegypti under laboratory and a semi-field trial. Parasites Vectors 17, 391 (2024). https://doi.org/10.1186/s13071-024-06465-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13071-024-06465-5