Abstract
Background
Neuropsychiatric symptoms (NPS) are a core feature of most neurodegenerative and cerebrovascular diseases. White matter hyperintensities and brain atrophy have been implicated in NPS. We aimed to investigate the relative contribution of white matter hyperintensities and cortical thickness to NPS in participants across neurodegenerative and cerebrovascular diseases.
Methods
Five hundred thirteen participants with one of these conditions, i.e. Alzheimer’s Disease/Mild Cognitive Impairment, Amyotrophic Lateral Sclerosis, Frontotemporal Dementia, Parkinson’s Disease, or Cerebrovascular Disease, were included in the study. NPS were assessed using the Neuropsychiatric Inventory – Questionnaire and grouped into hyperactivity, psychotic, affective, and apathy subsyndromes. White matter hyperintensities were quantified using a semi-automatic segmentation technique and FreeSurfer cortical thickness was used to measure regional grey matter loss.
Results
Although NPS were frequent across the five disease groups, participants with frontotemporal dementia had the highest frequency of hyperactivity, apathy, and affective subsyndromes compared to other groups, whilst psychotic subsyndrome was high in both frontotemporal dementia and Parkinson’s disease. Results from univariate and multivariate results showed that various predictors were associated with neuropsychiatric subsyndromes, especially cortical thickness in the inferior frontal, cingulate, and insula regions, sex(female), global cognition, and basal ganglia-thalamus white matter hyperintensities.
Conclusions
In participants with neurodegenerative and cerebrovascular diseases, our results suggest that smaller cortical thickness and white matter hyperintensity burden in several cortical-subcortical structures may contribute to the development of NPS. Further studies investigating the mechanisms that determine the progression of NPS in various neurodegenerative and cerebrovascular diseases are needed.
Similar content being viewed by others
Background
Neuropsychiatric symptoms (NPS) (such as depression, anxiety, apathy, psychosis, and disinhibition) are commonly reported in neurodegenerative and cerebrovascular diseases [1]. Their high frequency and increased severity are associated with higher patient distress, increased caregiver burden, and higher rates of institutionalised care [2, 3]. Moreover, the frequency of NPS varies across the various neurodegenerative and cerebrovascular disease. Affective symptoms like anxiety and depression are more prevalent in Alzheimer’s disease (AD) and vascular dementia (VaD) [4,5,6,7]. Apathy is most commonly reported in AD and frontotemporal dementia (FTD), and associated with functional impairment and disease progression [8, 9]. But the pattern of apathy presentation differs such that AD-related apathy is indicative of depression, cognitive dysfunction, and conversion from amnestic mild cognitive impairment (aMCI) to AD [10, 11], whilst FTD-related apathy is associated with measures of social cognition and executive dysfunction [11,12,13]. Moreover, the apathy symptoms observed in FTD have also been reported in ALS with behavioural variant FTD (bvFTD) [14, 15]. In Parkinson’s disease (PD), depression and anxiety are also present in addition to apathy, fatigue, sleep disturbances, and psychosis [16, 17]. Since the manifestation of NPS likely represents brain abnormalities and may reflect progression of disease, it is important to recognise the neural basis of NPS in neurodegenerative and cerebrovascular diseases.
Regional brain changes have been implicated in NPS in neurodegenerative and cerebrovascular diseases [5, 12, 15, 18,19,20,21,22,23]. Symptoms of apathy, anxiety, and depression reported in aMCI have been linked to smaller cortical thickness and volume in the frontal, temporal, and parietal regions [5, 19, 21]. In PD, lower frontal lobe volume was related to affective, psychotic, and apathy symptoms [22], whilst a smaller cortical thickness and volume of the frontotemporal, insular, and limbic regions was related to apathy and disinhibition in FTD and ALS [12, 15, 23]. In cerebrovascular disease (CVD), appetite/eating behaviour, depression, and apathy has been associated with smaller hippocampal, middle, and posterior cingulate volumes [24,25,26]. Although these studies suggest that NPS, particularly affective, apathy, and psychotic symptoms are most frequently associated with grey matter alterations in the fronto-subcortical circuitries, white matter lesions such as white matter hyperintensities (WMH) have also been implicated in NPS [27,28,29,30,31,32].
WMH have traditionally been attributed to either cerebrovascular disease [33], ageing [34], or neuroinflammatory processes [35]. In most neurodegenerative diseases, they are attributed to small vessel disease (SVD) [33]. However, these assumptions are being questioned as there is increasing evidence that non-vascular pathology such as tau-mediated secondary demyelination or microglial dysfunction may also contribute to WMH in neurodegenerative diseases [36]. In the context of presumed vascular origin, WMH in the frontal, parieto-occipital, and basal ganglia areas have been related to psychotic symptoms in AD [27]. Furthermore, greater WMH load (particularly in the frontal lobe) has been associated with greater delusions, hallucinations, anxiety, apathy, and depression in both AD and VaD [28,29,30,31,32, 37], as well as severe apathy and night time behaviour in FTD [37], and depression in PD with dementia [38].
Whilst changes in brain thickness, volume, and WMH burden have been associated with NPS, not many studies have investigated their contributions to NPS across multiple neurodegenerative and cerebrovascular diseases. This limitation may be partly due to the lack of transdiagnostic datasets, as previous research have focused on analyses within a single disease [39] or multiple diseases, mainly consisting of VaD, AD/MCI, and mixed dementia [31, 40], occasionally PD and FTD [30, 37], and none on ALS. Thus, the aims of the present study were to compare the frequency of NPS across multiple neurodegenerative and cerebrovascular diseases and to determine its relationship with WMH burden and cortical thickness across all cohorts. We hypothesised that all cohorts would display high frequency of NPS, particularly in participants with FTD and it will be associated with both WMH burden and a smaller focal cortical thickness.
Methods
Participants and study design
Study participants were enrolled as part of Ontario Neurodegenerative Disease Research Initiative (ONDRI), a multi-centre, multiple assessment, longitudinally observational study conducted in nine tertiary care academic medical centres in Ontario, Canada. Detailed inclusion and exclusion criteria for each diagnostic cohort (dx) are reported elsewhere [41, 42]. Briefly, AD/MCI participants met National Institute on Aging Alzheimer’s Association criteria for probable or possible AD, or aMCI [43, 44]; ALS participants met El Escorial World Federation of Neurology diagnostic criteria for possible, probable, or definite familial or sporadic ALS [45]; the latest criteria were used for possible or probable bvFTD [46], for agrammatic/non-fluent and semantic variants of primary progressive aphasia (nfvPPA and svPPA) [47] and possible or probable progressive supranuclear palsy (PSP) and corticobasal syndrome (CBS) [48]; PD participants met criteria for idiopathic PD defined by the United Kingdom’s Parkinson’s Disease Society Brain Bank clinical diagnostic criteria [49]; and CVD participants had experienced a mild or moderate ischemic stroke event (documented on MRI or CT) 3 or more months prior to enrolment in compliance with the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonisation standards [50]. The study was approved by each participating institution’s Research Ethics Board and performed in accordance with the Declaration of Helsinki. All participants provided informed consent and subsequently underwent clinical evaluation and MRI, in addition to the other assessments as part of the full ONDRI protocol described elsewhere [41]. The current project only used data from the baseline evaluation.
Measures
Neuropsychiatric symptoms (NPS) assessment
The Neuropsychiatric Inventory-Questionnaire (NPI-Q) was used to assess NPS observed in dementia [51]. Specifically, the study partners completed a questionnaire, where they indicated the presence and severity (mild, moderate, and severe) of 12 common NPS. This questionnaire also measured the level of distress (on a 5-point scale) the NPS caused the study partner. A total NPI-Q severity score was the sum of all the individual symptom severity scores and the total NPI-Q study partner distress score was the sum of all the individual symptom study partner distress scores. For the current study, we classified the symptoms into four neuropsychiatric subsyndrome groups in accordance with the European Alzheimer’s Disease Consortium [52], and the score for each subsyndrome was the sum of all the symptom severity scores in the subsyndrome: hyperactivity subsyndrome (agitation/aggression, euphoria/elation, irritability/lability, disinhibition, and aberrant motor behaviour); psychotic subsyndrome (hallucinations, delusions, and night time behaviours); affective subsyndrome (depression/dysphoria and anxiety); and apathy subsyndrome (apathy/indifference and appetite/eating).
Global cognitive and functional assessments
Global cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) on all participants [53] for which the total score was adjusted for educational attainment, and they were rated on instrumental activity of daily living (iADLs) and activity of daily living (ADLs) by their study partners [54].
Vascular risk factors
Participants were considered to have vascular risk factors if they reported to have received a diagnosis of hypertension, diabetes, and/or high cholesterol during medical history interview in addition to smoking history. Furthermore, we created a total measure of vascular risk factors burden per participant by counting the occurrences where they indicated a diagnosis of hypertension, diabetes, and/or high cholesterol, and having ever smoked for 3 or more months.
MRI acquisition
MRI scans were acquired using 3 Tesla MRI systems. MRI protocols details are published elsewhere [55, 56] and harmonised with the Canadian Dementia Imaging Protocol (CDIP) [57]. Briefly, the structural MRI used in this specific analysis of ONDRI data included the following sequences: high-resolution three-dimensional T1-weighted, interleaved proton density, T2-weighted, and T2 fluid-attenuated inversion recovery.
Image processing
White matter hyperintensity estimation
A detailed description of ONDRI structural processing pipeline methods has been described elsewhere [56]. Briefly, ONDRI’s neuroimaging platform used previously published and validated methods [58,59,60,61,62,63,64] and outputs were further subjected to comprehensive quality control measures from ONDRI’s neuroinformatics platform [65]. The final output of the neuroimaging pipeline produced a skull-stripped brain mask with segmented voxels comprising normal appearing white matter, normal appearing grey matter, ventricular and sulcal cerebrospinal fluid, deep and periventricular lacunes, perivascular spaces, cortico-subcortical stroke lesion, periventricular WMH (pWMH), and deep WMH (dWMH). The 10 tissue classes were further combined with ONDRI’s 28 regional parcellation to create 280 distinct brain regions [56].
For the purpose of this study, we combined both pWMH and dWMH volumes. This was derived by extracting brain parcellations that intersected with WMH segmentation and adding them to create 5 regional WMH volumes: frontal, parietal, temporal, occipital, and basal ganglia/thalamus (BGT). Each regional WMH volume was brain volume corrected using supratentorial total intracranial volume (ST-TIV) and log transformed + small constant to achieve normal distribution:
-
Corrected and transformed regional WMH volume = log((x / STTIV) + 0.0001); where x = uncorrected and untransformed regional WMH volume.
Cortical thickness estimation
All scans were processed using the stable version of FreeSurfer (FS) (Linux FSv6.0). Details of FreeSurfer pipeline have been previously described [66, 67]. Briefly, the standard reconstruction steps included skull stripping, WM segmentation, intensity normalisation, surface reconstruction, subcortical segmentation, cortical parcellation, and thickness. A modified FreeSurfer pipeline was used that incorporated ONDRI’s skull stripped and lesion masks to decrease overall failure rates in participants with significant atrophy and SVD [68].
Cortical thickness was measured as the distance between the GM and WM boundaries (WM surface) to GM and CSF boundaries (pial surface) on the cortex in each hemisphere. We extracted the 68 cortical thickness regions from the Desikan-Killany atlas for further regression analyses [69].
Statistical analyses
Statistical analyses were conducted using R (v 3.4.1) and figures generated using ggplot2 package [70]. One-way ANOVA was used to determine group differences on age, education, MoCA score, ADLs, iADLs, NPI-Q total severity, and NPI-Q caregiver distress. Chi-square test was performed to look for group differences in sex, history of vascular risk factors, and frequencies of NPS across groups. One-way MANOVA was conducted to determine group differences on hyperactivity, psychotic, affective, and apathy subsyndromes. Sex differences in frequencies of NPS were performed using chi-square or Fisher’s exact tests where appropriate. Group differences on ST-TIV adjusted log transformed regional WMH volumes was analysed using one-way MANCOVA, whilst controlling for age. Bonferroni post hoc correction was used where applicable. We ran a linear regression to examine the association between total vascular risk factors burden and log transformed ST-TIV corrected total WMH load, adjusted for age and sex.
Elastic net models and partial least square correlation
The two approaches that we used to determine the relationships between neuropsychiatric subsyndromes, cortical thickness regions, and lobar WMH volumes have been described in details elsewhere [71]. Firstly, we employed a univariate approach with elastic net (LASSO + ridge penalised regression) which is a sparse (LASSO) and penalised (ridge) procedure that suppresses coefficients to zero and helps identify the best subset of explanatory variables for a dependent variable [72, 73]. Each elastic net model consisted of neuropsychiatric subsyndrome ~ sex + age + MoCA + 68 cortical thickness regions + 10 lobar WMH. Alpha was set to equals 1 which was the elastic net penalty parameter for LASSO and we used glmnet’s internal cross-validation to search over the lambda parameter (ridge). Using a repeated train-test procedure, 75% of the data was used for internal cross-validation to identify the lambda parameter with k-folds equals 10, whilst the remaining 25% were used to test the model and report the lambda values with the mean square error (MSE). The above steps were repeated 500 times to construct a consensus of variables with the lowest MSE from the test step. We identified all models from the 500 repeats where a lambda value corresponded to the lowest MSE approximately 5% of the time. That is, models corresponding to lambda values that appeared approximately 25/500 times were retained, and those variables saved. We preserved the sex-by-dx distribution of the entire sample for the repeated splits.
Secondly, a multivariate approach with partial least square correlation (PLSc) was used to model the relationship between all four neuropsychiatric subsyndromes and the independent variables (sex, age, MoCA, cortical thickness regions, and lobar WMH volumes). Two resampling methods were used to help identify which components to interpret (permutation) [74,75,76], and to identify which variables were the most stable contributors to the components (bootstrap) [74, 77, 78]. We also preserved the sex-by-dx distribution of the entire sample for resampling.
Results
Participant demographic and clinical characteristics
A total of 513 participants (AD/MCI (N = 126), ALS (N = 40), FTD (N = 52), PD (N = 140), and CVD (N = 155)) with available baseline MRIs were included in this analysis. In the FTD group, 21 (40.4%) were diagnosed with bvFTD, 8 (15.4%) were diagnosed with nfvPPA, 4 (7.7%) were diagnosed with svPPA, 16 (30.8%) were diagnosed with PSP-Richardson syndrome, and 3 (5.8%) were diagnosed with CBS. Participants’ demographic and clinical characteristics are displayed in Table 1. All groups differed in terms of age, education, sex, MoCA, ADLs, iADLs, hypertension, and high cholesterol.
Results after Bonferroni post hoc correction showed that there were significant differences across all five dx groups on four lobar WMH volumes adjusting for age, with the CVD group showing the highest lobar WMH volumes (Table 1). There was a significant association between total vascular risk factors and WMH load (β = 0.176; p < 0.001; CI = 0.094–0.244), i.e. having a larger number of vascular risk factors burden was related to increased WMH load after adjusting for age and sex.
NPS across dx groups
Although NPS were common across the five disease groups, participants with FTD had the highest frequencies (Table 2; Fig. 1). Agitation, anxiety, apathy, appetite, disinhibition, euphoria, irritability, aberrant motor behaviour, and nighttime behaviours were significantly different across the groups (Table 2; Fig. 1). Depressive symptoms were the most common symptom across all the groups (Table 2). Table 3 shows the group comparison results for significant NPS.
Comparing neuropsychiatric subsyndromes across groups showed that hyperactivity, apathy, and affective subsyndromes were highest in FTD compared to other groups, whilst psychotic subsyndrome was high in both FTD and PD (Table 1; Fig. 2).
Sex differences in frequencies of NPS
Figure 3 shows the sex comparisons of the frequency of individual NPS across the entire sample. Overall, a significantly higher number of males exhibited irritability (39.2% vs 29.7%, p = 0.038, χ2 (1) = 4.28) and nighttime behaviours (43.0% vs 29.0%, p = 0.003, χ2 (1) = 8.97) than females, respectively. The frequency of other NPS was not significant between sexes.
In participants with AD/MCI, depression was significantly higher in females (42.3% vs 24.6%, p = 0.042, χ2 (1) = 4.12) than males, whilst irritability was significantly higher in males (48.4% vs 25.0%, p = 0.009, χ2 (1) = 6.69) than females.
In participants with FTD, the following NPS were significantly higher in males than females: delusions (21.9% vs 0.0%, p = 0.037, Fisher’s exact test), depression (46.9% vs 16.7%, p = 0.033, χ2 (1) = 4.56), apathy (68.8% vs 33.3%, p = 0.015, χ2 (1) = 5.86), and nighttime behaviours (65.6% vs 36.8%, p = 0.046, χ2 (1) = 3.99).
Lastly in participants with PD, the following NPS were significantly higher in males than females: depression (42.1% vs 22.6%, p = 0.048, χ2 (1) = 3.88), apathy (28.0% vs 6.5%, p = 0.012, χ2 (1) = 6.29), and nighttime behaviours (58.9% vs 32.3%, p = 0.009, χ2 (1) = 6.84). No sex differences were observed for CVD and ALS.
Relationship amongst neuropsychiatric subsyndromes, cortical thickness regions, and WMH volumes
All cases with complete data across the five dx and variables of interest, i.e. sex, age, cortical thickness regions, and lobar WMH, were used for both the elastic net and PLSc analyses (N = 490). Table 4 represents the distribution of males and females per dx. For these 490 participants, the mean age = 68.67, median age = 68.78, min/max age = 40.12/87.80; the mean MoCA = 24.40, median MoCA = 25.00, min/max MoCA = 13.00/30.00.
Elastic net models
The psychotic subsyndrome model produced eight lambda values that occurred greater than or equal to 5% of all resamples (i.e. > ~ 25/500). Table 5 shows the results for the psychotic subsyndrome models. One large lambda value (1000) occurred 70/500 times which was the full sample of data that produced an intercept only model. The other seven lambda values occurred a total of 200 out of 500 times and all values were generally in the same range (0.057–0.072). All lambda values produced the same variables for selection in the full sample: age, sex (female), MoCA, left hemisphere precuneus thickness, and right hemisphere (isthmus cingulate thickness, pars-triangularis thickness, and BGT WMH). Left pars-orbitalis, left posterior cingulate, and right caudal anterior cingulate thickness did not appear across all models.
Table 6 shows the results for the apathy subsyndrome models. The apathy subsyndrome model produced seven lambda values that occurred greater than or equal to 5% of all resamples (i.e. > ~ 25/500). The seven lambda values occurred a total of 264 out of 500 times, and all values were generally in the same range (0.066–0.095). All lambda values produced the same variables for selection in the full sample: age, MoCA, left hemisphere (rostral middle frontal and frontal pole thickness), and right hemisphere (entorhinal, middle temporal, pars-opercularis, and pars-triangularis thickness). Note sex (female), left hemisphere (cuneus thickness, transverse temporal thickness, and frontal WMH), and right hemisphere (isthmus cingulate, transverse temporal, and medial orbitofrontal) occurred less frequently across all models.
The affective subsyndrome model produced eight lambda values that occurred greater than or equal to 5% of all resamples (i.e. > ~ 25/500). Table 7 shows the results for the affective subsyndrome models. One large lambda value (1000) occurred 89/500 times which was the full sample of data that produced an intercept only model. The other five lambda values occurred a total of 257 out of 500 times and all values were generally in the same range (0.047–0.066). All lambda values produced the same variables for selection in the full sample: age, sex (female), sex (male), MoCA, left hemisphere (lateral occipital thickness, lateral orbitofrontal thickness, lingual thickness, pericalcarine thickness, posterior cingulate thickness, and occipital WMH), and right hemisphere (caudal anterior cingulate thickness, pars-triangularis thickness, temporal pole thickness, and BGT WMH). Also note, left superior temporal thickness, right middle temporal thickness, and right parietal WMH occurred but not in all models.
Lastly, Table 8 shows the results for the hyperactivity subsyndrome models. The hyperactivity subsyndrome model produced seven lambda values that occurred greater than or equal to 5% of all resamples (i.e. > ~ 25/500). The seven lambda values occurred a total of 281 out of 500 times and all values were generally in the same range (0.144–0.190). All lambda values produced the same variables for selection in the full sample: MoCA, left hemisphere (rostral anterior cingulate, superior temporal, and insula thickness), and right hemisphere (caudal anterior cingulate thickness, lateral orbitofrontal thickness, medial orbitofrontal thickness, pars-triangularis thickness, and temporal pole thickness). Left fusiform thickness and right BGT WMH were less frequent across all models.
PLSc
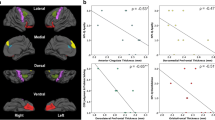
The PLSc produced four components that explained: 87.07% (component 1), 6.69% (component 2), 4.09% (component 3), and 2.16% (component 4) of the variance. The p-values for the four components using permutation were as follows: 0.0004 (component 1), 0.1496 (component 2), 0.0236 (component 3), and 0.1092 (component 4). Although we visualised components’ 1 and 2, we only reported on component 1 due to its large variance and very low permutation p-value. All neuropsychiatric subsyndromes were in the same direction with apathy showing the highest amount of variance on component 1 (Fig. 4). Psychotic and affective subsyndromes were not stable contributors to component 1 (Table 9). Many predictor variables (i.e. age, sex, MoCA, and cortical thickness) were also stable contributors to component 1 (Table 10), and they go in the opposite direction as the neuropsychiatric subsyndrome scores (see Fig. 5), thus, indicating a negative correlation between dependent and predictors variables (e.g. cortical thickness). Although there were many stable predictors, it was important to highlight those that regularly appeared in the elastic net results: sex (female), MoCA, and right hemisphere pars-triangularis and anterior cingulate. They were some of the strongest contributors to component 1. Moreover, the relationship of the participants with regard to the latent variables was shown in Fig. 6 and coloured by their corresponding dx. With the exception of a few FTD participants, most of the dx were clustered together. This reflects the homogeneity in the neural correlates of NPS amongst the study participants and suggests a disease spectrum.
Partial least square correlation diagram for stable contributors component scores. Notes: the stable contributors go in the opposite direction as the neuropsychiatric subsyndromes scores, indicating a negative correlation between them. LH, left hemisphere; MoCA, Montreal Cognitive Assessment; RH, right hemisphere
Relationship between diagnosis, neuropsychiatric subsyndromes, and contributors. Notes: FS, FreeSurfer cortical thickness (68 regions); WMH, lobar white matter hyperintensities (10 regions); MoCA = Montreal Cognitive Assessment; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; CVD, cerebrovascular disease; FTD, frontotemporal disease; MCI, mild cognitive impairment; PD, Parkinson’s disease
Discussion
In this study, we sought to compare NPS rates across multiple neurodegenerative and cerebrovascular diseases and determine the relative contribution of white matter lesion load and cortical thickness to NPS. The major findings included (1) NPS were common across all diseases which was consistent with the literature and (2) a smaller focal cortical thickness was significantly associated with NPS subsyndromes across all disease groups. Moreover, although there was a significant association between WMH burden and NPS subsyndromes in the univariate analyses, it was not maintained in the multivariate analyses signifying that across these diseases, focal atrophy contributed more to NPS.
We observed that participants with FTD had higher rates of agitation, anxiety, apathy, appetite changes, delusions, disinhibition, euphoria, irritability, aberrant motor behaviour, and nighttime behaviours than the other neurodegenerative or cerebrovascular groups. This is consistent with other research that showed that FTD was associated with higher rates of NPS than other neurodegenerative diseases [79,80,81,82,83]. This finding may be related to the early alterations in the fronto-subcortical structures seen in FTD that are responsible for various behavioural functions [79]. Such alterations are often observed in bvFTD which accounted for 40% of our FTD and are present at early stages [46, 84]. As expected, nighttime behaviours were significantly higher in PD with a trend for higher hallucinations in that group. This is consistent with the literature wherein visual hallucinations and sleep disorders are more common in PD and Dementia with Lewy Bodies (DLB) [85,86,87]. Psychotic symptoms are strong indicators of PD and DLB although DLB patients were excluded from our study [88,89,90].
The most prevalent NPS across all groups in our study was depression, which is consistent with prior studies that reported no significant difference in depressive symptoms across neurodegenerative diseases [80,81,82, 91]. Both anxiety and depression can be presenting symptoms of neurodegenerative disease as well as strong predictors of cognitive decline [92,93,94,95,96,97].
Sex differences were observed in our study with irritability and nighttime behaviours seen more frequently in males across the entire sample. Additionally, males with FTD or PD were more likely to experience NPS, such as delusions, apathy, and depression whilst females with AD/MCI were more likely to experience depression. Studies examining the sex/gender differences in presentation of NPS in dementia have mostly been in AD/MCI and have reported inconsistent findings [98,99,100,101,102]. The higher frequency of depression in females with AD/MCI is in keeping with previous studies that reported that more females suffer from affective disorders [100, 103,104,105]. One study found that depression was associated with a twofold greater risk of AD in females but not males [106]. The higher frequency of irritability, nighttime behaviours, delusion, and apathy in males is in keeping with some studies that also reported higher frequencies of the aforementioned NPS in males [98, 107,108,109], whilst contradicting others that have showed the opposite [98, 99, 105, 108]. These sex differences may be attributed to multiple factors such as disease severity across studies, the use of different NPS assessments, the genetic predisposition to AD including the interaction between sex and apoE4 in AD/MCI [99, 110], sex-related hormonal levels, or the use of pharmacological treatments [109]. Also, some diseases have sex differences in distribution and are associated with specific NPS, for example 50% of individuals with PD or DLB experience psychotic symptoms as compared to 30% of individuals with AD/MCI [111], but PD and DLB are more prevalent in males [112, 113]. Moreover, bvFTD appears to be more prevalent in males [114], and they have more apathy and psychotic symptoms and less empathy [71, 92, 115]. A recent study found that females with bvFTD displayed fewer NPS, particularly less apathy, sleep disturbance, and appetite changes than males, despite showing a similar amount of atrophy [116], which may support the neuroprotective role of oestrogen hormone in females [117].
A smaller cortical thickness was implicated in NPS across all groups. Although we obtained several brain regions within each NPS subsyndrome, we concentrated on those that appeared across subsyndromes and analyses such as the pars-triangularis, prefrontal, cingulate, temporal and frontal poles, and insula cortices. Apathy is a multifaceted syndrome representing deficits in cognition, emotion, and initiation [118]. It is not surprising that several studies report similar neuroanatomical correlates of apathy regardless of the underlying pathologies. Apathy is associated with changes in the fronto-striatal circuits (the dorsal anterior cingulate cortex and ventral striatum) in addition to the orbitofrontal cortex and basal ganglia [119]. In PD, the neural correlates of apathy have been structurally and functionally linked to a broad range of regions modulated by dopamine like the ventral striatum and prefrontal cortex [120,121,122,123,124,125,126]. Likewise in AD/MCI, Guercio et al. [20] found apathy was associated with smaller inferior temporal and increased anterior cingulate thickness in MCI whilst other studies found lower grey matter volume in the anterior cingulate, prefrontal, and subcortical areas were associated with apathy in AD [39, 127,128,129]. These findings in AD/MCI have been corroborated in some functional imaging studies that observed a relationship between apathy and hypometabolism in the anterior cingulate cortex and medial prefrontal cortex [130,131,132] in addition to being linked with increased neurofibrillary tangles in the anterior cingulate cortex [133].
The change in the anterior cingulate cortex has been implicated in apathy in FTD, ALS, and CVD. In FTD, apathy was related to atrophy in the subcortical areas in addition to anterior cingulate, and fronto-insular cortices in bvFTD [12, 134, 135]. Similar regions were associated with apathy in participants with ALS-FTD [136] and ALS without dementia [15]. Additionally, lesions in the fronto-striatal circuits has been implicated in apathy or related-disorder abulia [119, 137, 138] thus, indicating that it is a common symptom of both ischaemic and haemorrhagic strokes [138]. Moreover, functional neuroimaging has demonstrated decreased functional connectivity in the cingulo-opercular network due to dysfunction of the connecting regions [139]. Together, these results imply that the manifestation of apathy across multiple neurodegenerative and cerebrovascular diseases results from the disruption of critical and interconnected regions—mainly anterior cingulate cortex and ventral striatum—that are necessary for goal-oriented behaviours.
Psychosis was also associated with a smaller cortical thickness in fronto-cingulate and left precuneus regions. The inferior frontal and precuneus cortices have been implicated in visual hallucinations and delusions [140]. Lower grey matter volume in multiple regions including the right frontoparietal cortex were associated with delusions in AD [128] whilst decreased cortical thickness in the supramarginal gyrus was found in both AD and PD with visual hallucinations [19, 141]. Additionally, Sanchez-Castaneda et al. [142] found visual hallucinations were associated with atrophy in the precuneus and inferior frontal areas in DLB and orbitofrontal area in PD with dementia. Dysregulation amongst the frontoparietal networks has been implicated in psychosis across neurodegenerative diseases but different patterns are evident. Shine et al. [143] found an increase in connectivity between the default mode network (DMN) and ventral attention networks and a decrease in the DMN in patients with PD with visual hallucinations compared to patients without. In AD/MCI, Qian et al. [144] reported decreased connectivity between the inferior parietal lobule, superior temporal, and orbitofrontal with greater delusion severity in patients with AD compared to those without. These results suggest that disruption between top-down dorsal attention and bottom-up ventral attention and DMN processing can result in psychosis [145]. In relation to FTD, ALS, and CVD, only a few studies have explored the neural mechanism of psychosis [146, 147]. Devenney et al. [146] reported a predominant frontal and temporal pattern of atrophy extending to cerebellum and anterior thalamus across all the FTD-ALS continuum, particularly in C9orf72 carriers. Whilst Stangeland et al. [147] found the majority of post-stroke patients with psychosis had right hemisphere lesions mainly in frontoparietal and basal ganglia regions. Since these are network-based diseases, it is possible that psychosis can result from dysfunction of core neural networks that are associated with perception and beliefs in addition to interacting with other associative networks, thereby leading to disease-specific psychotic symptoms [148].
We found that increased right basal ganglia/thalamus WMH volume was associated with psychotic, affective, and hyperactivity subsyndromes whilst increased left frontal WMH volume was associated with apathy subsyndrome, albeit a lesser contributor than cortical thickness. Our results are in contrast to two recent longitudinal studies that showed that WMH contributed more to the progression of NPS subsyndromes than decreased grey matter volume in individuals with AD/MCI [39, 149]. Previous studies have demonstrated that lacunes and WMHs in the fronto-striatal circuitry were correlated with affective disorders [29, 40, 150,151,152], psychosis [31, 153], and reduction in goal-oriented behaviours [29, 32, 154] in neurodegenerative and cerebrovascular diseases disease. Kim et al. [32]. reported that lacunes and WMH, especially in the frontal lobe and basal ganglia and thalamic areas, were associated with depression and apathy in subcortical-vascular cognitive impairment. Similarly, a study on individuals with autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) found that basal ganglia and thalamic lesions were associated with apathy [154] whilst another reported an association between depression and frontal and temporal WMHs in community dwelling older adult [150]. In probable AD, increased frontal WMH was associated with apathy whilst increased right parietal WMH was associated with depression [29] which was supported in an autopsy-confirmed FTD and AD study [155].
The few studies that have investigated the association between WMH and NPS in PD have reported mixed results. Kraft et al. [156] found no association between global and occipital WMH with visual hallucinations in PD but two studies found that increased WMH was associated with depression and anxiety in PD [38, 157], particularly in the fronto-striatal region [38]. Another study found that baseline WMH volume was a risk factor for worsening apathy in PD [158]. These inconsistences in the localisation of WMH in relation to NPS echoes the notion that injury to multiple sites in a network may contribute to the disruption of cortico-subcortical circuits and the manifestation of NPS across many clinical constructs [159], as well as difficulty in capturing multiple NPS as a singular concept, e.g. affective.
Limitations and strengths
The current study has several limitations and strengths. Firstly, the generalisability of our findings might be impacted due to the lack of healthy controls in our study. Secondly, we were limited from addressing the cause-effect relationships amongst WMH, cortical thickness, and NPS due to the cross-sectional nature of our study. However, as discussed above from a recent longitudinal study, WMH may contribute more to NPS progression than decreased cortico-subcortical grey matter volumes, at least in individuals with AD/MCI [39, 149]. This may suggest that at baseline, smaller cortical thickness may have the greatest influence on NPS but that WMH may impact NPS progression. Thirdly, focussing on changes in cortical thickness estimation may lead to the exclusion of the potential involvement of subcortical structures to the manifestation of NPS. Fourthly, we did not account for the use of antipsychotics, antidepressants, anticholinergics, and stimulants for treatment of NPS (which might affect symptom severity in our cohorts). Lastly, since clinical and neuroimaging parameters were used to make the diagnoses of disease categories without diagnostic biomarkers, some observed relationships in our cohorts might have been influenced by mixed pathology because it is very common and increasingly recognised in neurodegenerative diseases [160].
A main strength of our study was the inclusion of multiple neurodegenerative disease groups, especially participants with ALS, FTD, and PD. Prior research examining grey matter loss and/or WMH correlates of NPS have mostly focused on AD/MCI and CVD [31, 32, 39], occasionally on PD and FTD [155, 157], and rarely on ALS [136]. Thus, our study provides an opportunity to investigate these associations across several disease groups. Also, we were able to adjust for several factors associated with NPS in our models.
Conclusions
Our findings demonstrate the high prevalence of NPS in neurodegenerative and cerebrovascular diseases, especially in FTD. Using both univariate and multivariate models, we showed that smaller cortical thickness and white matter lesion burden are associated with NPS subsyndromes across disease groups. In this cross-sectional study, a smaller cortical thickness was a more stable predictor than WMH in NPS across disease groups, particularly in the fronto-cingulate regions. These results underline the need for future longitudinal studies to include multiple neurodegenerative and cerebrovascular diseases when examining the interactive effects of WMH and grey matter loss on NPS. Moreover, SVD is associated with modifiable vascular risk factors, like hypertension, type 2 diabetes, and smoking that can be significantly reduced via healthy lifestyles changes. These interventions may help in managing vascular diseases that can contribute to the development of NPS in individuals with neurodegenerative and cerebrovascular diseases.
Availability of data and materials
The datasets presented in this article are readily available through an application process to ONDRI. For more information on the ONDRI project, please visit: http://ondri.ca/. Requests to access the datasets should be directed to http://ondri.ca/.
References
Aalten P, de Vugt ME, Lousberg R, Korten E, Jaspers N, Senden B, Jolles J, Verhey FRJ. Behavioral problems in dementia: a factor analysis of the neuropsychiatric inventory. Dement Geriatr Cogn Disord. 2003;15:99–105.
Zhao Q-F, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L, Xu W, Li J-Q, Wang J, Lai T-J, Yu J-T. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–71.
Matsumoto N, Ikeda M, Fukuhara R, Shinagawa S, Ishikawa T, Mori T, Toyota Y, Matsumoto T, Adachi H, Hirono N, Tanabe H. Caregiver burden associated with behavioral and psychological symptoms of dementia in elderly people in the local community. Dement Geriatr Cogn Disord. 2007;23:219–24.
Caputo M, Monastero R, Mariani E, Santucci A, Mangialasche F, Camarda R, Senin U, Mecocci P. Neuropsychiatric symptoms in 921 elderly subjects with dementia: a comparison between vascular and neurodegenerative types. Acta Psychiatr Scand. 2008;117:455–64.
Hayata TT, Bergo FPG, Rezende TJ, Damasceno A, Damasceno BP, Cendes F, Stella F, Balthazar MLF. Cortical correlates of affective syndrome in dementia due to Alzheimer’s disease. Arq Neuropsiquiatr. 2015;73:553–60.
Ballard C, Neill D, O’Brien J, McKeith IG, Ince P, Perry R. Anxiety, depression and psychosis in vascular dementia: prevalence and associations. J Affect Disord. 2000;59:97–106.
Lavretsky H, Zheng L, Weiner MW, Mungas D, Reed B, Kramer JH, Jagust W, Chui H, Mack WJ. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–50.
van Reekum R, Stuss DT, Ostrander L. Apathy: why care? J Neuropsychiatry Clin Neurosci. 2005;17:7–19.
Levy JA, Chelune GJ. Cognitive-behavioral profiles of neurodegenerative dementias: beyond Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2007;20:227–38.
Palmer K, Di Iulio F, Varsi AE, Gianni W, Sancesario G, Caltagirone C, Spalletta G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimer’s Dis. 2010;20:175–83.
Chow TW, Binns MA, Cummings JL, Lam I, Black SE, Miller BL, Freedman M, Stuss DT, van Reekum R. Apathy symptom profile and behavioral associations in frontotemporal dementia vs dementia of Alzheimer type. ArchNeurol. 2009;66:888–93.
Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M. Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol. 2012;25:127–36.
Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38:143–62.
Strong MJ, Abrahams S, Goldstein LH, Woolley S, Mclaughlin P, Snowden J, Mioshi E, Roberts-South A, Benatar M, HortobáGyi T, Rosenfeld J, Silani V, Ince PG, Turner MR. Amyotrophic lateral sclerosis - frontotemporal spectrum disorder (ALS-FTSD): revised diagnostic criteria. Amyotroph Lateral Scler Front Degener. 2017;18:153–74.
Consonni M, Cappa SF, Dalla Bella E, Contarino VE, Lauria G. Cortical correlates of behavioural change in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2019;90:380–6.
Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24:2175–86.
Kehagia AA. Neuropsychiatric symptoms in Parkinson’s disease: beyond complications. Front Psychiatry. 2016;7:110.
Finger E, Zhang J, Dickerson B, Bureau Y, Masellis M. Disinhibition in Alzheimer’s disease is associated with reduced right frontal pole cortical thickness. J Alzheimer’s Dis. 2017;60:1161–70.
Donovan NJ, Wadsworth LP, Lorius N, Locascio JJ, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Regional cortical thinning predicts worsening apathy and hallucinations across the Alzheimer disease spectrum. Am J Geriatr Psychiatry. 2014;22:1168–79.
Guercio BJ, Donovan NJ, Ward A, Schultz A, Lorius N, Amariglio RE, Rentz DM, Johnson KA, Sperling RA, Marshall GA. Apathy is associated with lower inferior temporal cortical thickness in mild cognitive impairment and normal elderly individuals. J Neuropsychiatry Clin Neurosci. 2015;27:e22–7.
Mah L, Binns MA, Steffens DC. Anxiety symptoms in amnestic mild cognitive impairment are associated with medial temporal atrophy and predict conversion to Alzheimer disease. Am J Geriatr Psychiatry. 2015;23:466–76.
Alzahrani H, Venneri A. Cognitive and neuroanatomical correlates of neuropsychiatric symptoms in Parkinson’s disease: a systematic review. J Neurol Sci. 2015;356:32–44.
Zamboni G, Huey ED, Krueger F, Nichelli PF, Grafman J. Apathy and disinhibition in frontotemporal dementia: insights into their neural correlates. Neurology. 2008;71:736–42.
Tiel C, Sudo FK, Alves CEO, Alves GS, Ericeira-Valente L, Moreira DM, Laks J, Engelhardt E. Behavioral and psychological symptoms and hippocampal atrophy in subcortical ischaemic vascular disease. Dement Neuropsychol. 2012;6:175–9.
Lyu H, Wang J, Xu J, Zheng H, Yang X, Lin S, Chen J, Zhou L, Hu Y, Guo Z. Structural and functional disruptions in subcortical vascular mild cognitive impairment with and without depressive symptoms. Front Aging Neurosci. 2019;11:241.
Wang J, Lyu H, Chen J, Lin S, Zheng H, Li J, Kong F, Gao J, Yu H, Hu Y, Guo Z. Cortical alterations are associated with depression in subcortical vascular mild cognitive impairment revealed by surface-based morphometry. J Alzheimer’s Dis : JAD. 2020;78(2):673–81. https://doi.org/10.3233/JAD-200156.
Lee DY, Choo ILH, Kim KW, Jhoo JH, Youn JC, Lee UY, Woo JI. Psychotic symptoms in Alzheimer’s disease patients. J Neuropsychiatr. 2006;18(2):191–8. https://doi.org/10.1176/jnp.2006.18.2.191.
Park KH, Lee J-Y, Na DL, Kim SY, Cheong H-K, Moon SY, Shim YS, Park KW, Ku BD, Choi SH, Joo H, Lee JS, Go SM, Kim SH, Kim SangYun, Cha KR, Lee J, Seo SW. Different associations of periventricular and deep white matter lesions with cognition, neuropsychiatric symptoms, and daily activities in dementia. J Geriatr Psychiatry Neurol. 2011;24:84–90.
Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2009;21:259–65.
Soennesyn H, Oppedal K, Greve OJ, Fritze F, Auestad BH, Nore SP, Beyer MK, Aarsland D. White matter hyperintensities and the course of depressive symptoms in elderly people with mild dementia. Dement Geriatr Cogn Dis Extra. 2012;2:97–111.
Anor CJ, O’Connor S, Saund A, Tang-Wai DF, Keren R, Tartaglia MC. Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegener Dis. 2017;17:127–34.
Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, Oh SJ, Kim JS, Choe YS, Lee KH, Noh Y, Cho H, Yoon CW, Chin J, Cummings JL, Lee JH, Na DL, Seo SW. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: a study among patients with subcortical vascular cognitive impairments. Neurobiol Aging. 2013;34:1913–20.
Wardlaw JM, Valdés Hernández MC, Muñoz-Maniega S. What are white matter hyperintensities made of? J Am Heart Assoc. 2015;4:e001140.
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet. 2000;356:628–34.
Zhong Y, Utriainen D, Wang Y, Kang Y, Haacke EM. Automated white matter hyperintensity detection in multiple sclerosis using 3D T2 FLAIR. Int J Biomed Imaging. 2014;2014:1–7.
Woollacott IOC, Bocchetta M, Sudre CH, Ridha BH, Strand C, Courtney R, Ourselin S, Cardoso MJ, Warren JD, Rossor MN, Revesz T, Fox NC, Holton JL, Lashley T, Rohrer JD. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia. Neurocase. 2018;24:166–74.
Desmarais P, Gao A, Keith J, Lanctôt K, Rogaeva E, Ramirez J, Herrmann N, Stuss DT, Black S, Masellis M. White matter hyperintensities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer’s disease. 2020.
Petrovic IN, Stefanova E, Kozic D, Semnic R, Markovic V, Daragasevic NT, Kostic VS. White matter lesions and depression in patients with Parkinson’s disease. J Neurol Sci. 2012;322:132–6.
Misquitta K, Dadar M, Louis Collins D, Tartaglia MC. White matter hyperintensities and neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2020;28:102367.
O’brien J, Perry R, Barber R, Gholkar A, Thomas A. The association between white matter lesions on magnetic resonance imaging and noncognitive symptoms. Ann N Y Acad Sci. 2000;903:482–9.
Farhan SMK, Bartha R, Black SE, Corbett D, Finger E, Freedman M, Greenberg B, Grimes DA, Hegele RA, Hudson C, Kleinstiver PW, Lang AE, Masellis M, McIlroy WE, McLaughlin PM, Montero-Odasso M, Munoz DG, Munoz DP, Strother S, Swartz RH, Symons S, Tartaglia MC, Zinman L, Strong MJ. The Ontario Neurodegenerative Disease Research Initiative (ONDRI). Can J Neurol Sci. 2017;44:196–202.
Sunderland KM, Beaton D, Arnott SR, Kleinstiver P, Kwan D, Lawrence‐Dewar JM, Ramirez J, Tan B, Bartha R, Black SE, Borrie M, Brien D, Casaubon LK, Coe BC, Cornish B, Dilliott AA, Dowlatshahi D, Finger E, Fischer C, Frank A, Fraser J, Freedman M, Greenberg B, Grimes DA, Hassan A, Hatch W, Hegele RA, Hudson C, Jog M, Kumar S, Lang A, Levine B, Lou W, Mandzia J, Marras C, McIlroy W, Montero‐Odasso M, Munoz DG, Munoz DP, Orange JB, Park DS, Pasternak SH, Pieruccini‐Faria F, Rajji TK, Roberts AC, Robinson JF, Rogaeva E, Sahlas DJ, Saposnik G, Scott CJM, Seitz D, Shoesmith C, Steeves TDL, Strong MJ, Strother SC, Swartz RH, Symons S, Tang‐Wai DF, Tartaglia MC, Troyer AK, Turnbull J, Zinman L, McLaughlin PM, Masellis M, Binns MA, Abrahao A, Adamo S, Berezuk C, Black A, Breen DP, Bulman D, Chen Y, El‐Defrawy S, Farhan S, Ghani M, Gonder J, Haddad SMH, Holmes M, Huang J, Leontieva E, Mandelcorn E, Margolin E, Nanayakkara N, Ozzoude M, Peltsch AJ, Pollock B, Raamana P, Rashkovan N, Yanina, Southwell A, Sujanthan S, Tayyari F, Van Ooteghem K, Woulfe J, Zamyadi M, Zou G. Characteristics of the Ontario neurodegenerative disease research initiative cohort. Alzheimers Dement. 2022;19(1):226–243. https://doi.org/10.1002/alz.12632.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and th. J Neurol Sci. 1994;124 Suppl:96–107.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14.
Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–9.
Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52.
Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, Decarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41.
Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–9.
Aalten P, Verhey FRJ, Boziki M, Bullock R, Byrne EJ, Camus V, Caputo M, Collins D, De Deyn PP, Elina K, Frisoni G, Girtler N, Holmes C, Hurt C, Marriott A, Mecocci P, Nobili F, Ousset PJ, Reynish E, Salmon E, Tsolaki M, Vellas B, Robert PH. Neuropsychiatric syndromes in dementia. Dement Geriatr Cogn Disord. 2007;24:457–63.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Scott CJM, Arnott SR, Chemparathy A, Dong F, Solovey I, Gee T, Schmah T, Lobaugh N, Nanayakkara N, Liang S, Zamyadi M, Ozzoude M, Holmes MF, Szilagyi GM, Ramirez J, Symons S, Black SE, Bartha R, Strother S. An overview of the quality assurance and quality control of magnetic resonance imaging data for the Ontario Neurodegenerative Disease Research Initiative ( ONDRI ): pipeline development and neuroinformatics. bioRxiv. 2020.
Ramirez J, Holmes MF, Scott CJM, Ozzoude M, Adamo S, Szilagyi GM, Gao F, Arnott SR, Dewar JML, Beaton D, Strother SC, Douglas P, Masellis M, Swartz RH, Bartha R, Symons S, Black SE, Investigators O. Ontario Neurodegenerative Disease Research Initiative (ONDRI): structural MRI methods & outcome measures. Front Neurol. 2020;11:847.
Duchesne S, Chouinard I, Potvin O, Fonov VS, Khademi A, Bartha R, Bellec P, Collins DL, Descoteaux M, Hoge R, McCreary CR, Ramirez J, Scott CJM, Smith EE, Strother SC, Black SE, CIMA-Q group and the CCNA group. The Canadian Dementia Imaging Protocol: Harmonizing National Cohorts. J Magn Reson Imaging. 2019;49:456–65.
Dade LA, Gao FQ, Kovacevic N, Roy P, Rockel C, O’Toole CM, Lobaugh NJ, Feinstein A, Levine B, Black SE. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–502.
Gibson E, Gao F, Black SE, Lobaugh NJ. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J Magn Reson. 2010;31:1311–22.
Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE. A robust method for extraction and automatic segmentation of brain images. Neuroimage. 2002;17:1087–100.
Ramirez J, McNeely AA, Scott CJM, Masellis M, Black SE. White matter hyperintensity burden in elderly cohort studies. The Sunnybrook Dementia study, Alzheimer disease Neuroimaging Initiative, and Three-City Study. Alzheimers Dement. 2015;12: 203–10.
Ramirez J, McNeely AA, Scott CJ, Stuss DT, Black SE. Subcortical hyperintensity volumetrics in Alzheimer’s disease and normal elderly in the Sunnybrook Dementia Study: correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimers Res Ther. 2014;6:49. https://doi.org/10.1186/alzrt279.
Ramirez J, McNeely AA, Scott CJ, Stuss DT, Black SE. Subcortical hyperintensity volumetrics in Alzheimer’s disease and normal elderly in the Sunnybrook Dementia Study: correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimer’s Res Ther. 2014;6(4):49. https://doi.org/10.1186/alzrt279.
Ramirez J, Gibson E, Quddus A, Lobaugh NJ, Feinstein A, Levine B, Scott CJM, Levy-Cooperman N, Gao FQ, Black SE. Lesion Explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963–73.
Sunderland KM, Beaton D, Fraser J, Kwan D, McLaughlin PM, Montero-Odasso M, Peltsch AJ, Pieruccini-Faria F, Sahlas DJ, Swartz RH, Strother SC, Binns MA, Binns MA. The utility of multivariate outlier detection techniques for data quality evaluation in large studies: an application within the ONDRI project. BMC Med Res Methodol. 2019;19:102.
Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81.
Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80.
Ozzoude M, Ramirez J, Raamana PR, Holmes MF, Walker K, Scott CJM, Gao F, Goubran M, Kwan D, Tartaglia MC, Beaton D, Saposnik G, Hassan A, Lawrence-Dewar J, Dowlatshahi D, Strother SC, Symons S, Bartha R, Swartz RH, Black SE. Cortical thickness estimation in individuals with cerebral small vessel disease, focal atrophy, and chronic stroke lesions. Front Neurosci. 2020;14:598868.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Wickham H. ggplot2: elegant graphics for data analysis. 2009.
Ozzoude M, Varriano B, Beaton D, Ramirez J, Holmes MF, Scott CJM, Gao F, Sunderland KM, McLaughlin P, Rabin J, Goubran M, Kwan D, Roberts A, Bartha R, Symons S, Tan B, Swartz RH, Abrahao A, Saposnik G, Masellis M, Lang AE, Marras C, Zinman L, Shoesmith C, Borrie M, Fischer CE, Frank A, Freedman M, Montero-Odasso M, Kumar S, Pasternak S, Strother SC, Pollock BG, Rajji TK, Seitz D, Tang-Wai DF, Turnbull J, Dowlatshahi D, Hassan A, Casaubon L, Mandzia J, Sahlas D, Breen DP, Grimes D, Jog M, Steeves TDL, Arnott SR, Black SE, Finger E, Strong M, Kleinstiver P, Lawrence-Dewar J, Rashkovan N, Bronskil S, Fraser J, McIlroy B, Cornish B, Van Ooteghem K, Faria F, Sarquis-Adamson Y, Black A, Greenberg B, Hatch W, Hudson C, Leontieva E, Margolin E, Mandelcorn E, Tayyari F, Defrawy S, Brien D, Chen Y, Coe B, Munoz D, Southwell A, Bulman D, Dilliott AA, Ghani M, Hegele R, Robinson J, Rogaeva E, Farhan S, Haddad SMH, Nanayakkara N, Berezuk C, Adamo S, Binns M, Lou W, Theyers A, Uthirakumaran A, Zou GG, Sujanthan S, Zamyadi M, Munoz D, Dixon RA, Woulfe J, Levine B, Orange JB, Peltsch A, Troyer A, Chum M, Tartaglia MC. Investigating the contribution of white matter hyperintensities and cortical thickness to empathy in neurodegenerative and cerebrovascular diseases. Geroscience. 2022;44:1575–98.
Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Statistical Methodol. 2005;67:301–20.
Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22.
Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Comput Stat Data Anal. 2014;72:176–89.
Berry KJ, Johnston JE, Mielke PW. Permutation methods. Wiley Interdiscip Rev Comput Stat. 2011;3:527–42.
Peres-Neto PR, Jackson DA, Somers KM. How many principal components? stopping rules for determining the number of non-trivial axes revisited. Comput Stat Data Anal. 2005;49:974–97.
Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1.
Hesterberg T. Bootstrap. Wiley Interdiscip Rev Comput Stat. 2011;3:497–526.
Mukherjee A, Biswas A, Roy A, Biswas S, Gangopadhyay G, Das SK. Behavioural and psychological symptoms of dementia: correlates and impact on caregiver distress. Dement Geriatr Cogn Dis Extra. 2017;7:354–65.
Srikanth S, Nagaraja AV, Ratnavalli E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J Neurol Sci. 2005;236:43–8.
Brodaty H, Connors MH, Xu J, Woodward M, Ames D. The course of neuropsychiatric symptoms in dementia: a 3-year longitudinal study. J Am Med Dir Assoc. 2015;16:380–7.
Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78.
Kapustin D, Zarei S, Wang W, Black SE, Finger E, Freedman M, Hink H, Kwan D, Lang A, Masellis M, McLaughlin P, Pollock BG, Saposnik G, Strother SC, Sunderland KM, Swartz RH, Tan B, Tang‐Wai DF, Tartaglia C, Turnbull J, Zinman L, Rajji TK, Fischer CE, Kumar S. Neuropsychiatric symptom burden across neurodegenerative disorders and its association with function. Alzheimers Dement. 2020;16.
Banks SJ, Weintraub S. Neuropsychiatric symptoms in behavioral variant frontotemporal dementia and primary progressive aphasia. J Geriatr Psychiatry Neurol. 2008;21:133–41.
Bertram K, Williams DR. Visual hallucinations in the differential diagnosis of parkinsonism: Table 1. J Neurol Neurosurg Psychiatry. 2012;83:448–52.
Cooper AD, Josephs KA. Photophobia, visual hallucinations, and REM sleep behavior disorder in progressive supranuclear palsy and corticobasal degeneration: a prospective study. Parkinsonism Relat Disord. 2009;15:59–61.
Rabey JM. Hallucinations and psychosis in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:S105–10.
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Bryne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller ML, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB). Neurology. 1996;47:1113–24.
Nomura T, Inoue Y, Takigawa H, Nakashima K. Comparison of REM sleep behaviour disorder variables between patients with progressive supranuclear palsy and those with Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:394–6.
Armstrong RA. Visual signs and symptoms of corticobasal degeneration. Clin Exp Optom. 2016;99:498–506.
Starkstein SE, Sabe L, Vázquez S, Tesón A, Petracca G, Chemerinski E, Di Lorenzo G, Leiguarda R. Neuropsychological, psychiatric, and cerebral blood flow findings in vascular dementia and Alzheimer’s disease. Stroke. 1996;27:408–14.
Santacruz Escudero JM, Beltrán J, Palacios Á, Chimbí CM, Matallana D, Reyes P, Perez-Sola V, Santamaría-García H. Neuropsychiatric symptoms as predictors of clinical course in neurodegeneration. A Longitudinal Study. Front Aging Neurosci. 2019;11:176.
Kitching D. Depression in dementia. Aust Prescr. 2015;38:209–11.
Varghese M, Muliyala K. The complex relationship between depression and dementia. Ann Indian Acad Neurol. 2010;13:69.
Becker E, Orellana Rios CL, Lahmann C, Rücker G, Bauer J, Boeker M. Anxiety as a risk factor of Alzheimer’s disease and vascular dementia. Br J Psychiatry. 2018;213:654–60.
Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35.
Rasmussen H, Rosness TA, Bosnes O, Salvesen Ø, Knutli M, Stordal E. Anxiety and depression as risk factors in frontotemporal dementia and Alzheimer’s disease: the HUNT study. Dement Geriatr Cogn Dis Extra. 2018;8:414–25.
Tao Y, Peters ME, Drye LT, Devanand DP, Mintzer JE, Pollock BG, Porsteinsson AP, Rosenberg PB, Schneider LS, Shade DM, Weintraub D, Yesavage J, Lyketsos CG, Munro CA. Sex differences in the neuropsychiatric symptoms of patients with Alzheimer’s disease. Am J Alzheimer’s Dis Other Dementiasr. 2018;33:450–7.
Xing Y, Tang Y, Jia J. Sex differences in neuropsychiatric symptoms of Alzheimer’s disease: the modifying effect of apolipoprotein E ε 4 status. Behav Neurol. 2015;2015:1–6.
Colombo D, Caltagirone C, Padovani A, Sorbi S, Spalletta G, Simoni L, Ori A, Zagni E. Gender differences in neuropsychiatric symptoms in mild to moderate Alzheimer’s disease patients undergoing switch of cholinesterase inhibitors: a post hoc analysis of the EVOLUTION study. J Womens Health (Larchmt). 2018;27:1368–77.
Milani SA, Cantu PA, Berenson AB, Kuo YF, Markides KS, Raji MA. Gender differences in neuropsychiatric symptoms among Community-Dwelling Mexican Americans aged 80 and older. Am J Alzheimers Dis Other Demen. 2021;36:153331752110429.
Hsieh S-W, Chen C-H, Huang L-C, Chang Y-H, Yang Y-H. Gender differences in presentation of behavioral and psychological symptoms in Alzheimer’s disease in Taiwan. Aging Ment Health. 2020;24:1342–7.
Cohen D, Eisdorfer C, Gorelick P, Luchins D, Freeh S, Semla T, Paveza G, Shaw H, Ashford JW. Sex Differences in the psychiatric manifestations of Alzheimer’s disease. J Am Geriatr Soc. 1993;41:229–32.
Lee J, Lee KJ, Kim H. Gender differences in behavioral and psychological symptoms of patients with Alzheimer’s disease. Asian J Psychiatr. 2017;26:124–8.
Kitamura T, Kitamura M, Hino S, Tanaka N, Kurata K. Gender differences in clinical manifestations and outcomes among hospitalized patients with behavioral and psychological symptoms of dementia. J Clin Psychiatry. 2012;73:1548–54.
Artero S, Ancelin M-L, Portet F, Dupuy A, Berr C, Dartigues J-F, Tzourio C, Rouaud O, Poncet M, Pasquier F, Auriacombe S, Touchon J, Ritchie K. Risk profiles for mild cognitive impairment and progression to dementia are gender specific. J Neurol Neurosurg Psychiatry. 2008;79:979–84.
Wolfova K, Creese B, Aarsland D, Ismail Z, Corbett A, Ballard C, Hampshire A, Cermakova P. Gender/Sex Differences in the Association of Mild Behavioral Impairment with Cognitive Aging. J Alzheimer’s Dis : JAD. 2022;88(1):345–55. https://doi.org/10.3233/JAD-220040.
Eikelboom WS, Pan M, Ossenkoppele R, Coesmans M, Gatchel JR, Ismail Z, Lanctôt KL, Fischer CE, Mortby ME, van den Berg E, Papma JM. Sex differences in neuropsychiatric symptoms in Alzheimer’s disease dementia: a meta-analysis. Alzheimers Res Ther. 2022;14:48.
Lövheim H, Sandman P-O, Karlsson S, Gustafson Y. Sex differences in the prevalence of behavioral and psychological symptoms of dementia. Int Psychogeriatrics. 2009;21:469.
Kim J, Fischer CE, Schweizer TA, Munoz DG. Gender and pathology-specific effect of apolipoprotein e genotype on psychosis in Alzheimer’s disease. Curr Alzheimer Res. 2017;14:834–40.
Gallagher D, Fischer CE, Iaboni A. Neuropsychiatric symptoms in mild cognitive impairment. Can J Psychiatry. 2017;62:161–9.
Nelson PT, Schmitt FA, Jicha GA, Kryscio RJ, Abner EL, Smith CD, Van Eldik LJ, Markesbery WR. Association between male gender and cortical Lewy body pathology in large autopsy series. J Neurol. 2010;257:1875–81.
Elbaz A, Bower JH, Maraganore DM, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55:25–31.
Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology. 2002;58:1615–21.
Ranasinghe KG, Rankin KP, Lobach IV, Kramer JH, Sturm VE, Bettcher BM, Possin K, Christine You S, Lamarre AK, Shany-Ur T, Stephens ML, Perry DC, Lee SE, Miller ZA, Gorno-Tempini ML, Rosen HJ, Boxer A, Seeley WW, Rabinovici GD, Vossel KA, Miller BL. Cognition and neuropsychiatry in behavioral variant frontotemporal dementia by disease stage. Neurology. 2016;86:600–10.
Illán-Gala I, Casaletto KB, Borrego-Écija S, Arenaza-Urquijo EM, Wolf A, Cobigo Y, Goh SYM, Staffaroni AM, Alcolea D, Fortea J, Blesa R, Clarimon J, Iulita MF, Brugulat-Serrat A, Lladó A, Grinberg LT, Possin K, Rankin KP, Kramer JH, Rabinovici GD, Boxer A, Seeley WW, Sturm VE, Gorno-Tempini ML, Miller BL, Sánchez-Valle R, Perry DC, Lleó A, Rosen HJ. Sex differences in the behavioral variant of frontotemporal dementia: A new window to executive and behavioral reserve. Alzheimer’s Dement. 2021;17:1329–41.
Flaherty C V., Zangeneh AS, Harrison MA, Marikunte S. Gender differences in Frontotemporal Lobar Degeneration (FTLD) support an estrogenic model of delayed onset. In: Sex Hormones in Neurodegenerative Processes and Diseases. InTech; 2018. https://www.intechopen.com/chapters/59558.
Radakovic R, Abrahams S. Developing a new apathy measurement scale: dimensional apathy scale. Psychiatry Res. 2014;219:658–63.
Le Heron C, Apps MAJ, Husain M. The anatomy of apathy: a neurocognitive framework for amotivated behaviour. Neuropsychologia. 2018;118:54–67.
Martin GP, McDonald KR, Allsop D, Diggle PJ, Leroi I. Apathy as a behavioural marker of cognitive impairment in Parkinson’s disease: a longitudinal analysis. J Neurol. 2020;267:214–27.
Pagonabarraga J, Kulisevsky J, Strafella AP, Krack P. Apathy in Parkinson’s disease: clinical features, neural substrates, diagnosis, and treatment. Lancet Neurol. 2015;14:518–31.
Le Heron C, Plant O, Manohar S, Ang Y-S, Jackson M, Lennox G, Hu MT, Husain M. Distinct effects of apathy and dopamine on effort-based decision-making in Parkinson’s disease. Brain. 2018;141:1455–69.
Carriere N, Besson P, Dujardin K, Duhamel A, Defebvre L, Delmaire C, Devos D. Apathy in Parkinson’s disease is associated with nucleus accumbens atrophy: a magnetic resonance imaging shape analysis. Mov Disord. 2014;29:897–903.
Reijnders JSAM, Scholtissen B, Weber WEJ, Aalten P, Verhey FRJ, Leentjens AFG. Neuroanatomical correlates of apathy in Parkinson’s disease: a magnetic resonance imaging study using voxel-based morphometry. Mov Disord. 2010;25:2318–25.
Thobois S, Ardouin C, Lhommee E, Klinger H, Lagrange C, Xie J, Fraix V, Coelho Braga MC, Hassani R, Kistner A, Juphard A, Seigneuret E, Chabardes S, Mertens P, Polo G, Reilhac A, Costes N, LeBars D, Savasta M, Tremblay L, Quesada JL, Bosson JL, Benabid AL, Broussolle E, Pollak P, Krack P. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–27.
Baggio HC, Segura B, Garrido-Millan JL, Marti M-J, Compta Y, Valldeoriola F, Tolosa E, Junque C. Resting-state frontostriatal functional connectivity in Parkinson’s disease-related apathy. Mov Disord. 2015;30:671–9.
Apostolova LG, Akopyan GG, Partiali N, Steiner CA, Dutton RA, Hayashi KM, Dinov ID, Toga AW, Cummings JL, Thompson PM. Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:91–7.
Bruen PD, McGeown WJ, Shanks MF, Venneri A. Neuroanatomical correlates of neuropsychiatric symptoms in Alzheimer’s disease. Brain. 2008;131:2455–63.
Tunnard C, Whitehead D, Hurt C, Wahlund LO, Mecocci P, Tsolaki M, Vellas B, Spenger C, Kłoszewska I, Soininen H, Lovestone S, Simmons A, AddNeuroMed Consortium. Apathy and cortical atrophy in Alzheimer’s disease. Int J Geriatr Psychiatry. 2011;26:741–8.
Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer disease. Arch Neurol. 2007;64:1015–20.
Robert PH, Darcourt G, Koulibaly MP, Clairet S, Benoit M, Garcia R, Dechaux O, Darcourt J. Lack of initiative and interest in Alzheimer’s disease: a single photon emission computed tomography study. Eur J Neurol. 2006;13:729–35.
Lanctôt KL, Moosa S, Herrmann N, Leibovitch FS, Rothenburg L, Cotter A, Black SE. A SPECT study of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:65–72.
Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;21:144–7.
Rosen HJ, Allison SC, Schauer GF, Gorno-Tempini ML, Weiner MW, Miller BL. Neuroanatomical correlates of behavioural disorders in dementia. Brain. 2005;128:2612–25.
Gonçalves SAB, Caramelli P, Mariano LI, Guimarães HC, Gambogi LB, Resende EPF, Teixeira AL, de Souza LC. Apathy in frontotemporal dementia is related to medial prefrontal atrophy and is independent of executive dysfunction. Brain Res. 2020;1737:146799.
Mioshi E, Lillo P, Yew B, Hsieh S, Savage S, Hodges JR, Kiernan MC, Hornberger M. Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology. 2013;80:1117–23.
Maeda K, Idehara R, Shiraishi T. Micrographia and abulia due to frontal subcortical infarction. Intern Med. 2012;51:1953–4.
Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2013;35:23–39.
Siegel JS, Snyder AZ, Metcalf NV, Fucetola RP, Hacker CD, Shimony JS, Shulman GL, Corbetta M. The circuitry of abulia: Insights from functional connectivity MRI. NeuroImage Clin. 2014;6:320–6.
Jellinger KA. Cerebral correlates of psychotic syndromes in neurodegenerative diseases. J Cell Mol Med. 2012;16:995–1012.
Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson’s Progression Markers Initiative. J Neurol Neurosurg Psychiatry. 2017;88:325–31.
Sanchez-Castaneda C, Rene R, Ramirez-Ruiz B, Campdelacreu J, Gascon J, Falcon C, Calopa M, Jauma S, Juncadella M, Junque C. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25:615–22.
Shine JM, Halliday GM, Gilat M, Matar E, Bolitho SJ, Carlos M, Naismith SL, Lewis SJG. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum Brain Mapp. 2014;35:2206–19.
Qian W, Fischer CE, Churchill NW, Kumar S, Rajji T, Schweizer TA. Delusions in Alzheimer disease are associated with decreased default mode network functional connectivity. Am J Geriatr Psychiatry. 2019;27:1060–8.
Shine JM, Halliday GM, Naismith SL, Lewis SJG. Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov Disord. 2011;26:2154–9.
Devenney EM, Tu S, Caga J, Ahmed RM, Ramsey E, Zoing M, Kwok J, Halliday GM, Piguet O, Hodges JR, Kiernan MC. Neural mechanisms of psychosis vulnerability and perceptual abnormalities in the ALS-FTD spectrum. Ann Clin Transl Neurol. 2021;8:1576–91.
Stangeland H, Orgeta V, Bell V. Poststroke psychosis: a systematic review. J Neurol Neurosurg Psychiatry. 2018;89:879–85.
Naasan G, Shdo SM, Rodriguez EM, Spina S, Grinberg L, Lopez L, Karydas A, Seeley WW, Miller BL, Rankin KP. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144:999–1012.
Anor CJ, Dadar M, Collins DL, Tartaglia MC. The longitudinal assessment of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease and their association with white matter hyperintensities in the National Alzheimer’s Coordinating Center’s Uniform Data Set. Biol Psychiatry Cogn Neurosci Neuroimaging. 2020;6:70–8.
O’Brien JT, Firbank MJ, Krishnan MS, van Straaten ECW, van der Flier WM, Petrovic K, Pantoni L, Simoni M, Erkinjuntti T, Wallin A, Wahlund L-O, Inzitari D. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–41.
Steffens DC, Helms MJ, Krishnan KRR, Burke GL. Cerebrovascular disease and depression symptoms in the cardiovascular health study. Stroke. 1999;30:2159–66.
Vataja R, Pohjasvaara T, Leppävuori A, Mäntylä R, Aronen HJ, Salonen O, Kaste M, Erkinjuntti T. Magnetic resonance imaging correlates of depression after ischemic stroke. Arch Gen Psychiatry. 2001;58:925.
Lee DY, Choo IH, Kim KW, Jhoo JH, Youn JC, Lee UY, Woo JI. White matter changes associated with psychotic symptoms in Alzheimer’s disease patients. J Neuropsychiatry Clin Neurosci. 2006;18:191–8. https://doi.org/10.1176/jnp.2006.18.2.191.
Reyes S, Viswanathan A, Godin O, Dufouil C, Benisty S, Hernandez K, Kurtz A, Jouvent E, O’Sullivan M, Czernecki V, Bousser MG, Dichgans M, Chabriat H. Apathy: a major symptom in CADASIL. Neurology. 2009;72:905–10.
Desmarais P, Gao AF, Lanctôt K, Rogaeva E, Ramirez J, Herrmann N, Stuss DT, Black SE, Keith J, Masellis M. White matter hyperintensities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer’s disease. Alzheimers Res Ther. 2021;13:129.
Kraft E, Winkelmann J, Trenkwalder C, Auer DP. Visual hallucinations, white matter lesions and disease severity in Parkinson’s disease. Acta Neurol Scand. 1999;99:362–7.
Lee J-Y, Kim JS, Jang W, Park J, Oh E, Youn J, Park S, Cho JW. Association between white matter lesions and non-motor symptoms in Parkinson disease. Neurodegener Dis. 2018;18:127–32.
Zhang Y, Zhang GY, Zhang ZE, He AQ, Gan J, Liu Z. White matter hyperintensities: a marker for apathy in Parkinson’s disease without dementia? Ann Clin Transl Neurol. 2020;7:1692–701.
Tay J, Tuladhar AM, Hollocks MJ, Brookes RL, Tozer DJ, Barrick TR, Husain M, de Leeuw F-E, Markus HS. Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology. 2019. https://doi.org/10.1212/WNL.0000000000007095.
Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–86.
Acknowledgements
We would like to thank the ONDRI participants for the time, consent, and participation in our study.
ONDRI Investigators; Members: Michael Strong, Peter Kleinstiver, Jane Lawrence-Dewar, Natalie Rashkovan, Susan Bronskil, Julia Fraser, Bill McIlroy, Ben Cornish, Karen Van Ooteghem, Frederico Faria, Yanina Sarquis-Adamson, Alanna Black, Barry Greenberg, Wendy Hatch, Chris Hudson, Elena Leontieva, Ed Margolin, Efrem Mandelcorn, Faryan Tayyari, Sherif Defrawy, Don Brien, Ying Chen, Brian Coe, Doug Munoz, Alisia Southwell, Dennis Bulman, Allison Ann Dilliott, Mahdi Ghani, Rob Hegele, John Robinson, Ekaterina Rogaeva, Sali Farhan, Seyyed Mohammad Hassan Haddad, Nuwan Nanayakkara, Courtney Berezuk, Malcolm Binns, Wendy Lou, Athena Theyers, Abiramy Uthirakumaran, Guangyong (GY) Zou, Sujeevini Sujanthan, Mojdeh Zamyadi, David Munoz, Roger A. Dixon, John Woulfe, Brian Levine, JB Orange, Alicia Peltsch, Angela Troyer, Marvin Chum.
Funding
This research was conducted with the support of the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Matching funds were provided by participant hospital and research foundations, including the Baycrest Foundation, Bruyere Research Institute, Centre for Addiction and Mental Health Foundation, London Health Sciences Foundation, McMaster University Faculty of Health Sciences, Ottawa Brain and Mind Research Institute, Queen’s University Faculty of Health Sciences, the Thunder Bay Regional Health Sciences Centre, the University of Ottawa Faculty of Medicine, University Health Network, Sunnybrook, and the Windsor/Essex County ALS Association. The Temerty Family Foundation provided the major infrastructure matching funds.
Author information
Authors and Affiliations
Consortia
Contributions
MO: Conceptualisation, Data Curation, Formal Analysis, Investigation, Methodology, Project Administration, Software, Visualisation, and Writing (draft, review, and editing). BV: Conceptualisation, Investigation, Methodology, Project Administration, Writing (review, and editing). DB: Data Curation, Formal Analysis, Supervision, Methodology, Software, Visualisation, and Writing (draft, review, and editing). JR: Data Curation, Supervision, and Writing (review and editing). SA, MFH, PM, AR, CJMS, and BT: Data Curation and Writing (review and editing). KS: Data Curation, Software, Writing (review and editing). JR, SA, and MG: Writing (review and editing). DK: Data Curation, Project Administration, Writing (review and editing). FG: Data Curation, Validation, Resources, Writing (review and editing). RB: Data Curation, Resources, Funding Acquisition, Writing (review and editing). AR, SS, RHS, AA, GS, MM, AEL, CM, SEB, LZ, CS, MM, CF, AF, MF, MMO, SK, SP, BP, TKR, DS, DT, MC, JT, DD, AH, LC, JM, DS, DPB, DG, MJ, TS, and EF: Resources, Funding Acquisition, Writing (review and editing). MCT: Conceptualisation, Investigation, Methodology, Supervision, Resources, Funding Acquisition, Writing (review and editing). All authors contributed to the article and approved the submitted version. All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by ONDRI. Study participants were recruited at various health centres across Ontario, Canada: London Health Science Centre and Parkwood Institute in London; Hamilton General Hospital and McMaster Medical Centre in Hamilton; The Ottawa Civic Hospital in Ottawa; Thunder Bay Regional Health Sciences Centre in Thunder Bay; St. Michael’s Hospital, Sunnybrook Health Sciences Centre, Baycrest Health Sciences, Centre for Addiction and Mental Health, and Toronto Western Hospital (University Health Network) in Toronto. Ethics approval was obtained from all participating institutions and performed in accordance with the Declaration of Helsinki. All participants and study partners provided informed consent. The patients/participants provided their written informed consent to participate in this study.
Consent for publication
Not applicable.
Competing interests
TKR has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. TKR also received in-kind equipment support for an investigator-initiated study from Magstim, and in-kind research accounts from Scientific Brain Training Pro. SK has recieved research support from Brain and Behavior Foundation, National institute on Ageing, BrightFocus Foundation, Brain Canada, Canadian Institute of Health Research, Canadian Consortium on Neurodegeneration in Aging, Centre for Ageing and Brain Health Innovation, Centre for Addiction and Mental Health, and an Academic Scholars Award from the Department of Psychiatry, University of Toronto. He has also received equipment support from Soterix Medical. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ozzoude, M., Varriano, B., Beaton, D. et al. White matter hyperintensities and smaller cortical thickness are associated with neuropsychiatric symptoms in neurodegenerative and cerebrovascular diseases. Alz Res Therapy 15, 114 (2023). https://doi.org/10.1186/s13195-023-01257-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13195-023-01257-y