Abstract
Background
Klippel–Feil syndrome is a rare condition described in 1912 by Maurice Klippel and André Feil. It is defined as a congenital cervical fusion of at least two vertebrae, associated with a classical triad of clinical signs: short neck, low posterior hairline, and limited range of movement. However, Klippel–Feil syndrome manifests with a vast spectrum of phenotypes, ranging from no symptoms to complete triad, with or without other associated malformations. Most commonly, CCF results from sporadic mutations, even though autosomal recessive, autosomal dominant, or even X-linked inheritance can be detected. The ATP-binding cassette subfamily B member 4 is only expressed in the liver and is involved in biliary phospholipid secretion. The clinical spectrum includes various hepatobiliary pathologies, including low phospholipid-associated cholelithiasis, and has never been associated with musculoskeletal anomalies.
Case presentation
A 55-year-old male Caucasian patient presenting with low phospholipid-associated cholelithiasis syndrome with ATP-binding cassette subfamily B member 4 mutation and liver cirrhosis was referred to our clinic for a liver transplant. A period of 6 months before, the patient underwent a T7–T9 posterior fixation for a T8 osteoporotic fracture. Postoperatively, he was tetraparetic, whereas he was neurologically intact before the operation. At admission to our hospital, he was still tetraparetic and presented with clinical signs of cervical myelopathy. Moreover, he suffered a limitation of cervical range of motion in all directions, short neck, and low posterior hairline. Imaging showed multiple cervical and thoracic vertebral bodies fusion, as well as cervical spine stenosis. Based on the available data, we diagnosed a type 3 Klippel–Feil syndrome according to Samartzis’ classification.
Conclusions
The heterogeneity of KFS and the various potential hereditary links that are known indicate that it is important to highlight all potential cases related to known genetic defects. At present, no association between ATP-binding cassette subfamily B member 4 mutation and congenital cervical fusions has been reported. The other important clinical focus of this case is the appearance of spontaneous tetraparesis after thoracic spine surgery. This mechanism remains unclear, but considering different spinal anatomy it might have been due to difficult intubation and patient’s positioning during his previous operation.
Similar content being viewed by others
Background
Klippel–Feil syndrome (KFS) is a relatively uncommon condition first described in 1912 by Maurice Klippel and André Feil [1]. According to a recent demographic study, KFS prevalence is between 0.58% and 0.71%, and it appears to be more common in females with a female-to-male ratio of 4:1 [2]. It is defined as a congenital cervical fusion (CCF) of at least two vertebrae, associated with a classical triad of clinical signs, namely short neck, low posterior hairline, and limited range of movement [3]. However, it has been estimated that only 34–74% of patients present with this constellation of findings [4]. Indeed, KFS manifests with a vast spectrum of phenotypes, ranging from asymptomatic cases and patients presenting an incomplete triad, and cases that include a larger array of congenital anomalies. In the symptomatic population, neck pain is one of the most common symptoms [5].
CCF is believed to result from an abnormal somite segmentation process between the 2nd and the 8th weeks of gestation. There exists specific signaling cascades, which are responsible for a successful somite segmentation and an aberration of any of these elements can lead to congenital deformity [6]. Most commonly, CCF results from sporadic mutations; even autosomal recessive, autosomal dominant, or even X-linked inheritance are also described in the literature. At present, several genes have been identified to play a role in spinal development [7]. For instance, MEOX1 gene is known as the most common autosomal recessive transmission for CCF [8], and mutations in the notch pathway disrupt somite segmentation.
The ATP-binding cassette subfamily B member 4 (ABCB4), also known as multidrug resistance protein 3 (MDR3), encoded by ABCB4, is involved in biliary phospholipid secretion, protecting hepatobiliary system from deleterious detergent and lithogenic properties of the bile. Well-established phenotypes of ABCB4 deficit are progressive familial intrahepatic cholestasis type 3, low phospholipid-associated cholelithiasis (LPAC) syndrome, high gamma-glutamyl transferase intrahepatic cholestasis of pregnancy, chronic cholangiopathy, and adult biliary fibrosis/cirrhosis. Moreover, ABCB4 aberrations may be involved in some cases of drug induced cholestasis, transient neonatal cholestasis, and parenteral nutrition-associated liver disease [9].
However, in current literature there is no report of association between ABCB4 mutations and KFS, or more generally CCF. In this case report, we describe a patient affected by LPAC syndrome with Child–Pugh type C cirrhosis, who was waiting for liver transplant and presented with a classical triad of KFS and mutation of ABCB4.
Case presentation
A 55-year-old male Caucasian patient affected by LPAC syndrome with Child–Pugh type C liver cirrhosis presented to our hospital to perform a preoperative work-up for liver transplant.
A period of 6 months before, the patient underwent a T7–T9 posterior fixation and decompression at another hospital for a T8 osteoporotic fracture classified as OF5 according to the classification proposed by the German Society for Orthopedics and Trauma [10]. The operation was complicated by a cement leakage in T8 epidural space. Surprisingly, after surgery the patient presented with tetraparesis that was more severe on the left side and associated with an ipsilateral sensitive deficit. Notably, the patient was neurologically intact before the intervention and the cement leakage did not explain the postoperative neurological deficit of the upper limbs. For this reason, he was admitted to a neuro-reeducation clinic for 5 months and thereafter, he was transferred to our hospital to undergo a liver transplantation. At admission, he was still tetraparetic and due to the persistence of neurological deficit, our colleagues sought an expert neurosurgical consultation.
On physical examination, the patient was tetraparetic and stable compared with the immediate postoperative neurological status described in the clinical documentation. He also showed clinical signs of cervical myelopathy (bilaterally positive Hoffman’s sign, four limbs with symmetrical hyperreflexia, and bilateral ankle clonus). Moreover, he suffered a limitation of cervical motion range in all directions and showed a short neck and low posterior hairline, namely the classical clinical triad of KFS.
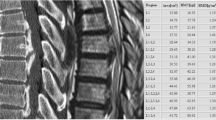
Imaging performed at our hospital, showed the fusion of multiple cervical and thoracic vertebral bodies, namely in C2–C4, C5–C6, and C7–T2 [Fig. 1], without congenital cervical spine stenosis spinal cord occupation ratio (SCOR) index of 43% (Fig. 2) (11). T2 sequences underlined a spinal cord hypersignal potentially due to prior injury at the level of the C3 body; however, it was impossible to clearly determine its origin due to the lack of comparative imaging (Fig. 3). At the level of T7–T9 thoracic fixation, no stenotic consequences on the spinal cord due to the cement leakage were found (Fig. 4). According to radiological presentation, a diagnosis of type 3 KFS was made [4]. Even though a T2 hypersignal was detected at C3 level, there was no cervical stenosis at this segment, and it was considered as an ischemic sequalae. Based on these assumptions, an acute spinal cord compression at stenotic levels was unlikely. Since no compression of the spinal cord at T8 was identified and the tetraparesis is not compatible with a spinal cord lesion at T8, our hypothesis was that surgical installation and/or intubation before surgery for T8 fracture might have produced a cervical spinal cord injury (SCI) and consequently produced the neurological deficit.
Dorsal spine T2-sequence magnetic resonance imaging showing the kyphoplasty at T8 level with posterior fixation at T7 and T9. A axial view showing intracanal cement leakage at T8 level, without visible compressive consequences on the dorsal spinal cord. B sagittal view showing no myelopathic consequences at T7-T9 level
Based on these considerations, the absence of instability, and stability of neurological findings over the last few months, no surgical treatment was undertaken. Instead, we proposed a radiological follow-up concerning the C6–C7 discopathy and cervical canal stenosis.
Discussion and conclusions
The main objective of this article is to present a case of syndromic KFS associated to an ABCB4 genetic mutation. To the best of our knowledge, this case represents the first patient with KFS and ABCB4 mutation. While we cannot determine if the presentation of this genetic mutation and KFS are related, the KFS heterogeneity and the various potential hereditary links that are known, indicate that it is important to highlight all potential cases related to known genetic defects. For example, it is known that congenital fusion of vertebrae can occur in many different genetic syndromes, such as mucupolysaccaride disease and vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities (VACTERL) syndrome, many of which have been pointed out by Giampietro et al. [12].
Genetically, our patient presented with an ABCB4 mutation, which is involved in biliary phospholipid secretion. This gene is located on chromosome 7q21.1 and it is exclusively expressed in the liver. ABCB4/MDR3 is organized as a full transporter and acts as an energy-dependent “floppase,” translocating phospholipids of the phosphatidylcholine family from the inner to the outer leaflet of the lipid bilayer of the canalicular membrane to be extracted by bile salts [8]. The main clinical spectrum of ABCB4 deficiency-associated diseases includes various hepatobiliary pathologies. However, as far as we know, no association is reported between ABCB4 mutation and CCF or other musculoskeletal problems, which might indicate that this is only a rare coincidence. Moreover, the absence of a family history of spine deformities indicates that KFS in this patient was perhaps a sporadic mutation.
The second important clinical focus of this case was the appearance of spontaneous tetraparesis. The manifestation of this matter remains unclear, but it is possible that it occurred due to a difficult intubation and patient positioning during his previous operation. Unfortunately, the diagnosis of KFS was not known previously, which is often the case, and therefore makes surgical preparation more challenging. Indeed, there exists literature showing how short neck and body habitus in KFS make intubation and positioning more difficult compared with the general population [13, 14]. On the other hand, since the SCI occurred behind what appeared to be a fused segment, it is virtually impossible to outline a clear connection between patient positioning and the development of SCI during the intervention.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- ABCB4:
-
ATP-binding cassette subfamily B member 4
- CCF:
-
Congenital cervical fusions
- KFS:
-
Klippel–Feil syndrome
- LPAC:
-
Low phospholipid-associated cholelithiasis
- MDR3:
-
Multidrug resistance protein 3
- MRI:
-
Magnetic resonance imaging
- OF:
-
Osteoporotic fracture
- SCI:
-
Spinal cord injury
References
Klippel FA. Un cas d’absence des vertébres cervicales cage thoracique remontant jusqu’a la base du crâne. Bull et Mém de la Soc d’Anthropol de Paris. 1912;3(1):101–2.
Nouri A, Patel K, Evans H, Saleh M, Kotter MRN, Heary RF, et al. Demographics, presentation and symptoms of patients with Klippel-Feil syndrome: analysis of a global patient-reported registry. Eur Spine J. 2019;28(10):2257–65.
Hensinger RN, Lang JE, MacEwen GD. Klippel-Feil syndrome; a constellation of associated anomalies. J Bone Joint Surg Am. 1974;56(6):1246–53.
Samartzis DD, Herman J, Lubicky JP, Shen FH. Classification of congenitally fused cervical patterns in Klippel-Feil patients: epidemiology and role in the development of cervical spine-related symptoms. Spine. 2006;31(21):E798-804.
Patel K, Evans H, Sommaruga S, Vayssiere P, Qureshi T, Kolb L, et al. Characteristics and management of pain in patients with Klippel-Feil syndrome: analysis of a global patient-reported registry. J Neurosurg Spine. 2019;13:1–6.
Chen JW, Zahid S, Shilts MH, Weaver SJ, Leskowitz RM, Habbsa S, et al. Hoxa-5 acts in segmented somites to regulate cervical vertebral morphology. Mech Dev. 2013;130(4–5):226–40.
Saga Y, Takeda H. The making of the somite: molecular events in vertebrate segmentation. Nat Rev Genet. 2001;2(11):835–45.
Bayrakli F, Guclu B, Yakicier C, Balaban H, Kartal U, Erguner B, et al. Mutation in MEOX1 gene causes a recessive Klippel-Feil syndrome subtype. BMC Genet. 2013;28(14):95.
Sticova E, Jirsa M. ABCB4 disease: many faces of one gene deficiency. Ann Hepatol. 2020;19(2):126–33.
Schnake KJ, Blattert TR, Hahn P, Franck A, Hartmann F, Ullrich B, et al. Classification of osteoporotic thoracolumbar spine fractures: recommendations of the spine section of the German society for orthopaedics and trauma (DGOU). Global Spine J. 2018;8(2_suppl):46S-49S.
Nouri A, Tetreault L, Nori S, Martin AR, Nater A, Fehlings MG. Congenital cervical spine stenosis in a multicenter global cohort of patients with degenerative cervical myelopathy: an ambispective report based on a magnetic resonance imaging diagnostic criterion. Neurosurgery. 2018;83(3):521–8.
Giampietro PF, Raggio CL, Blank RD, McCarty C, Broeckel U, Pickart MA. Clinical, genetic and environmental factors associated with congenital vertebral malformations. Mol Syndromol. 2013;4(1–2):94–105.
Khawaja OM, Reed JT, Shaefi S, Chitilian HV, Sandberg WS. Crisis resource management of the airway in a patient with Klippel-Feil syndrome, congenital deafness, and aortic dissection. Anesth Analg. 2009;108(4):1220–5.
Hase Y, Kamekura N, Fujisawa T, Fukushima K. Repeated anesthetic management for a patient with Klippel-Feil syndrome. Anesth Prog. 2014;61(3):103–6.
Acknowledgements
Not applicable.
Funding
Open access funding provided by University of Geneva The authors declare that they did not receive any funding for this article.
Author information
Authors and Affiliations
Contributions
Data collection: MDB. Manuscript drafting: MDB and AN. Critical revision: all authors. Manuscript approval: all authors. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Da Broi, M., Nouri, A., Patet, G. et al. Tetraparesis following thoracic spine surgery in a patient with Klippel–Feil syndrome and ABCB4 mutation: a case report. J Med Case Reports 17, 528 (2023). https://doi.org/10.1186/s13256-023-04263-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-023-04263-8