Abstract
Background
Mycoplasma hominis is typically found on the mucosal epithelium of the human genital tract, with infections being rare. However, when the mucosal barrier is compromised or in individuals with weakened immune systems, this microorganism can trigger infections in both intragenital and extragenital sites. This study offers a comprehensive overview of infections caused by the rare pathogen M. hominis. This overview helps laboratories identify M. hominis infections in a timely manner, thereby enabling earlier clinical intervention for patients.
Case presentation
A 75-year-old Taiwanese man with type 2 diabetes mellitus initially underwent a left lower extremity amputation following a severe infection caused by necrotizing fasciitis. Subsequently, a poorly healing wound developed at the site of amputation. Upon culturing the wound abscess, M. hominis was isolated and identified as the causative agent.
Conclusions
Through this case, we present clinical and microbiological observations along with a review of the literature to deepen our understanding of M. hominis. Our findings can be used to develop laboratory diagnostic protocols and innovative therapeutic approaches.
Similar content being viewed by others
Introduction
Mycoplasma hominis, a commensal bacterium residing in the genital tract, lacks a conventional bacterial cell wall and is characterized by a negative Gram stain. Cultivation of M. hominis requires specific conditions, such as blood and chocolate agar plates, incubated at 37 °C with 5–10% CO2.The colonies are small (0.2 mm in diameter), translucent, and easily mistaken for droplets, necessitating prolonged incubation for proper development. When conventional Gram staining fails to detect microbes, suspicions of M. hominis presence arise, prompting subculture on specialized media. Various commonly used agar media, such as SP4 agar supplemented with arginine, Hayflick agar, and A7 agar, often incorporate penicillin G for enhanced selectivity. The incubation of agar plates under anaerobic conditions at 35 °C for a minimum of 5 days is recommended. Colony observation under stereomicroscopy aids identification based on their characteristic “fried egg” appearance [1]. However, reliance solely on blood or chocolate agar may not always yield reliable results, as demonstrated in our case, where bacterial growth was not detected in the second wound culture. Molecular diagnostic methods, such as matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI–TOF MS) or 16S rRNA sequencing, can be used to accurately identify M. hominis.
Although infections with M. hominis are rare, they predominantly affect the mucosal epithelium of the genital tract. However, compromised mucosal integrity or immune function can lead to infections in both intragenital and extragenital regions. An understanding of Mycoplasma infections requires an understanding of the effects of various factors, such as glycemic index, mineral balance, and oxidative stress; the evolutionary pathways of intracellular pathogens; and the interaction of these pathogens with our immune response—in addition to the genetic bases underlying these mechanisms. These factors play a critical role in the selection of pathogen populations over time, both locally and systemically, during the course of infection. Although M. hominis is commonly found in the urinary tract of humans, reports of this rare pathogen in diabetes-related foot infections are scarce in the English language literature, possibly due to cross-contamination. This study aimed to provide a comprehensive description of infections caused by M. hominis to assist laboratories in timely identification, which is crucial for early and effective treatment.
Case report
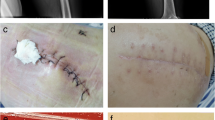
We present the case of a 75-year-old Taiwanese man with a medical history of end-stage renal disease undergoing hemodialysis via a left arm arteriovenous fistula. He also presented with hypertension, hyperlipidemia, and type II diabetes mellitus (DM) with retinopathy as comorbidities. Due to painful, erythematous changes and a nonhealing wound over left ankle persisting for 3 months, he sought assistance in our emergency room. His vital signs were within normal limits, with a blood pressure of 114/54 mmHg, temperature of 37.3 °C, a pulse of 71 beats per minute, and respiratory rate of 16 per minute. A physical examination revealed a 2-cm chronic wound with turbid, orange discharge over the left leg and necrotic tissue over the left ankle (Fig. 1a). Laboratory tests revealed a white blood cell count of 7080/μL, with differential counts showing neutrophils at 38%, lymphocytes at 23%, monocytes at 18%, eosinophils at 12%, and basophils at 3%. His C-reactive protein level was elevated to 10.2 mg/dL. A computed tomography scan of the left lower extremity revealed gas formation within the subcutaneous and intermuscular fascial planes over the foot and leg, raising suspicion of necrotizing fasciitis. Consequently, empirical antibiotic therapy with piperacillin/tazobactam was initiated, and the patient was admitted for further management. Subsequent fasciectomy revealed necrosis of the ankle joint capsule, joint pus formation, necrosis of the plantar and dorsal foot periosteum (Fig. 1b), and necrosis of the lateral compartment muscles of the lower leg (Fig. 1c). Wound culture led to the identification of Proteus mirabilis and Peptostreptococcus anaerobius infections, prompting a change in antibiotic therapy to cefoxitin based on sensitivity testing. However, the wound emitted purulent discharge and a foul odor, and poor circulation was noted in the left lower extremity. Therefore, below-knee amputation was performed. Although initial wound cultures exhibited no bacterial growth, purulent discharge persisted from the medial part of the amputated stump 3 days later, prompting a third wound culture. Given the negative result of the second culture, the possibility of a rare pathogen was considered, leading to incubation of the third wound culture on anaerobic blood agar at 35 °C under 5% CO2 for 5 days, which revealed pinpoint-sized colonies resembling water droplets (Fig. 1d). These colonies tested negative on a gram stain, but MALDI–TOF MS findings led to the identification of these colonies as M. hominis. Genetic sequencing confirmed this identification (Fig. 1e). Accordingly, antibiotic therapy was changed to intravenous levofloxacin, which resulted in resolution of purulent discharge and closure of the wound. The patient was then discharged without complications. The clinical treatment process is detailed in the supplementary file.
a A chronic wound , 2 cm in size (arrow). b Operative finding showed ankle joint capsule necrosis (1), joint pus formation (2), and plantar and dorsal foot periosteum necrosis (3). c Lateral compartment of lower leg part muscle necrosis (arrow). d Formation of pinpoint-sized colonies resembling water droplets on agar. e Result of gene sequencing performed by Genomics Bioscience and Tech. Co., Ltd. The solid blue dots (M1110221-140) in the phylogram tree belong to the Mycoplasma hominis group in our case
Discussion
M. hominis is difficult to culture in microbiology laboratories due to its slow growth, which may lead to human error. In a case initially managed with piperacillin/tazobactam, the antibiotic regimen was changed to cefoxitin following the detection of Proteus mirabilis and Peptostreptococcus anaerobius in wound cultures. Despite this adjustment, the wound infection persisted until M. hominis was isolated, emphasizing delayed treatment potentially attributable to concurrent bacterial infections. Subsequent treatment with levofloxacin led to a resolution of the infection.
Infections caused by M. hominis can occur through both intragenital and extragenital routes. In genital tract infections, transmission to newborns during birth is a concern [2]. Extragenital infections can manifest in various forms, such as septic arthritis [3], prosthetic joint infections [4], central nervous system infections [5], infective endocarditis [6], abscess formation, secondary infections in joint replacement surgery [7], and wound infections [8]. Catheterization has also been associated with M. hominis, potentially due to the use of indwelling catheters used during surgery or hematogenous transmission to surgical sites [9]. However, identifying the source of infection in such cases can often be challenging.
Poorly controlled glycemia in patients with diabetes poses a risk of M. hominis infection, potentially affecting wound healing processes. The elusive nature of the bacterium prompts suspicion of infection when wound repair is suboptimal and conventional abscess cultures yield negative results. Detecting M. hominis necessitates specialized culture conditions, including 5–10% CO2 or anaerobic conditions at 35 °C for several days. Molecular techniques such as MALDI–TOF MS and gene sequencing aid in its identification.
Recent research has uncovered the influence of various factors on immune function and cellular permeability, thereby affecting homeostasis. These factors include Glutathione (GSH)/Glutathione disulfide (GSSG) [10], vitamin E, magnesium [11], and cellular ion concentrations [12]. Additionally, cellular aging and cycling can affect stress-tolerant cell subpopulations, immune function, infection risk, and antibiotic resistance [13]. Phenotypic heterogeneity in immune cells among individuals with diabetes may contribute to increased susceptibility to infections [14]. Antioxidants such as glutathione and plasma copper levels may play a pivotal role in immune modulation [15], particularly in patients with diabetes.
Conclusions
The literature provides therapeutic insights for fungal infections [16] and suggests innovative strategies for treating Naegleria and intracellular pathogens, including M. hominis infections, particularly in immune-compromised patients with diabetes, autoimmune diseases, different types of neoplasia, and neurodegenerative diseases. These insights offer the potential for complete cure. These theoretical perspectives can be used to revolutionize future research approaches and deepen our understanding of immune modulation along with regeneration pathways, thereby informing treatment strategies for M. hominis infections and a wide range of other illnesses.
Availability of data and materials
Not applicable.
References
Stabler S, Faure E, Duployez C, et al. The brief case: Mycoplasma hominis extragenital abscess. J Clin Microbiol. 2021;59(4):e02343-e2420. https://doi.org/10.1128/JCM.02343-20.
Jonduo ME, Vallely LM, Wand H, Sweeney EL, Egli-Gany D, Kaldor J, et al. Adverse pregnancy and birth outcomes associated with Mycoplasma hominis, Ureaplasma urealyticum and Ureaplasma parvum: a systematic review and meta-analysis. BMJ Open. 2022;12(8): e062990. https://doi.org/10.1136/bmjopen-2022-062990.
Luttrell LM, Kanj SS, Corey GR, et al. Mycoplasma hominis septic arthritis: two case reports and review. Clin Infect Dis. 1994;19(6):1067–70. https://doi.org/10.1093/clinids/19.6.1067.
Rieber H, Frontzek A, Fischer M. Periprosthetic joint infection associated with Mycoplasma hominis after transurethral instrumentation in an immunocompetent patient. Unusual or underestimated? A case report and review of the literature. Int J Infect Dis. 2019;82:86–8. https://doi.org/10.1016/j.ijid.2019.03.012.
McNaughton RD, Robertson JA, Ratzlaff VJ, Molberg CR. Mycoplasma hominis infection of the central nervous system in a neonate. Can Med Assoc J. 1983;129(4):353–4.
Gagneux-Brunon A, Grattard F, Morel J, Suy F, Fuzellier JF, Verhoeven P, Cazorla C, Guglielminotti C, Fresard A, Lucht F, et al. Mycoplasma hominis, a rare but true cause of infective endocarditis. J Clin Microbiol. 2015;53(9):3068–71. https://doi.org/10.1128/JCM.00827-15.
Xiang L, Lu B. Infection due to Mycoplasma hominis after left hip replacement: case report and literature review. BMC Infect Dis. 2019;19(1):50. https://doi.org/10.1186/s12879-019-3686-z.
Cuchý E, Cherta I, Garau J. Mycoplasma hominis catheter-related infection in a patient with multiple trauma. Clin Microbiol Infect. 2000;6(2):115. https://doi.org/10.1046/j.1469-0691.2000.00022.x.
Qamar Z, Tjoumakaris S, Pattengill MA, Ahmed M, Hess B. Intracranial Mycoplasma hominis infection following emergent craniectomy. IDCases. 2021;25:e01175. https://doi.org/10.1016/j.idcr.2021.e01175.
Paolisso G, Di MG, Pizza G, D’Amore A, Sgambato S, Tesauro P, Varricchio M, D’Onofrio F. Plasma GSH/GSSG affects glucose homeostasis in healthy subjects and non-insulin-dependent diabetics. Am J Physiol. 1992;263(3 Pt 1):E435–40. https://doi.org/10.1152/ajpendo.1992.263.3.E435.
Mario B, Ligia JD, Maria RT, Lawrence MR, Giuseppe P. Effects of vitamin E and glutathione on glucose metabolism. Hypertension. 1999;34(4 Pt 2):1002–6. https://doi.org/10.1161/01.hyp.34.4.1002.
Barbagallo M, Gupta RK, Dominguez LJ, Resnick LM. Cellular ionic alterations with age relation to hypertension and diabetes. J Am Geriatr Soc. 2000;48(9):1111–6. https://doi.org/10.1111/j.1532-5415.2000.tb04788.x.
Edward RS, Simon VA. Phenotypic heterogeneity: differential stress resistance among individual cells of the yeast Saccharomyces cerevisiae. Microbiology (Reading). 2002;148(Pt 2):345–51.
Amy LB, Faiza AR, Edward RS, Simon VA. Phenotypic heterogeneity can enhance rare-cell survival in ‘stress-sensitive’ yeast populations. Mol Microbiol. 2007;63(2):507–20. https://doi.org/10.1111/j.1365-2958.2006.05504.x.
Matthew CAS, Edward RS, Simon VA. Glutathione and Gts1p drive beneficial variability in the cadmium resistances of individual yeast cells. Mol Microbiol. 2007;66(3):699–712. https://doi.org/10.1111/j.1365-2958.2007.05951.x.
Elena MM, Cindy V, Sara LH, Simon VA. Novel, synergistic antifungal combinations that target translation fidelity. Sci Rep. 2015;17(5):16700. https://doi.org/10.1038/srep16700.
Acknowledgements
This manuscript was edited by Wallace Academic Editing.
Funding
This research is provided by Kaohsiung Veterans General Hospital (KSVGH-113-085).
Author information
Authors and Affiliations
Contributions
The investigation was carried out by Li-Chen Kuo, Yu-Hsiang Tseng, and Lee-Wei Chen. Writing—original draft preparation was performed by Li-Chen Kuo, Tso-Ping Wang, and Ciao-Shan Chen. Writing, review and editing, was performed by Li-Chen Kuo and Herng-Sheng Lee. Supervision was provided by Herng-Sheng Lee.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the KSVGH (KSVGH23-CT2-12).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
All authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kuo, LC., Tseng, YH., Chen, LW. et al. Infection of Mycoplasma hominis in the left lower leg amputation wound of a patient with diabetes: a case report. J Med Case Reports 18, 380 (2024). https://doi.org/10.1186/s13256-024-04718-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13256-024-04718-6