Abstract
Background
Mesenchymal stromal cells (MSCs) therapy for acute respiratory distress syndrome (ARDS) is an emerging treatment, but most of the current trials of MSCs stay in the animal experimental stage, and the safety and efficacy of MSCs in clinical application are not clear. We aimed to analyze the safety, efficacy and biomarkers of mesenchymal stromal cells in the treatment of ARDS.
Methods
For this systematic review and meta-analysis, we searched PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of science, CNKI, VIP and Wan Fang data, studies published between database inception and Mar 17, 2022. All randomized controlled trials (RCT) of stem cell interventions for ARDS were included, without language or date restrictions. We did separate meta-analyses for mortality, subjects with adverse events (AEs) and subjects with serious adverse events (SAEs). Since the trials data are dichotomous outcomes, the odds ratio (OR) is adopted for meta-analysis. The quality of the evidence was assessed with the Cochrane risk of bias tool.
Findings
In total, 5 trials involving 171 patients with ARDS were included in this meta-analysis. A total of 99 individuals were randomly assigned to receive MSCs treatment, and 72 were randomly assigned to receive placebo treatment. Treatment with MSCs appeared to increase the occurrence of adverse events, but this result was not statistically significant (OR, 1.58; 95%CI, 0.64–3.91; P = 0.32). The occurrence of serious adverse events was lower in the MSCs group than in the placebo group (OR, 0.57; 95%CI, 0.14–2.32; P = 0.43); there seems to be no significant difference between the two groups in terms of 28 days mortality (OR, 0.93; 95%CI, 0.45–1.89); oxygenation index and biomarkers showed a tendency to improve in treatment, but there was a lack of more statistically significant clinical evidence to support them.
Interpretation
Based on the current clinical trials, MSCs intervention has some safety for ARDS patients, but its effectiveness and predictive value of airspace biomarkers need to be determined by more large-scale, standard randomized controlled trials.
Similar content being viewed by others
Introduction
ARDS is a type of acute diffuse, inflammatory lung injury [1]. The pathological features of ARDS are a diffuse alveolar injury that includes endothelial and epithelial injury in the lung [2]. Lung parenchymal injury is caused by multiple mechanisms, such as direct injury, immune-related injury, and mechanical ventilation-related injury [2]. Physiologically, refractory hypoxemia due to ventilation-blood imbalance and intrapulmonary shunting is a major characteristic of ARDS [2]. At present, the treatment of ARDS mainly focuses on mechanical ventilation and some emerging pharmacotherapy. Mechanical ventilation includes conventional small tidal volume ventilation, prone position ventilation, lung-protective ventilation, airway pressure release ventilation (APRV) and extracorporeal membrane oxygenation (ECMO) [3,4,5,6]. Compared with traditional small tidal volume ventilation, prone position ventilation and lung-protective ventilation can reduce the mortality of patients with moderate to severe ARDS, the application of early APRV can improve oxygenation and respiratory system compliance, reduce Pplat and reduce the duration of both mechanical ventilation and ICU stay [3,4,5]. While ECMO is mainly used in patients with severe ARDS, it is controversial whether ECMO can improve the mortality of ARDS patients [6]. Pharmacotherapies include corticosteroids, surfactants, N-acetylcysteine, statins, beta-agonists, DNase, granulocyte-colony stimulating factor (GM-CSF), human ACE2, and so on, and there is no evidence that these drugs can improve mortality in ARDS [7,8,9]. Although the treatment of ARDS is more comprehensive and effective with advances in medicine, the mortality rate of ARDS is still high. Based on the current state, improving mortality in ARDS patients is still an urgent need.
MSCs, as a member of stem cells, have been found to have strong immune regulation and anti-inflammatory function in the past decade in addition to proliferation and division function and are increasingly used in various diseases [10, 11]. Laffey has summarized the relevant mechanisms of action of MSCs in reviewing decades of cell therapy for ARDS: 1. Immune modulation; 2. Enhanced bacterial clearance and antimicrobial effects; 3. Injury and inflammation resolution; and 4. Restoration of capillary barrier function [2].
In recent years, lots of animal experiments have conducted in-depth research and discussion on the mechanism and efficacy of MSCs in the treatment of ARDS [12,13,14,15,16]. In 2017, Thomas' animal study found that MSCs improve lung injury in vivo through (extracellular vesicles) EV-mediated mitochondrial transfer promoting an anti-inflammatory and hyperphagocytic macrophage phenotype in the setting of ARDS [16]. In 2019, Lu et al. conducted a further study on the relationship between MSCs, hepatocyte growth factor (HGF) and DCregs and concluded that MSCs alleviate early ALI by inducing mature dendritic cells (MDCs) to differentiate into regulatory dendritic cells (DCs) through paracrine hepatocyte growth factor (HGF), and the mechanism of HGF-induced differentiation of mDCs into tolerogenic dendritic cells (DCregs) is related to the activation of the Akt pathway [14]. Recently, Zhang and Peng have studied the mechanism of anti-fibrotic and epithelial repair effects of MSCs, respectively, and studies have shown that high expression of epithelial–mesenchymal transition (EMT) is associated with early lung fibrosis in ARDS, mesenchymal stem cell microvesicles (MSC MVs) could inhibit the expression of EMT [13], and MSCs can also reduce the inflammatory response by inhibiting T helper cell differentiation and promoting p63 + cell proliferation and lung injury repair, the effect is associated with transcriptional inhibition of interleukin 6-phosphorylation and activation of tumor protein 63-jagged 2 signaling [12]. However, the clinical application of MSCs in the treatment of ARDS is still full of more uncertainty, and there is a lack of clear clinical evidence for both safety and efficacy. In 2020, Qu reviewed all clinical studies (including 2 cases, 3 RCTs, and 4 non-RCT studies) on MSCs for ARDS between 1990 and March 2020 to conduct a meta-analysis of safety and efficacy to propose that MSCs may have potential efficacy for COVID-19-associated ARDS, but it still needs to be confirmed by more clinical trials [17]. The aim of this study was to review all randomized controlled studies on MSCs in the treatment of ARDS as of March 17, 2022, and to conduct a comprehensive systematic review and meta-analysis of adverse events, mortality, improvement of oxygenation index, and biomarkers during MSCs treatment to fully assess the safety and efficacy of MSCs in clinical application, and to conduct a detailed evaluation of biomarkers with assessed MSCs efficacy to promote the clinical application of MSCs in the future.
Methods
Eligibility criteria
The inclusion criteria were as follows: (1) Population: We included randomized controlled trials of adult (age ≥ 18 years) patients with Berlin criteria-defined ARDS; (2) Interventions: Interventions were mesenchymal stromal cell, mesenchymal stem cell, stem cell, MSCs/MSC or progenitor cell; (3) Comparators: Placebo; (4) Outcomes: While each study had different outcomes measure, the main outcome measures we included were as follows: subjects with adverse events (AEs), subjects with serious adverse events (SAEs), mortality, PaO2/FiO2, ventilation‑free days to D28; and (5) Study types: randomized controlled trials (RCT), blind or not. We applied no language restrictions (Table 1).
The exclusion criteria were as follows: (1) conference papers and abstracts; (2) data cannot be extracted; (3) Not RCT; (4) clinical protocols; (5) animal; and (6) case series.
Information sources
On March 17, 2022, we searched PubMed, Embase, Cochrane Central Register of Controlled Trials, Web of science, CNKI, VIP and Wan Fang data, studies published between database inception and Mar 17, 2022.
Search strategy
The search strategy was to use the following terms: ARDS; Acute Respiratory Distress Syndrome; ALI; acute lung injury; Shock Lung; Respiratory Distress Syndrome; mesenchymal stromal cell; stromal cell; mesenchymal stem cell; stem cell; Mesenchymal Progenitor Cell; Progenitor Cell; MSCs/MSC; and randomized controlled trial. We also provide the specific search strategy of four English databases, and the details are shown in Additional file 1: Table 1.
Selection process
Two researchers independently screened titles and abstracts of all articles retrieved. In case of disagreement, consensus on which articles to screen full text was reached by discussion. If necessary, the third researcher was consulted to make the final decision. Next, two researchers independently screened full-text articles for inclusion. Again, in case of disagreement, consensus was reached on inclusion or exclusion by discussion, and if necessary, the third researcher was consulted.
Data collection process
We designed a data extraction form, which two review authors used to extract data from eligible studies. Extracted data were compared, with any discrepancies being resolved through discussion.
Data items
Eligible outcomes were broadly categorized as follows: subjects with adverse events (AEs), subjects with serious adverse events (SAEs), mortality, PaO2/FiO2, MSCs source, administration route, dose of MSCs, biomarkers (Additional file 2: Table 2 and Additional file 3: Table 3).
Study risk of bias assessment
We assessed risk of bias in the included studies using the revised Cochrane ‘Risk of bias’ tool for randomized trials (RoB 2.0). RoB 2.0 addresses six specific domains: (1) randomization method; (2) allocation concealment; (3) blinding of participants and personnel or assessment; (4) incomplete outcome data; (5) selective reporting; and (6) other bias. According to the results of each study, the judgment on low risk, high risk and unclear risk is made for the six items. The three items [ (1) (2) (5)] are used to evaluate the risk of bias of each included study, and the other three items are evaluated according to the different results of each included study, emphasizing that the different results in the same study are affected by bias to different extents. Two review authors independently applied the tool to each included study. Any discrepancies in judgments of risk of bias or justifications for judgements were resolved by discussion to reach consensus between the two review authors, with a third review author acting as an arbiter if necessary (Table 1). We used the GRADE approach (Grading of recommendations, development assessment and Evaluation) to rate certainty in the effect of MSCs on mortality, AEs and SAEs (Additional file 5: Table 5 and Additional file 6: Table 6).
Effect measures
Since the trials data are dichotomous outcomes, we planned to analyze dichotomous outcomes by calculating the odds ratio (OR) of a successful outcome for each trial.
Synthesis methods
We used the odds ratio to pool results to estimate the safety and efficacy of different kinds of stem cells in the treatment of ARDS and performed meta-analysis using Reman 5.3 software. The heterogeneity between studies was tested by Cochrane Q test and I2 test. If P > 0.1, when I2 ≤ 50%, it means that the homogeneity between studies is better, and then, the fixed-effects model is used; if P < 0.1, when I2 > 50%, it means that there is heterogeneity between the studies, and then, the random-effects model is used. If the number of included studies is more than 10, the publication bias analysis is performed by drawing a funnel chart, linear regression method, and rank correlation test. If the number of included studies is less than 10, then the publication bias is considered inevitable, and no correlation analysis is performed. Publication bias was considered inevitable because less than 10 trials were included in this study. Use sensitivity analysis to evaluate the stability of the research results. The results are statistically significant when P < 0.05.
Results
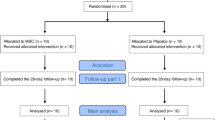
After searching the database, we found 1839 records. After removing duplicates, 1416 records were screened, of which 89 full texts were reviewed, and finally, 5 records were included [18,19,20,21,22]. Subsequently, we also searched the references of other current meta-analyses on MSCs studies. However, no additional articles meeting the inclusion criteria were found in these searches (Fig. 1).
Table 2 shows a summary of the patient, country, study type, age, percentage of males, PaO2/FiO2, SOFA score, primary ARDS Cause and lung injury score characteristics for each trial. Data from all included trials were obtained from published manuscripts. Also, Table 2 presents the specific details of the interventions and reports detailed data on mortality, subjects of adverse events or serious adverse events in each study.
The risk of bias for each of included studies was assessed by the RoB2.0 tool (Fig. 2). Table 1 provides a summary of these assessments. In terms of overall risk of bias, the majority of studies (4/5) have different degrees of risk. We have also provided a detailed description of the justification for each assessment (Additional file 4: Table 4).
Adverse events and Serious Adverse events
Four [18, 20,21,22] of the five included articles reported the occurrence of adverse events during follow-up and three [20,21,22] reported the occurrence of serious adverse events. A total of 99 individuals were randomly assigned to receive MSCs treatment, and 72 were randomly assigned to receive placebo treatment. One study [19] was excluded from analysis of adverse events due to lack of follow-up data on adverse events. In addition, two studies [18, 19] were excluded due to lack of follow-up data on serious adverse events. Of the 59 patients treated with MSCs, 46 experienced adverse events at follow-up, accounting for approximately 78% (46/59), of the 52 patients treated with placebo, 36 experienced adverse events at follow-up, accounting for approximately 69.2% (36/52), and there were three studies with OR > 1, accounting for approximately 75% (3/4) of the total studies, treatment with MSCs appeared to increase the occurrence of adverse events, but this result was not statistically significant (OR, 1.58; 95%CI, 0.64–3.91; P = 0.32), there was no significant heterogeneity in the adverse events across the 4 trials (χ2 = 5.22; P = 0.16; I2 = 42%) [18, 20,21,22]; of the 53 patients treated with MSCs, 20 experienced serious adverse events at follow-up, accounting for approximately 37.7% (20/53), of the 46 patients treated with placebo, 20 experienced serious adverse events at follow-up, accounting for approximately 55.6% (20/36), there were two studies [21, 22] with OR ≥ 1, accounting for about 66.7% (2/3) of the total studies, the study [20] with OR < 1 had certain statistical significance(OR, 0.10; 95%CI, 0.01–0.69; P = 0.02), but due to the small sample size, it did not indicate that MSCs treatment could reduce the occurrence of serious adverse reactions, in total, there appeared to be more serious adverse events with placebo treatment, but the results were not statistically significant (OR, 0.57; 95%CI, 0.14–2.32; P = 0.43), there was heterogeneity in the serious adverse events across the 3 trials (χ2 = 4.73; P = 0.09; I2 = 58%) [20,21,22] (Fig. 3).
Mortality
Five studies [18,19,20,21,22] reported 28-day all-cause mortality, one study [19] reported 60-day mortality, and one study [21] reported 1-year mortality. Two of the studies had an OR > 1 for 28-day mortality [19, 22], accounting for approximately 40% (2/5) of the total studies. 24 of the 98 patients in the MSCs group died, accounting for 24.5% (24/96), 18 of the 72 patients in the placebo group died, accounting for 25% (18/72). There seems to be no significant difference between the two groups in terms of 28 days mortality, but it lacks statistical significance (OR, 0.93; 95%CI, 0.45–1.89; P = 0.84), there was no significant heterogeneity in the mortality across the 5 trials (χ2 = 5.70; P = 0.22; I2 = 30%). Longer follow-up time (60 days) was reported in Matthay's study[19]. During the period from 28 to 60 days, the number of deaths increased by 3 [10.7% (3/28)] in MSCs group and 2 [11.8% (2/17)] in placebo group. Overall, the 60-day mortality was 37.5% (15/40) in the MSCs group and 25% (5/20) in the placebo group. Statistically, the two groups of data were not statistically significant (OR, 1.80; 95%CI, 0.54–5.96; P = 0.34). The study in Bellingan [21] was followed up for 1 year, and the follow-up result was that the mortality rate of the MSCs group [40% (8/20)] was lower than that of the placebo group [50% (5/10)], but its result was not statistically significant (OR, 0.67; 95%CI, 0.14–3.07; P = 0.60). Also, the study of Bellingan [21] gave the change of mortality in each group when PaO2/FiO2 was < 150 mmHg. Eight patients in the MSCs group met PaO2/FiO2 < 150 mmHg, and eight patients in the placebo group were eligible. There were 2 deaths [25% (2/8)] in the treatment group and 4 deaths [50% (4/8)] in the control group at follow-up 28. The mortality rate was lower in the critical treatment group, but this result was not statistically different possibly due to the small sample size (OR, 0.33; 95%CI, 0.04–2.77; P = 0.31) (Fig. 4). In summary, based on the current clinical study results, no statistical difference in mortality was observed between the treatment group and the control group whether short-term or long-term follow-up, which may be related to the small number of included studies, imbalance of baseline data in individual studies and only a few studies reporting long-term follow-up data.
PaO2/FiO2
Four studies [18, 19, 21, 22] explained the changes in oxygenation index (PaO2/FiO2), all of which indicated that the increase in oxygenation index in the experimental group was higher than that in the control group, but also indicated that the difference between the two groups of data was not statistically significant. Refer to Additional file 3: Table 3 for the description of oxygenation index for each study.
Biomarkers
All studies [18,19,20,21,22] reported on plasma biomarkers in patients treated with MSCs versus placebo in detail. First, Zheng's study [18] in 2014 pointed out that SP-D, IL-6 or IL-8 levels were similar between day 0 and day 5 in the placebo group, while the inflammatory factors levels (SP-D, IL-6 or IL-8) in the MSCs group were significantly decreased compared with the baseline data on day 0, and the decrease in SP-D was statistically significant. However, the study [18] also showed that the changes in inflammatory factors were not statistically different between the two groups. Then, Matthay's study [19] in 2019, at 6 h after the start of infusion, the decrease in angiopoietin 2 concentration in plasma was significantly greater in the MSC group than in the placebo group (P = 0.005). Recently, Lanzoni's studies [20] observed only the UC-MSC-treated group showed a consistent decrease in inflammatory markers. At day 6, a significant difference in the concentration of GM-CSF, IFNg, IL-5, IL-6, IL-7, TNFa, TNFb, PDGF-BB, and RANTES was observed in a comparison between groups. Bellingan's study [21] also demonstrated an average decrease in inflammatory biomarkers (IFN-gamma, IL-1 beta, IL-1R2, IL 6, IL 12, KGF, PD-1, RAGE, and TNF-alpha) in the cells group on day 7, but Bellingan's study did not statistically analyze the changes. Monsel's study [22] provides a volcano plot of plasma biomarkers from 0 to 14 days, as can be seen from the plot, by the 14th day, IL-7, IL-10, IP-10, IL-18, RAGE and MCP-2 were decreased in all groups, while IL-9, IL-10 and IL-17F were decreased in the cell group and statistically different from the control group. (Additional file 3: Table 3).
Discussion
In this study, we analyzed the associations between MSCs and ARDS using a meta-analysis to obtain a powerful conclusion, and we summarized the latest RCT clinical studies on MSCs in ARDS on the basis of previous studies and performed a detailed analysis of their biomarkers to update the previous meta-analysis. Although Qu has done a similar meta-analysis on the safety and efficacy of MSCs in the treatment of COVID-19-associated ARDS in 2020, Qu's study included few RCTs and lacked a detailed analysis of biomarkers [17].
Meta-analysis showed that MSCs treatment reduced the occurrence of serious adverse events in ARDS patients compared with placebo, but was accompanied by an increased risk of adverse events (SAEs, OR < 1; AEs, OR > 1), although this result was not statistically different (SAEs, P = 0.32; AEs, P = 0.43), and all five included studies stated that the occurrence of adverse events and serious adverse events in the treatment group was mainly related to the patient's current disease, without significant association with MSCs [18,19,20,21,22]. In Monsel's study [22], only one of the adverse events (diarrhea) was considered related to the treatment process of MSCs; in Belligan's study [21], the baseline data of the two groups were not completely balanced, the modified SOFA score of the cell group was lower, the oxygenation index was higher, and these two were statistically different from the placebo group, and there was one adverse event (grade 1 fever) considered related to MSCs treatment in Belligan's study; in Lanzoni's study [20], the baseline data of the two groups were basically balanced, only one adverse event was considered related to infusion within 6 h in the cell group and two in the placebo group, and it was considered that MSCs treatment could reduce the occurrence of SAEs in the cell group; in Zheng's study [18], only two adverse events (diarrhea, rash) were considered related to MSCs treatment in the cell group, and these two adverse events resolved spontaneously at 48 h and 24 h, respectively. In general, although the incidence of adverse events in the cell group was considered to be higher in the included studies, but it was considered to be unrelated to the treatment with MSCs after respective analysis, a few related adverse events were mild in severity, had a lower incidence, and had a tendency of self-healing; for serious adverse events, the study suggested that the treatment with MSCs had the potential efficacy of reducing the occurrence of serious adverse events, but this needs to be corroborated by standard randomized controlled trials with larger sample size and follow-up time. At present, it is believed that the safety of MSCs in the treatment of ARDS is fair and basically consistent with the conclusions of previous studies [23,24,25,26], and these studies all acknowledged the safety of MSCs in the treatment of ARDS.
As of April 2, 2022, a total of 476 million COVID-19 cases have been diagnosed worldwide, with a cumulative number of 6.12 million deaths reported and a mortality rate of approximately 1.3%. [Data were obtained from the National Health Commission of China, the World Health Organization, official epidemic notification and authoritative media reports from various countries (regions), it is summarized by the official media of China and published on the epidemic map platform.] With the worldwide pandemic of COVID-19, the number of patients with ARDS has increaed dramatically [27]. According to an epidemiological study from 50 countries in 2016, the prevalence of ARDS in ICU is 10.4%, hospital mortality was 34.9% for those with mild, 40.3% for those with moderate, and 46.1% for those with severe ARDS [28]. Arguably, in the context of the current COVID-19 pandemic, reducing in-hospital mortality in ARDS is a great challenge and urgent need today. The result of this study indicates the potential efficacy of MSCs therapy to reduce in-hospital mortality in moderate and severe ARDS, although there is no statistical support for this result. And, most of the included studies (3/5) considered the cell group to have a lower mortality rate [18, 20, 21]. Although Matthay's study [19] results concluded that the mortality rate of the cell group was higher than that of the placebo group [MSCs group, 30% (12/40); placebo group, 15% (3/20)], this result may be related to the more severe disease in the cell group, which can be confirmed by the lower oxygenation index (MSCs group, 135.8 ± 32.3; placebo group, 143.3 ± 39) and higher SOFA score (MSCs group, 8.1 ± 3.3; placebo group, 6.9 ± 2.7) in the cell group than in the placebo group. In another study [22], which stated that the mortality rate of the cell group was higher than that of the placebo group, the authors stated that there was no statistical difference in mortality between the two groups. And the topic of Monsel's study is COVID-19-related ARDS, which is not completely consistent with the disease development process of ARDS in the traditional sense, such as the prevalence of coagulopathy and venous thrombosis in COVID-19-related ARDS [29,30,31,32]. In severe cases of ARDS, Bellingan's study [21] found that when PaO2/FiO2 was < 150 mmHg, the mortality rate was significantly lower after MSCs treatment, and although it was not statistically different because of the small sample size, the potential therapeutic effect of MSCs was undeniable. It has also been shown in several large animal trials and clinical trials that MSCs therapy can reduce the mortality of ARDS [33,34,35]. The results of this study suggest that MSCs therapy has the possibility of reducing the mortality of ARDS, which is consistent with previous animal experiments and clinical trials. However, it is also recommended to conduct a clinical trial with a large sample size to fully confirm this efficacy.
Oxygenation index, as an important indicator of respiratory function and diagnostic criteria for ARDS, was reported and analyzed as a secondary outcome measure in four of the five included studies [18, 19, 21, 22]. The study showed that the oxygenation index was improved after treatment in both the cell group and the placebo group, and there was no statistical difference between the two groups of data. Since both groups of patients received mechanical ventilation, and the improvement of oxygenation index by mechanical ventilation has been confirmed by most studies [3, 4], it is difficult to say from the current research evidence that MSCs have a clear therapeutic effect of improving oxygenation index. And whether oxygenation index can play a role in judging the prognostic indicators is still somewhat controversial [36], mainly because oxygenation index is highly susceptible to limitations such as atmospheric pressure and FiO2 and so on [37]. Monsel's study [22] suggested that MSCs have the ability to promote lung tissue repair and improve oxygenation, the lack of significant difference in the change of oxygenation index between the two groups was interpreted as the severity of lung injury exceeded the repair effect of MSCs when the patient's respiratory failure was severe enough to require invasive mechanical ventilation or ventilator support. According to the pooled results of this study, there are many intervention factors for oxygenation index. Although it has been shown from animal tests that MSCs have the effect of improving lung injury, which can indirectly predict that MSCs may improve oxygenation by improving lung injury, and there is no direct evidence that MSCs have the effect of improving oxygenation.
The results vary widely between studies on biomarkers, and there is no uniform standard for the types of biomarkers observed. The most important reason for the difference may be that the biomarkers were from plasma rather than from the lungs. A recent study has shown that the use of nonbronchoscopic bronchoalveolar lavage as airspace biomarker may represent a lung-specific therapeutic effect than plasma biomarkers [38]. The five studies [18,19,20,21,22] had some contradictions to the reporting of biomarkers, for example, Zheng's study [18] indicated that SP-D decreased in the cell group and was different from the placebo group, while Belligan's study [21] found that SP-D increased in the cell group but decreased in the placebo group. In general, plasma biomarkers such as IL-6, IL-8, RAGE, and angiopoietin-2 (Ang-2) decreased more significantly in cells groups agreed with most of the included studies. Ang-2, on the other hand, is recognized as a mediator and biomarker of pulmonary and systemic vascular injury. And the concentration of Ang-2 has important predictive value for the development of ARDS [39,40,41]. Wick designed a randomized controlled study on airspace biomarkers and plasma biomarkers to determine whether airspace biomarkers can increase the value of plasma biomarkers and whether airspace biomarkers provide mechanistic evidence for MSCs in the treatment of ARDS [38]. Wick’ study has shown that the concentrations of airspace biomarkers were significantly different from those in plasma. The concentrations of IL-8, IL-6, and RAGE were significantly lower in plasma than in airspaces, while the concentrations of sTNFR-1 and Ang-2 were significantly lower in airspaces than in plasma [38]. Both Airspace Ang-2 and airspace RAGE were positively correlated with airspace total protein [38]; in experimental models of MSC therapy for ARDS and clinical studies of ARDS, the concentration of total protein in the airspaces is a good biomarker of lung endothelial cell and epithelial protein permeability [42,43,44,45,46]. Compared to plasma Ang-2 and plasma RAGE, higher airspace Ang-2 is associated with fewer ventilator-free days (VFDs), while higher airspace RAGE is associated with higher radiographic assessment of lung oedema (RALE) score [38]. There was no significant statistical difference in plasma biomarkers between the treatment placebo group, which may be related to the fact that plasma biomarkers are not only derived from lung tissues, but also from other tissues [47]. Therefore, for some biomarkers, airspace biomarkers may reflect different biological processes compared to plasma biomarkers [38]. Plasma IL-8 is an important biomarker for the assessment of ARDS [48], but IL-8 in plasma does not accurately reflect the inflammatory environment in the lungs. Similarly, there is a lack of correlation between airspace and plasma Ang-2 concentrations [49]. At 48 h after treatment, the values of airspace biomarkers in the MSCs group were very low, and most of them were statistically different compared with the placebo group, in which airspace Ang-2 levels were significantly reduced (P = 0.0076), while plasma biomarkers were not different from the placebo group [38]. So, plasma biomarkers may reflect the overall level of the disease, and airspace biomarkers represent lung-specific therapeutic effects [38]. Although based on the current study, it could not be fully demonstrated that the plasma biomarkers in the MSCs group were significantly different from those in the placebo group after treatment, in terms of airspace biomarkers, it has been proposed that MSCs treatment can significantly reduce airspace biomarkers within 48 h. Among them, airspace Ang-2, airspace RAGE have some specificity in assessing the therapeutic effect of lung injury. In future biomarker studies, more attention to airspace biomarkers may be a better option.
In this study, based on the latest clinical randomized controlled study, the safety, efficacy and biomarkers of MSCs in the treatment of ARDS were comprehensively and comprehensively analyzed, providing a more comprehensive theoretical basis for future studies. This study also has some limitations. First, only five studies were included, although all five studies were high-quality randomized controlled studies; second, the number of subjects included in each study was small, resulting in that the total number of subjects included in the meta-analysis was only 171, which made the results of this study possibly inconsistent with the results of future studies; finally, two of the included studies indicated the use of steroids during treatment [21, 22], which have a similar effect to MSCs and have a cytotoxic effect on MSCs, which may have some impact on the efficacy of MSCs, but in some studies, it was pointed out that steroids (e.g., dexamethasone) have a small effect on the activity of cells [50], and in one study, MSCs have been used as rescue therapy for acute graft-versus-host disease with severe steroid resistance [51]. So although there is steroid use, this does not deny the therapeutic effect of MSCs.
Conclusion
Compared with the placebo group, MSCs have considerable safety in the treatment of ARDS and have the potential to reduce the mortality of moderate and severe ARDS. Airspace biomarkers represent lung-specific treatment efficacy than plasma biomarkers, and airspace Ang-2 and airspace RAGE have some specificity in assessing the therapeutic effect of ARDS. More and larger studies are also necessary to further confirm the safety and efficacy of MSCs and the predictive value of airspace biomarkers.
Flow chart of the selection process. Flow chart describing the selection steps of the systematic review and meta-analysis of comparing the safety, efficacy and biomarkers of mesenchymal stem cells in patients with ARDS, showing the number of studies excluded at each step, as well as the reasons for exclusion. *PubMed (n = 108), Embase (n = 381), Central (Cochrane library) (n = 176), Web of Science (n = 624), VIP (n = 132), Wanfang Data (n = 338), and China National Knowledge Infrastructure (n = 80). Ultimately, a total of 1839 were retrieved from the seven database. Of these 1839 studies, five articles were finally identified, including 5 quantitative studies
Forest plot of Subjects with AEs and SAEs. There was no statistical difference in the number of patients with AEs or SAEs after MSCs treatment compared with placebo. Three studies related to SAEs have some heterogeneity, and a random-effects model was used for statistical analysis. In the plane rectangular coordinate system, the forest plot takes a vertical invalid line (scale of abscissa is 0) as the center, describes the effect quantity and 95% CI of each study by using multiple line segments parallel to the horizontal axis, and describes the effect quantity and confidence interval of multiple studies by using a diamond. AEs, adverse events; SAEs, serious adverse events; MSCs, mesenchymal stem cells; CI, confidence interval
Forest plot of mortality. Mortality was not statistically significant between the two groups, either at short-term or long-term follow-up. No significant heterogeneity was observed in any of the five groups, and a fixed-effects model was used for statistical analysis. In the plane rectangular coordinate system, the forest plot takes a vertical invalid line (scale of abscissa is 0) as the center, describes the effect quantity and 95% CI of each study by using multiple line segments parallel to the horizontal axis, and describes the effect quantity and confidence interval of multiple studies by using a diamond. AEs, adverse events; SAEs, serious adverse events; MSCs, mesenchymal stem cells; CI, confidence interval
Availability of data and materials
Template data collection forms, data extracted from included studies and data used for all analyses have been included in the Supplementary Information.
Abbreviations
- MSCs:
-
Mesenchymal stromal cells
- ARDS:
-
Acute respiratory distress syndrome
- RCT:
-
Randomized controlled trial
- AEs:
-
Adverse events
- SAEs:
-
Serious adverse events
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- MSC MVs:
-
Mesenchymal stem cell microvesicles
- EMT:
-
Epithelial–mesenchymal transition
- COVID-19:
-
Coronavirus disease 2019
- SOFA score:
-
Sequential organ failure assessment score
- ALI:
-
Acute lung injury
- GRADE:
-
Grading of recommendations, development assessment and Evaluation
- APRV:
-
Airway pressure release ventilation
- AMs:
-
Alveolar macrophages
- ECMO:
-
Extracorporeal membrane oxygenation
- HGF:
-
Hepatocyte growth factor
- DCs:
-
Dendritic cells
References
Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Laffey JG, Matthay MA. Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med. 2017;196(3):266–73.
Griffiths MJD, McAuley DF, Perkins GD, Barrett N, Blackwood B, Boyle A, Chee N, Connolly B, Dark P, Finney S, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Respir Res. 2019;6(1): e000420.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–63.
Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, Wang B, Kang Y. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43(11):1648–59.
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–75.
Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7(7):Cd004477.
Lee YY, Park HH, Park W, Kim H, Jang JG, Hong KS, Lee JY, Seo HS, Na DH, Kim TH, et al. Long-acting nanoparticulate DNase-1 for effective suppression of SARS-CoV-2-mediated neutrophil activities and cytokine storm. Biomaterials. 2021;267: 120389.
Park HH, Park W, Lee YY, Kim H, Seo HS, Choi DW, Kwon HK, Na DH, Kim TH, Choy YB, et al. Bioinspired DNase-I-coated melanin-like nanospheres for modulation of infection-associated NETosis dysregulation. Adv Sci (Weinheim, Baden-Wurttemberg, Germany). 2020;7(23):2001940.
Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12(5):383–96.
Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13(4):392–402.
Peng W, Chang M, Wu Y, Zhu W, Tong L, Zhang G, Wang Q, Liu J, Zhu X, Cheng T, et al. Lyophilized powder of mesenchymal stem cell supernatant attenuates acute lung injury through the IL-6-p-STAT3-p63-JAG2 pathway. Stem Cell Res Ther. 2021;12(1):216.
Zhang X, Ye L, Tang W, Ji Y, Zheng L, Chen Y, Ge Q, Huang C. Wnt/β-catenin participates in the repair of acute respiratory distress syndrome-associated early pulmonary fibrosis via mesenchymal stem cell microvesicles. Drug Des Dev Ther. 2022;16:237–47.
Lu Z, Chang W, Meng S, Xu X, Xie J, Guo F, Yang Y, Qiu H, Liu L. Mesenchymal stem cells induce dendritic cell immune tolerance via paracrine hepatocyte growth factor to alleviate acute lung injury. Stem Cell Res Ther. 2019;10(1):372.
Younes N, Zhou L, Amatullah H, Mei SHJ, Herrero R, Lorente JA, Stewart DJ, Marsden P, Liles WC, Hu P, et al. Mesenchymal stromal/stem cells modulate response to experimental sepsis-induced lung injury via regulation of miR-27a-5p in recipient mice. Thorax. 2020;75(7):556–67.
Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O’Kane CM, Krasnodembskaya AD. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196(10):1275–86.
Qu W, Wang Z, Hare JM, Bu G, Mallea JM, Pascual JM, Caplan AI, Kurtzberg J, Zubair AC, Kubrova E, et al. Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med. 2020;9(9):1007–22.
Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15(1):39.
Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7(2):154–62.
Lanzoni G, Linetsky E, Correa D, Messinger Cayetano S, Alvarez RA, Kouroupis D, Alvarez Gil A, Poggioli R, Ruiz P, Marttos AC, et al. Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: a double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl Med. 2021;10(5):660–73.
Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, Young D, Bentley A, McVerry BJ, Wunderink RG, et al. Safety and efficacy of multipotent adult progenitor cells in acute respiratory distress syndrome (MUST-ARDS): a multicentre, randomised, double-blind, placebo-controlled phase 1/2 trial. Intensive Care Med. 2022;48(1):36–44.
Monsel A, Hauw-Berlemont C, Mebarki M, Heming N, Mayaux J, Nguekap Tchoumba O, Diehl JL, Demoule A, Annane D, Marois C, et al. Treatment of COVID-19-associated ARDS with mesenchymal stromal cells: a multicenter randomized double-blind trial. Crit Care (London, England). 2022;26(1):48.
Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3(1):24–32.
Yip HK, Fang WF, Li YC, Lee FY, Lee CH, Pei SN, Ma MC, Chen KH, Sung PH, Lee MS. Human umbilical cord-derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med. 2020;48(5):e391–9.
Gorman E, Shankar-Hari M, Hopkins P, Tunnicliffe WS, Perkins GD, Silversides J, McGuigan P, Krasnodembskaya A, Jackson C, Boyle R, et al. Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) trial: a phase 1 trial. EClinicalMedicine. 2021;41: 101167.
Wei FT, Kong DX, Li T, Li A, Tan Y, Fang JF, Zhuang XH, Lai C, Xu WH, Dong H, et al. Efficacy and safety of umbilical cord mesenchymal stem cells for the treatment of patients with COVID-19. Clinics. 2021;76:e2604.
Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–37.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800.
Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a “Typical” acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299–300.
Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, Thompson BT, Hardin CC. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560–4.
Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148–50.
Mangalmurti NS, Reilly JP, Cines DB, Meyer NJ, Hunter CA, Vaughan AE. COVID-19-associated Acute respiratory distress syndrome clarified: a vascular endotype? Am J Respir Crit Care Med. 2020;202(5):750–3.
Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, et al. Clinical Study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering (Beijing, China). 2020;6(10):1153–61.
Rojas M, Cárdenes N, Kocyildirim E, Tedrow JR, Cáceres E, Deans R, Ting A, Bermúdez C. Human adult bone marrow-derived stem cells decrease severity of lipopolysaccharide-induced acute respiratory distress syndrome in sheep. Stem Cell Res Ther. 2014;5(2):42.
Cardenes N, Aranda-Valderrama P, Carney JP, Sellares Torres J, Alvarez D, Kocyildirim E, Wolfram Smith JA, Ting AE, Lagazzi L, Yu Z, et al. Cell therapy for ARDS: efficacy of endobronchial versus intravenous administration and biodistribution of MAPCs in a large animal model. BMJ Open Respir Res. 2019;6(1): e000308.
Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–8.
El-Khatib MF, Jamaleddine GW: Clinical relevance of the PaO2/FiO2 ratio. Critical care (London, England) 2008, 12(1):407; author reply 407.
Wick KD, Leligdowicz A, Zhuo H, Ware LB, Matthay MA: Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight. 2021;6(12):e148983.
Bhandari V, Choo-Wing R, Lee CG, Zhu Z, Nedrelow JH, Chupp GL, Zhang X, Matthay MA, Ware LB, Homer RJ, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12(11):1286–93.
Parikh SM. Angiopoietins and Tie2 in vascular inflammation. Curr Opin Hematol. 2017;24(5):432–8.
Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, Cortez A, Abbott J, Liu KD, Calfee CS. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187(7):736–42.
Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32(1):116–25.
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells. 2010;28(12):2229–38.
Clark JG, Milberg JA, Steinberg KP, Hudson LD. Type III procollagen peptide in the adult respiratory distress syndrome. Association of increased peptide levels in bronchoalveolar lavage fluid with increased risk for death. Ann Intern Med. 1995;122(1):17–23.
Meduri GU, Kohler G, Headley S, Tolley E, Stentz F, Postlethwaite A. Inflammatory cytokines in the BAL of patients with ARDS. Persistent elevation over time predicts poor outcome. Chest. 1995;108(5):1303–14.
Agrawal A, Zhuo H, Brady S, Levitt J, Steingrub J, Siegel MD, Soto G, Peterson MW, Chesnutt MS, Matthay MA, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012;303(8):L634-639.
Calfee CS, Janz DR, Bernard GR, May AK, Kangelaris KN, Matthay MA, Ware LB. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015;147(6):1539–48.
Sinha P, Delucchi KL, McAuley DF, O’Kane CM, Matthay MA, Calfee CS. Development and validation of parsimonious algorithms to classify acute respiratory distress syndrome phenotypes: a secondary analysis of randomised controlled trials. Lancet Respir Med. 2020;8(3):247–57.
Fiedler U, Scharpfenecker M, Koidl S, Hegen A, Grunow V, Schmidt JM, Kriz W, Thurston G, Augustin HG. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103(11):4150–6.
Wyles CC, Houdek MT, Wyles SP, Wagner ER, Behfar A, Sierra RJ. Differential cytotoxicity of corticosteroids on human mesenchymal stem cells. Clin Orthop Relat Res. 2015;473(3):1155–64.
Fang B, Song Y, Liao L, Zhang Y, Zhao RC. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transpl Proc. 2007;39(10):3358–62.
Acknowledgments
The authors thank all those who participated in the manuscript.
Funding
Project supported by the Scientific Research Foundation of Fujian Provincial Health Commission (2020CXA046) and the Natural Science Foundation of the Fujian Province (2021J01258).
Author information
Authors and Affiliations
Contributions
All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. JW, YL, and FL were involved in study concept and design and study supervision. All authors contributed to acquisition, analysis, or interpretation of data. JW was involved in drafting of the manuscript. YL contributed to critical revision of the manuscript for important intellectual content. JW, FL, YS, YZ, KC, and DY were involved in statistical analysis. JW and FL contributed to administrative, technical, or material support. All authors read and approved the final manuscript
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have disclosed that they do not have any potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1
. Search strategy.
Additional file 2
. Data characteristics in Included Trials.
Additional file 3
. PaO2/FiO2 and Biomarkers characteristics in Included Trials.
Additional file 4
. Characteristics of included studies.
Additional file 5
. Quality of GRADE.
Additional file 6.
Summary of Findings.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Luo, F., Suo, Y. et al. Safety, efficacy and biomarkers analysis of mesenchymal stromal cells therapy in ARDS: a systematic review and meta-analysis based on phase I and II RCTs. Stem Cell Res Ther 13, 275 (2022). https://doi.org/10.1186/s13287-022-02956-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02956-3