Abstract
Importance
Cataract is one of the leading causes of childhood blindness in Africa. The management of this condition requires timely surgical extraction of the cataractous lens with immediate optical correction and long-term follow-up to monitor visual improvement and manage complications that may arise. This review provides an opportunity to benchmark outcomes and to shed light on the reasons for those outcomes.
Objectives
To review the published literature and report on the outcomes of paediatric cataract surgery with intraocular lens insertion in sub-Saharan Africa.
Data source
The EMBASE, PubMed, Scopus, and Web of Science were searched for relevant articles.
Study selection
We included all published primary studies from sub-Saharan Africa on cataract surgery outcomes in children aged 0–16 years with primary intraocular lens implantation conducted between 1990 and 2020. Eligible studies were those published in English or for which an English translation was available. In addition, reviewers screened the reference lists of all studies included in the full-text review for eligible studies. During the review, studies fitting the inclusion criteria above except for having been conducted in middle and high-income countries were tagged and placed in a comparison arm.
Data extraction and synthesis
Study eligibility was determined by two independent reviewers, and data extraction was conducted by one reviewer with entries checked for accuracy by another reviewer. Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines for data synthesis were followed. The Joanna Briggs Institute (JBI) critical appraisal checklist was used for quality appraisal of the studies. The statistical software R was used in the analysis, and data were pooled using a random-effects model. Forest plots were generated using the R package ‘metafor’.
Main outcomes and measures
The primary outcome was visual acuity (VA) after cataract surgery and the proportions of eyes that achieved good, borderline, or poor visual outcome according to the World Health Organisation (WHO) categorisation of post-operative visual acuity. The secondary outcome measures reported included lag time to surgery, rates of follow-up, and rate of complications.
Results
Eight out of 4763 studies were eligible for inclusion in this review, and seven were included in the quantitative analysis. There was a male preponderance in the study population, and the mean age at the time of cataract surgery ranged from 3.4 to 8.4 years. Visual outcomes were available for short-term visual outcomes (1 to 6 months) as the studies had a significant loss to follow-up. The pooled proportion of eyes that achieved a good visual acuity (i.e. equal to or greater than 6/18) in the short-term period was 31% (CI, 20–42). The comparative studies from middle and high-income countries reported proportions ranging from 41 to 91%, with higher thresholds for good visual acuity of 6/12 and 6/15.
Conclusion and relevance
This review reports that there is a lower proportion of eyes with good outcomes after undergoing paediatric cataract surgery in sub-Saharan Africa than in middle- and high-income countries. Furthermore, this review states that there is a high proportion of patients lost to follow-up and suboptimal refractive correction and amblyopia treatment after paediatric cataract surgery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The management of paediatric cataracts, i.e. the opacification of the crystalline lens in children, involves timely diagnosis and surgical intervention as delays can lead to permanent suboptimal functional vision due to amblyopia. Previously, corneal disease was the predominant anatomic cause of childhood blindness [1]. However, in recent years, with improved childhood immunisation coverage and vitamin A supplementation, visual impairment from cataracts has become an important cause [2].
Over the years, the technique for paediatric cataract surgery has undergone changes in order to improve visual outcomes and lower the rate of post-operative complications [3]. Substantial debate still exists among paediatric ophthalmologists regarding the best practice of intraocular lens implantation in children [4]. These include primary versus secondary implantation, intraocular lens power calculations, intraocular lens material selection, and associated safety profiles [4]. However, the general consensus is that primary intraocular lens implantation is an appropriate standard of care for children above the age of 2 years [5], with much less consensus on the implantation in infants, especially under the age of 1 year [6].
After surgical removal of the cataract, immediate correction of any refractive error is required to maximise the visual acuity and prevent amblyopia [7]. In patients with primary intraocular lens implantation, this is usually achieved using prescription spectacles. For children who are left aphakic, this can be done using aphakic glasses or more preferably contact lenses [8]. Although refractive correction alone can significantly enhance visual acuity, treatment for amblyopia is sometimes necessary. This is done by increasing visual stimulation of the amblyopic eye by intermittent occlusion of the dominant eye, either by means of patching (occlusion therapy) or atropine and optical penalisation [9].
There is a lack of comprehensive prospective studies on the outcomes of paediatric cataract surgery in sub-Saharan Africa (SSA). A few isolated reports suggest that paediatric cataract surgical outcomes in SSA are not in keeping with outcomes from other parts of the world. For example, using the World Health Organisation’s (WHO) visual acuity threshold of 6/18 for a good cataract surgical outcome, only 31.5% of eyes in a retrospective Nigerian study achieved a good visual outcome [10]. Another retrospective study conducted in Ethiopia reported an even lower proportion of 11% of the study eyes that achieved a good outcome.
Complications of paediatric cataract surgery are potentially visually significant, and they may be observed from the early post-operative period up to many years after the procedure [11]. The risk of post-operative complications is higher than in adult cataract surgery due to the more intense inflammatory response mounted by children after intraocular surgery [12]. Paediatric cataract management requires a multidisciplinary team that includes paediatric ophthalmologists, optometrists, and orthoptists to optimise outcomes [12]. Furthermore, it requires the dedication of the child’s carer to the numerous visits required to monitor for short- and long-term post-operative complications.
Rationale
This review synthesised studies that reported the outcomes of paediatric cataract with a minimum follow-up of 4 weeks. The findings from this review may provide a baseline for tracking paediatric surgical outcomes in Africa. This review will be one of the first to report on the outcomes of paediatric cataract surgery in SSA. There have been some isolated reports in parts of Africa. However, there has not been a comprehensive analysis of these data to add to the body of knowledge and inform clinical practice on the surgical management of paediatric cataract in the SSA region.
Objective
This review aimed to answer the following question: what is the level of vision achieved in children who underwent cataract surgery with intraocular lens insertion in SSA?
Methods
This study protocol and review were reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) guidelines [13]. The protocol was registered prospectively on PROSPERO (ID CRD42022309523).
Eligibility criteria
In 1990, the World Health Organisation (WHO) organised the inaugural meeting of experts on the prevention of blindness in children, where they estimated the global magnitude, classification, and causes of childhood blindness [14]. Following from this meeting, a new system for classifying the causes of blindness in children was developed [14]. We thus expected studies starting from 1990 onwards to be more likely to be relevant and comparable to contemporary healthcare practices in sub-Saharan Africa. We included all published primary studies on cataract surgery outcomes in children aged 0–16 years conducted in SSA between 1990 and 2020. Only studies with a minimum follow-up time of 4 weeks were included. Studies with mixed patient groups, for example, those with traumatic cataracts, were included if the data analysis regarding the visual outcomes of the aetiology was performed separately.
Articles were excluded from the analysis if the study design was a letter to the editor, a case report, or a systematic review. Studies that included children with pre-existing visually significant comorbidities such as glaucoma and retinal or corneal dystrophy were excluded. Furthermore, studies available only as conference abstracts or unpublished data and studies that were reported in languages other than English with no translation available were also excluded.
Information sources and search strategy
A search of PubMed (last searched on 21st March 2022), EMBASE (last searched on 28th March 2022), Scopus (last searched on 1st April 2022), and Web of Science (last searched on 10th April 2022) databases was done using a predefined search strategy. The full search strategy for PubMed (Additional file 1: Appendix 1) was modified as necessary for the other databases. The results from the four databases were uploaded to the online review management software Covidence [15]. Two independent reviewers (PPM and TLZ) performed the first and second rounds of article screening through this platform. The first round was the screening of titles and abstracts, and round two was full-text screening. The reference lists of full-text articles were also scrutinised for potentially eligible studies. All discrepancies in article selection were tagged by Covidence, and the conflicts were resolved within the software while the reviewers were blinded to each other’s conflict-resolving vote. In scenarios where both reviewers had voted to exclude an article but disagreed on the reason for exclusion, a discussion was held to reach a consensus on the reason. A third reviewer (HIN) was available as an arbitrator in case the two reviewers could not resolve any conflicts; however, the need for this did not arise. During the review, studies fitting the inclusion criteria but conducted in middle and high-income countries were tagged and placed in a comparison arm.
Data extraction
Data were extracted from studies retained from round two of screening by one reviewer (PPM) and checked by a second reviewer (HIN) for accuracy. One reviewer (PPM) conducted the data extraction for all the included studies, and a second reviewer (HIN) rechecked the results against the papers for accuracy. Discrepancies were resolved through a review of the article in question and a discussion between the two reviewers.
The primary outcome was visual acuity after cataract surgery, which was reported using the WHO categorisation of visual acuity; those with a visual acuity of 6/18 or better were categorised as ‘good outcome’, those with a visual acuity of less than 6/18 but greater than 6/60 were categorised as borderline, and those with a visual acuity less than 6/60 were categorised as poor outcome [16]. Depending on the duration after surgery, these outcomes were described as short-term outcomes (1 to 6 months), medium-term (7 to 12 months), or long-term (longer than 12 months). Data on the secondary outcome of the rate of post-operative complications such as uveitis, glaucoma, retinal detachment, and visual axis or posterior capsular opacification were also collected if reported. Where available, other data collected included publication characteristics, preoperative visual acuity, whether or not amblyopia treatment was given, and lag time. Lag time was defined as the time taken from noticing the cataract to surgery and ‘late presentation’ was defined as a delay to cataract surgery of more than 12 months.
Risk of bias and quality assessment
We applied the Joanna Briggs Institute (JBI) critical appraisal checklist [17] to the eight studies included in this review. The checklist had 11 questions with the options ‘yes’, ‘no’, and ‘unclear’. A response of ‘yes’ indicated that the study met that question’s quality criterion. Two reviewers (PPM, HIN) performed the risk of bias assessment independently, and conflicts were resolved through discussion. An arbitrator (TLZ) was on standby for conflicts that could not be resolved through dialogue. For the risk of bias and quality assessment, all the studies were classified as case series because the study population consisted of only participants who were sampled based on the presence of a specific outcome [18], i.e. visual outcomes after cataract surgery. The studies included consecutive participants who satisfied the inclusion criteria over a given period of time. Furthermore, the absence of a control group of patients that prevented the estimation of relative risk (the odds ratio) for the outcome [18] was also considered a criterion for classification as a case series.
Statistical analysis
The statistical software R was used in the analysis [19]. Forest plots were generated using the R package ‘metafor’ [20]. We used a random-effects model to evaluate pooled effects due to the high likelihood of heterogeneity among the selected studies. Heterogeneity between studies was assessed using the I2 statistic and the chi-squared test.
Differences between protocol and review
The age of inclusion in the review was adjusted from 0–15 to 0 to less than 16 years as some studies considered this range the paediatric population. We concluded that an additional 12 months would not significantly alter or adversely affect the results.
During the review process, similar studies from middle- and high-income countries were tagged and placed in a geographical comparison group. This was done in an attempt to contextualise the results on a global scale of paediatric cataract surgical outcomes.
Results
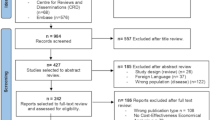
The search strategy extracted 6448 published articles of which 1685 were duplicates as described by the PRISMA flow chart (Additional file 1: Appendix 2). The full texts of 24 studies were evaluated, and 16 studies were excluded. A summary of excluded studies can be found in Additional file 1: Appendix 3. Eight studies were included in the quantitative analysis, seven of which were included in qualitative analysis. The study types that were included as reported by the authors were retrospective case series, retrospective chart review, prospective interventional, retrospective interventional case series, prospective longitudinal, hospital-based interventional study, prospective longitudinal hospital-based observational study, and retrospective survey. The PRISMA checklist for the review is available in Additional file 1: Appendix 4.
Clinical presentation
All the studies had a male preponderance in the patient population and the study durations ranged from 12 to 58 months. The mean age in the studies ranged from 3.4 to 8.4 years old. Only three of the studies provided the mean age along with the standard deviation. Therefore, combining the means of these studies alone, although considered, was deemed unlikely to yield meaningful results. Table 1 shows the characteristics of the included studies.
Six out of the eight studies documented the lag time of the study participants. For the studies that reported the lag time as mean, the longest mean delay was reported in Bowman et al. [22]: 44 months. Gogate et al. [25] and Mndeme et al. [28] reported similar mean delays in their study populations, 20.7 months (SD 18) and 21 months (SD 26.7), respectively. Furthermore, half of the study participants had a delay of more than 15 months in Gogate et al. [25] and more than 12 months in Mndeme et al. [28]. A larger proportion of patients with a long lag time were seen in Mboni et al. [26], with nearly two-thirds of the study population undergoing surgery after more than a 12-month delay.
Assessment and diagnosis
Six of the eight studies described the proportion of eyes that were blind prior to cataract surgery, that is, eyes with a visual acuity of less than 3/30. The remainder of the studies described the proportion of eyes that had a preoperative visual acuity of less than 6/60, and these were 98.7% and 88.4% in Mboni et al. [26] and Gogate et al. [25], respectively.
Five out of eight and six out of eight studies reported the proportion of eyes with preoperative strabismus and nystagmus, respectively. One study, Mndeme et al. [28], gave the combined proportion of patients with both findings. Six of the eight studies reported collecting data on systemic comorbidities as part of their methodology; however, only three studies reported the results of these findings (Table 2).
Treatment
We did not specify a single surgical procedure for cataract extraction in the eligibility criteria and allowed for some variation in the surgical procedure. The reason was the consideration of the differences in the availability of resources in the various sub-Saharan countries like surgical equipment and consumables. For example, in Gogate et al. [25], the participants underwent phacoaspiration with primary posterior capsulotomy (PPC) and anterior vitrectomy (AV) performed in participants below 6 years of age. On the other hand, the cataract removal procedure performed included lens aspiration, PPC and AV in Bowman et al. [22], and extra-capsular cataract extraction (ECCE) in Onabolu and Iwuora [23] as shown in Table 1.
Visual outcomes
All the studies except Mndeme et al. [28] reported quantifiable visual outcomes using the conventional 6 m. Although Mndeme et al. reported visual acuity in LogMar, the same WHO cut-offs for good borderline and poor categories were used. For example, the 0.48 LogMar vision for a good outcome equates to 6/18. Good short-term visual outcomes were reported for all eight studies. However, borderline and poor short-term outcomes were not available for Mndeme et al. [28] and Bowman et al. [22], and poor short-term outcomes were not available for Yorston et al. [21] (Fig. 1).
The proportion of eyes that achieved a good visual outcome after cataract surgery ranged from 16.5 to 62.0%. On the other hand, the proportion of eyes which attained poor visual outcome ranged from 0 to 51%. Only one study, Yorston et al. [21], reported medium- and long-term visual outcomes. The proportions of eyes that achieved a good visual outcome were 39.1% and 50.8% in the medium and long term, respectively. In the long term, only 6.1% of eyes in Yorston et al. [21] had maintained a poor visual outcome. The pooled proportion of eyes that achieved a good visual acuity in the short-term period is 31% (CI, 20–42), as shown in Fig. 2.
Amblyopia treatment
Four out of the eight studies (Yorston et al. [21], Bowman et al. [22], Umar et al. [24], and Gogate) indicated that they had instituted amblyopia treatment during the post-operative period. The method of amblyopia treatment in these studies was patching of the better eye for a specified duration according to the severity of the amblyopia. A comparison of visual acuity before and after amblyopia treatment was not reported.
Post-operative complications
Five out of the eight studies reported that some eyes developed acute fibrinous uveitis. The proportion of eyes that developed this complication ranged from 1.3 to 30.5% (Fig. 3). Analysis yielded I2 statistic of 96% (p-value < 0.01). This indicated the presence of large and significant heterogeneity in the effect sizes. Pooling the data yielded a proportion of 12% (CI, 2–21) with uveitis.
Six of the eight studies reported on the development of posterior capsular or visual axis opacification within 6 months post-surgery (Fig. 4). Analysis for those who developed PCO yielded I2 statistic of 93% (p-value < 0.01). This indicated the presence of large and significant heterogeneity in the effect sizes. Pooling the proportions yielded a proportion of 13% (CI, 5–22). Only one study, Onabolu and Iwuora [23], reported the complication of post-operative retinal detachment. No study observed the development of post-operative endophthalmitis in the participants' eyes. The rest of the post-operative complications are depicted in Additional file 1: Appendix 5.
Post-operative follow-up
Only three of the eight studies reported a statistically quantifiable follow-up time for the study eyes. Asferaw et al. [27] reported a median follow-up time of 2.8 months (range, 1–33). Bowman et al. [22] reported a mean follow-up time of 6 months (SD 9 months), with only 54% of participants seen after 3 months. On the other hand, Yorston et al. [21] reported both mean and median follow-up times of 15 and 17 months, respectively.
Quality of the evidence and subgroup analysis
The results of the quality assessment are presented in Additional file 1: Appendix 6. Analysis output for good visual acuity reveals that Q-statistic is 22.3225 with p-value equal to 0.002, and I2 statistic is 67.74% (CI, 23.0–91.5). This suggests the presence of heterogeneity in the effect sizes. The heterogeneity is between low to large. The heterogeneity in the effect sizes is uncertain.
An in-depth analysis of visual outcomes based on age was not possible due to the lack of homogeneity of age categories in the studies (Fig. 5). For example, Bowman et al. [22] did not report visual outcomes disaggregated by age, Yorston et al. [21] grouped the eyes in the categories 0–1, 2–5, and 6–10 years, and Umar grouped them 0–1, > 1–3, and > 8.
However, after stratifying the studies based on the average age of the participants, large and significant heterogeneity in the effect sizes was observed among the studies with mean age of participants of less than 6 (I2 statistic was 94% and p-value < 0.01), while among the studies that had a mean age of participants of greater than 6, heterogeneity was medium and not significant (I2 statistic was 57% and p-value = 0.10). Although the outcomes for those under the average age of 6 shown better visual outcomes, analysis did not yield a significant difference between the proportions (for good visual acuity) of those that were under the age of six (CI, 16–55) and those whose age was more than six (CI, 12–37).
Subgroup analysis based on whether biometry was done or not yielded an I2 statistic of 40% (p-value = 0.15) for the studies in which biometry was done indicating that heterogeneity in the effect sizes was low and not significant (Fig. 6). For studies in which biometry was done, the analysis yielded a proportion of good visual acuity of 24% (CI, 18–30). There was one study, Onabolu and Iwuora [23], which indicated that biometry was not done, and the proportion of good visual acuity was 22%(CI, 6–48).
Subgroup analysis was also performed based on whether single IOL or multiple IOLs types were used (Fig. 7). For studies that used single IOL, heterogeneity might be due to sampling error (I2 statistic was 0% with p-value = 0.5), while among studies that used multiple IOL, it was large and significant (I2 statistic was 81% with p-value < 0.01). The analysis yielded a proportion of 22% (CI, 18–27) for good visual acuity for studies that used single IOL, and a proportion of 46% (CI, 28–64) for those that used multiple IOLs. The proportion of good visual acuity for those who used multiple IOLs was significantly higher than that of those who used single IOL.
Discussion
In this review, we synthesised the available primary studies on the outcomes of paediatric cataract surgery with intraocular lens implantation in SSA.
Patient characteristics and presentation
All the studies in this review had a male preponderance of participants even though there is no biological evidence to support a sex-specific male predisposition in the prevalence of congenital or non-traumatic developmental cataracts [29]. Some studies [21, 24] reported one reason is that in a lot of African communities, boys are awarded a higher societal value than girls. Other studies on paediatric cataract surgery from Africa have reported the similar findings of gender discrepancy with similar explanations [30, 31]. These findings suggest a need to improve equitable access to paediatric cataract surgery in SSA.
The majority of the participants in the studies experienced a long preoperative delay which is not uncommon in SSA [25]. African studies that investigated the reasons for lag time and its association with visual outcomes defined delay as ‘more than 12 months’ before receiving a cataract operation [25, 26, 32]. This is likely due to pragmatic reasons, as it is a routine occurrence to have children with cataracts present late to the hospitals. In principle, for congenital and infantile cataracts, by the time the children are delayed for 12 months, the optimum time for surgery has passed [33, 34].
Mwende et al. [32] reported on the causes of delayed presentation for non-traumatic cataracts in Tanzania. They found that a longer distance from the eye care facility significantly increased the delay in presentation. Furthermore, there was a positive correlation between rising maternal socio-educational status and a reduction in delay in presentation. This is because these mothers are more likely to have some knowledge of the problem and the treatment that exists. They are also more likely to have the financial means to access eye care services and accept the surgical services offered.
The lag time from the studies in this review was not qualified; it is unclear the extent of delay that resulted from late recognition of the cataract by the children’s caregivers, delay in accessing eye care services, and the delay that resulted from waiting for surgery after presentation to an eye care facility. This information would be crucial in formulating an approach to dealing with the primary barriers that exist at the community level. One study from Southwest Nigeria that investigated the factors associated with early versus late presentation to tertiary eye care facility found that children whose cataract was detected by their mothers were more likely to present to the eye care facility within 3 months of detection [35]. In addition, these children were also more likely to present at a younger age than cataracts detected by other caregivers [35]. This suggests that educating and empowering mothers about cataract in children may be a tool in the arsenal of tackling blindness from childhood cataracts.
Preoperative assessment
This review found that the proportion of blind eyes preoperatively in all the studies ranged from 50 to 100%. Preoperative findings are essential in prognosticating the outcome of surgery. The presence of strabismus and/or nystagmus can adversely affect outcomes, and their prevalence varies widely among studies [36]. Strabismus prevents the development of binocular vision, and the amblyopia it causes can have adverse aesthetic and psychological effects on the child [36]. Moreover, the presence of nystagmus and strabismus are indications that substantial visual deprivation has occurred [24]. Other studies have reported an association between poor preoperative vision and limited improvement in post-operative visual acuity [22, 25, 28, 37].
Visual outcomes
During our literature review, we did not find standardised benchmark indicators for outcomes of paediatric cataract surgery comparable to the WHO guidelines established for adult cataract outcomes. Similarly, in their work on outcome indicators in paediatric cataract surgery, Nihalani et al. [38] did not identify any publication focused on benchmark indicators in paediatric cataract surgery. As such, the WHO categorisation was used in this review. The pooled proportion of eyes that achieved a good visual acuity in the short-term period was 31% (CI, 20–42). Although we did not find studies from high-income countries that defined the cut-off for ‘good visual outcomes’ as 6/18 like in the studies in our review to offer a direct comparison, there were several studies that used the cut-offs of 6/12 and 6/15 (Additional file 1: Appendix 7) The American studies Peterseim et al. [39], Wilson et al. [40], and Struck et al. [41] reported that 27 (91%) (CI, 80–97), 48 (72%) (CI, 59–82), and 13 (85.7%) (CI, 66–100) of eyes achieved a visual acuity of 6/12 or better good visual outcome at the last follow-up visit, respectively. In the UK, a study by Cassidy et al. [42], 25 (73.5%) (CI, 51–80) children achieved a visual acuity of 6/12 or better. Furthermore, the European study Ambroz et al. reported that 34 (54.0%) (CI, 41–67) of eyes achieved a post-operative acuity equivalent to 6/15 of better. Although these studies are not a direct comparison, the cut-offs of 6/15 and 6/12 are a higher standard of visual acuity. It thus suggests that outcomes that are achieved in middle- and high-income countries are superior to those from SSA.
It has been proposed that during visual development, there is a short, well-defined period in early life where the neuronal pathways are robustly restructured in response to sensory input [43, 44]. Years after this ‘critical period’ the same stimuli have less influence on visual development. What follows from these findings is that the occlusion of one eye during this critical period in early life results in the development of a suboptimal visual acuity in the deprived eye that persists into adulthood if left uncorrected [45]. Our expectation would be that children with developmental cataract would obtain better visual outcome because during the critical period, there was no sensory deprivation, and thus, they attained optimum vision prior to the cataract.
It should be noted that the visual outcomes in SSA may be better than those reported in this review. This is because of the lack of long-term follow-up for visual rehabilitation and maturation [25], especially in younger children. Moreover, the visual function is not limited to visual acuity and other factors such as contrast sensitivity and stereopsis need to be considered. However, no study in this review reported on post-operative contrast sensitivity testing, and only one study, Gogate et al. [25], measured stereopsis preoperatively and on follow-up visits. The majority of eyes had poor stereopsis, with only 9 (18%) children achieving better than or equal to 400 s of arc.
Post-operative follow-up
A comparison of the follow-up times from the studies in this review to those from studies in high-income countries revealed that the latter have longer follow-up times for patients. For example, in the American study Ledoux et al. [46], the median follow-up time was 3.65 years. Similarly, Repka et al. [47], in their multicentre study of 994 children, retained 88.4 and 66% of participants at 1 and 5 years. Follow-up in Africa is usually challenging for various reasons, such as the long distance from the tertiary eye care facility, which is coupled with poor road infrastructure, financial constraints, and lack of awareness of the importance of long-term follow-up [48]. Unfortunately, without appropriate post-operative follow-up, paediatric cataract surgery alone produces limited results [49]. The value of long-term follow-up visits can be evident if the child receives the appropriate care at each visit. In many parts of SSA, post-operative services from allied personnel such as orthoptists, refractionists, and low visual aid service providers are lacking [50]; thus, the care received is likely to be suboptimal.
Post-operative complications
Acute uveitis is more common in children as they mount a greater inflammatory reaction following intraocular surgery due to an immature blood-aqueous barrier [51]. Patients are typically prescribed topical steroid eye drops and cycloplegics post-operatively [51]. These were prescribed in all the studies included in this review. Literature shows a variable incidence of uveitis after paediatric cataract surgery. The proportion of eyes with acute uveitis in this review varied from zero in Gogate et al. [25] to 30.5% in Yorston et al. [21]. This is comparable to isolated studies from the west. In the American study by Ledoux et al. [46], no eyes in their series of 139 children had post-operative uveitis, whereas in the UK study by Cassidy et al. [42], uveitis occurred in 28.2% of eyes. However, the pooled proportion of uveitis in our study was found as 12% (CI, 2–21).
Glaucoma is a significant risk in paediatric cataract surgery. Recent multicentre prospective studies in high-income countries reported the incidence of glaucoma after paediatric cataract surgery to be 10% in the first year of follow-up, with the condition occurring in both aphakic and pseudophakic eyes [52, 53]. The highest proportion of eyes with elevated intraocular pressure was 2.9% in Mndeme et al. [28]. Glaucoma after paediatric cataract surgery is typically late-onset open-angle glaucoma [11], although it can be observed within the first few months following surgery. Most early-onset glaucoma is due to vitreous pupillary block or inflammation. But with advances in technology, changes in surgical techniques and the appropriate use of anti-inflammatory medication post-operatively, early-onset glaucoma is much more uncommon [54]. The low number of eyes that developed glaucoma in our review can be explained by the short follow-up time. Therefore, it can be anticipated that if there were a longer follow-up, there would be a larger proportion of eyes seen with glaucoma.
Eyes that have undergone cataract extraction are at an increased risk of retinal detachment. Like aphakic or pseudophakic glaucoma, retinal detachment is also a long-term complication. In Denmark, a study of 1043 eyes of children aged 0 to 17 years by Haargaard et al. [55] reported that 25 eyes developed retinal detachment after a mean duration of 9.1 years after surgery. They further reported an overall 20-year risk of retinal detachment of 7%. This highlights the need for lifelong monitoring in these patients. Our review has low numbers of eyes that developed retinal detachment for the same reason as the low number of glaucoma, which is the short follow-up time.
Heterogeneity in the effect sizes
Analysis output for good visual acuity revealed that Q-statistic was 55.83 with p-value less than 0.0001, and I2 statistic was 87% (CI, 78–93). This indicated the presence of large heterogeneity in the effect sizes. This may result from the clinical and methodological differences across studies in this review. As previously outlined, there were variations in how the surgical procedures were conducted. Furthermore, in some studies, there was one surgeon who performed paediatric cataract surgeries, whereas some studies had multiple surgeons. In addition, the small number of studies included in the review may be the reason heterogeneity in the effect sizes is uncertain. Subgroup analysis based on whether biometry was done or not, and whether single IOL or multiple IOL types were used revealed small heterogeneity which was not significant in any of the strata. This indicates that in addition to the small number of studies considered, heterogeneity in the effect sizes was largely due to variations in the methodological designs of the studies.
Challenges that result in inferior outcomes in sub-Saharan Africa
Early surgical intervention is recommended for bilateral congenital cataracts to improve visual outcomes [33], and for unilateral cataracts, this intervention is recommended even earlier [34]. As seen from this review, the majority of patients had a lag time of more than 12 months and, in some cases, more than 36 months. In high-income countries, there are surveillance programs for routine screening of neonates for early recognition of any lens opacity and thus provide timely surgical intervention [56]. On the other hand, in low-income countries, research suggests that long lag times are multifactorial, ranging from sociocultural barriers at the community level to logistical and organisational barriers within the health care system [57]. In some cases, the late presentation is due to poor health-seeking habits of the child’s guardians, as illustrated in Gogate et al. [25], where a quarter of guardians stated the reason for the delay in seeking help as ‘did not see the need to come to hospital.’ In situations where the symptoms are painless and not considered life-threatening, there may be a delay in presentation to tertiary eye facility for treatment [58].
Another reason for poor outcomes is the shortage of specialised paediatric ophthalmologists in SSA to cater to the immense burden of paediatric cataracts. Accessing sub-specialty training for paediatric ophthalmology is difficult, especially in Francophone Africa; thus, there is a continued lack of skilled eye care providers needed in tertiary hospitals [59]. In addition, there is a lack of visual rehabilitation facilities in SSA, especially for very young children. The standard of practice for managing these children is to prescribe contact lenses in lieu of intraocular lens implantation due to the increased risk of post-operative complications and higher reoperation rates in this patient group [60,61,62]. However, their use in SSA is impractical [21]. The majority reside in rural areas where clean running water is scarce, making personal and ocular hygiene a challenge [63]. This is further compounded by the high cost of the lenses and lens cleaning solutions. Other associated problems such as the risk of microbial keratitis and lens loss also limit the use of these methods [8]. There is a paucity of research on the safety and effectiveness of these interventions in the African context. With all the problems surrounding contact lens usage and the rise of published case series reporting promising results with intraocular lens implantation in younger children [39, 64,65,66], some paediatric surgeons are now moving to primary intraocular lens implantation in younger children.
Whether children are left aphakic or have intraocular lens implantation, they still require optical correction to maximise visual outcomes [67]. Although glasses are more appropriate for the African setting, there are few children who get the glasses even after being refracted. For instance, the Madagascan study Randrianotahina et al. [68], in their series of 86 children, found that despite three-quarters of patients having refraction performed, only 3.5% received glasses. Furthermore, the glasses may break or get lost [21], after which they may not be replaced. Other challenges in prescribing glasses to children include difficulties obtaining accurate refraction and the availability of suitable frames for very young children [21].
Strengths and limitations
This review has scope for novelty in adding to the knowledge gap regarding paediatric cataract surgery. To our knowledge, this is the first review focusing on collating outcomes of visual outcomes of paediatric cataract surgery across sub-Saharan Africa. The inclusion of primary research studies combined with rigorous article screening and quality assessment provides a comprehensive evaluation of the available evidence.
A limitation of this review is the presence of significant heterogeneity within the included studies. Given the context of the SSA setting, the nature of the study population, and intervention under investigation, identifying controlled trials for more accurate and reliable estimates proved challenging. Nevertheless, it is important to note that this review emphasised narrative synthesis of the results over quantitative analysis, aiming to highlight the underlying reasons behind the observed findings.
Caution is advised when interpreting our pooled estimate of good outcomes, as not all factors influencing post-operative visual acuity were systematically analysed. For example, the primary studies lacked information on the measures employed for visual rehabilitation in patients. Moreover, for those implementing amblyopia treatment, details on treatment compliance and the ultimate visual outcomes post-treatment were not reported. There was also no information provided on optical correction compliance for those who received glasses. Lastly, the follow-up period for most of the studies was very short; thus, visual acuity conducted on the young infants may not be reliable. A longer follow-up of patients is needed to further discuss the surgical outcomes of cataracts in SSA.
Conclusion
This review showed that paediatric cataract surgery outcomes in sub-Saharan Africa are lower compared to reports from high-income countries. We reported that the proportion of eyes that achieve a vision of 6/18 or better within 6 months of cataract surgery is 31%. All comparative studies from middle- and high-income countries reported proportions ranging from 41 to 91%, with a higher visual acuity cut-offs of 6/12 and 6/15. Furthermore, there are low rates of follow-up and suboptimal refractive correction and amblyopia treatment after surgery within the studies.
Recommendations
In order to improve outcomes, there is a need to focus on visual rehabilitation after paediatric cataract surgery. Therefore, we recommend cost-effectiveness studies to establish the best models that could be adopted for a sustainable provision of refractive services to children after undergoing cataract surgery in sub-Saharan Africa.
Furthermore, we propose that stakeholders and policymakers in international eye health should come up with guidelines and recommendations for the outcomes of paediatric cataract surgeries for benchmarking, ensuring quality, consistency, and continuous improvement in patient care.
References
Kong L, Fry M, Al-Samarraie M, Gilbert C, Steinkuller PG. An update on progress and the changing epidemiology of causes of childhood blindness worldwide. J AAPOS. 2012;16(6):501–7. https://doi.org/10.1016/j.jaapos.2012.09.004.
Ezegwui IR, Aghaji AE, Uche NJ, Onwasigwe EN. Challenges in the management of paediatric cataract in a developing country. Int J Ophthalmol. 2011;4(1):66–8. https://doi.org/10.3980/j.issn.2222-3959.2011.01.15.
Basti S, Greenwald MJ. Principles and paradigms of pediatric cataract management. Indian J Ophthalmol. 1995;43(4):159–76.
Lin AA, Buckley EG. Update on pediatric cataract surgery and intraocular lens implantation. Curr Opin Ophthalmol. 2010;21(1):55–9.
Fan DSP, Yip WWK, Yu CBO, Rao SK, Lam DSC. Updates on the surgical management of paediatric cataract with primary intraocular lens implantation. Ann Acad Med Singapore. 2006;35(8):564–70.
Plager DA, Lynn MJ, Buckley EG, Wilson ME, Lambert SR. Complications in the first 5 years following cataract surgery in infants with and without intraocular lens implantation in the Infant Aphakia treatment study. Am J Ophthalmol. 2015;158(5):892–8.
Jamison A, Mackinnon JR, Lavy TE, Manda C, Msukwa G. Establishing a pediatric ophthalmology service in Malawi: developments in childhood cataract surgery. Middle East Afr J Ophthalmol. 2019;26(2):77–82.
Lambert SR, Buckley EG, Drews-Botsch C, DuBois L, Hartmann E, Lynn MJ, et al. The infant aphakia treatment study: design and clinical measures at enrollment. Arch Ophthalmol. 2010;128(1):21–7.
Papageorgiou E, Asproudis I, Maconachie G, Tsironi EE, Gottlob I. The treatment of amblyopia: current practice and emerging trends. Vol. 257, Graefe’s Archive for Clinical and Experimental Ophthalmology. Graefe’s Archive for Clinical and Experimental Ophthalmology; 2019. p. 1061–78.
Abuh S, Brennan R, Congdon N, Jin L. Pediatric cataract surgery outcomes in Kano, Nigeria. Niger J Ophthalmol. 2018;26(1):62.
Gasper C, Trivedi RH, Wilson ME. Complications of pediatric cataract surgery. Dev Ophthalmol. 2016;57:69–84.
Pandey SK, Wilson ME, Trivedi RH, Izak AM, Macky TA, Werner L, et al. Pediatric cataract surgery and intraocular lens implantation: current techniques, complications, and management. Int Ophthalmol Clin. 2001;41(3):175–96.
Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle PSL. PRISMA-P (Preferred Reporting Items for Systematic review and Meta-Analysis Protocols) 2015 checklist: recommended items to address in a systematic review protocol. BMJ Br Med J. 2015;350:g7647.
Gilbert C, Muhit M. Twenty years of childhood blindness: what have we learnt? Community Eye Heal J. 2008;21(67):46–7. Available from: https://www.cehjournal.org/article/twenty-years-of-childhood-blindness-what-have-we-learnt/.
Australia Veritas Health Innovation Melbourne. Covidence systematic review software. [cited 2022 Jun 18]. Available from: https://www.covidence.org
Lewallen S, Schmidt E, Jolley E, Lindfield R, Dean WH, Cook C, et al. Factors affecting cataract surgical coverage and outcomes: a retrospective cross-sectional study of eye health systems in sub-Saharan Africa. BMC Ophthalmol. 2015;15(1):1–8. https://doi.org/10.1186/s12886-015-0063-6.
Briggs J. Critical appraisal checklist for case reports - critical appraisal tools for use in JBI systematic reviews. Jbi. 2020;1–5. Available from: https://joannabriggs.org/critical_appraisal_tools
Gierisch JM, Myers ER, Schmit KM, McCrory DC, Coeytaux RR, Crowley M, Chatterjee R, Kendrick AS, Sanders GD. Distinguishing case series from cohort studies. Ann Intern Med. 2014;160(6):407–14.
CoreTeam R. R: A Language and Environment for Statistical Computing. 2017;2. Available from: https://www.r-project.org/
Viechtbauer W. Conducting meta-analyses in R with the metafor Package. J Stat Softw. 2010;36(3):1–48 (https://www.jstatsoft.org/index.php/jss/article/view/v036i03).
Yorston D, Wood M, Foster A. Results of cataract surgery in young children in East Africa. Br J Ophthalmol. 2001;85(3):267–71.
Bowman RJC, Kabiru J, Negretti G, Wood ML. Outcomes of bilateral cataract surgery in Tanzanian children. Ophthalmology. 2007;114(12):2287–92.
Onabolu O, Iwuora A. Experience with extra-capsular cataract extraction and intra-ocular lens implant in children. West Afr J Med. 2010;29(5):309–13. https://doi.org/10.4314/wajm.v29i5.68250.
Umar MM, Abubakar A, Achi I, Alhassan MB, Hassan A. Pediatric cataract surgery in National Eye Centre Kaduna, Nigeria: outcome and challenges. Middle East Afr J Ophthalmol. 2015;22(1):92–6.
Gogate P, Parbhoo D, Ramson P, Budhoo R, Øverland L, Mkhize N, et al. Surgery for sight: Outcomes of congenital and developmental cataracts operated in Durban. South Africa Eye. 2016;30(3):406–12.
Mboni C, Gogate PM, Phiri A, Seneadza A, Ramson P, Manolakos-Tsehisi H, et al. Outcomes of pediatric cataract surgery in the Copperbelt Province of Zambia. J Pediatr Ophthalmol Strabismus. 2016;53(5):311–7.
Asferaw M, Mekonen SY, Woodruff G, Gilbert CE, Tesfaye S. Outcome of paediatric cataract surgery in Northwest Ethiopia: a retrospective case series. Br J Ophthalmol. 2019;103(1):112–8.
Mndeme FG, Mmbaga BT, Msina M, Mwende J, Vaitha SJ, Kim MJ, et al. Presentation, surgery and 1-year outcomes of childhood cataract surgery in Tanzania. Br J Ophthalmol. 2021;105(3):334–40.
Sheeladevi S, Lawrenson JG, Fielder AR, Suttle CM. Global prevalence of childhood cataract: a systematic review. Eye (Lond). 2016;30(9):1160–9.
Duke RE, Adio A, Oparah SK, Odey F, Eyo OA. Evaluation of a public child eye health tertiary facility for pediatric cataract in Southern Nigeria I : visual acuity outcome. Open Ophthalmol J. 2016;119–25.
Ngoy JK, Stahnke T, Dinkulu S, Makwanga E, Moanda A, Ngweme G, et al. Bilateral paediatric cataract surgery-outcomes of 298 children from Kinshasa, the democratic republic of the Congo. Afr Health Sci. 2020;20(4):1817–27.
Mwende J, Bronsard A, Mosha M, Bowman R, Geneau R, Courtright P. Delay in presentation to hospital for surgery for congenital and developmental cataract in Tanzania. Br J Ophthalmol. 2005;89(11):1478–82.
Birch EE, Cheng C, Stager DR, Weakley DR, Stager DR. The critical period for surgical treatment of dense congenital bilateral cataracts. J AAPOS. 2009;13(1):67–71. https://doi.org/10.1016/j.jaapos.2008.07.010.
Birch EE, Stager D, Leffler J, Weakley D. Early treatment of congenital unilateral cataract minimizes unequal competition. Invest Ophthalmol Vis Sci. 1998;39(9):1560–6.
Olusanya BA, Ugalahi MO, Adeyemo AO, Baiyeroju AM. Age at detection and age at presentation of childhood cataract at a tertiary facility in Ibadan, Southwest Nigeria. BMC Ophthalmol. 2020;20(1):1–6.
Hwang SS, Kim WS, Lee SJ. Clinical features of strabismus and nystagmus in bilateral congenital cataracts. Int J Ophthalmol. 2018;11(5):813–7.
Chaudhary S, Lavaju P, Govinda Shrestha B, Shah S, Chaudhary SK. Factors affecting the visual outcome of pediatric cataract surgery: a hospital based prospective study in eastern Nepal. Nepal J Ophthalmol. 2017;9(18):143–8. Available from: https://pdfs.semanticscholar.org/63ef/c0102fec710fa0a2bcf6b254bd3c4c60d4ec.pdf.
Nihalani BR, Vander Veen DK. Benchmarks for outcome indicators in pediatric cataract surgery. Eye. 2017;31(3):417–21.
Peterseim MW, Wilson ME. Bilateral intraocular lens implantation in the pediatric population. Ophthalmology. 2000;107(7):1261–6.
Wilson ME, Elliott L, Johnson B, Peterseim MM, Rah S, Werner L, et al. AcrySof acrylic intraocular lens implantation in children: clinical indications of biocompatibility. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2001;5(6):377–80.
Struck MC. Long-term results of pediatric cataract surgery and primary intraocular lens implantation from 7 to 22 months of life. JAMA Ophthalmol. 2015;133(10):1180–3.
Cassidy L, Rahi J, Nischal K, Russell-Eggitt I, Taylor D. Outcome of lens aspiration and intraocular lens implantation in children aged 5 years and under. Br J Ophthalmol. 2001;85(5):540–2. Available from: https://pubmed.ncbi.nlm.nih.gov/11316711.
Levelt CN, Ḧubener M. Critical-period plasticity in the visual cortex. Annu Rev Neurosci. 2012;35:309–30.
Hensch TK, Quinlan EM. Critical periods in amblyopia. Vis Neurosci. 2018;35(May):E014.
Morishita H, Hensch TK. Critical period revisited: impact on vision. Curr Opin Neurobiol. 2008;18(1):101–7.
Ledoux DM, Trivedi RH, Wilson ME, Payne JF. Pediatric cataract extraction with intraocular lens implantation: visual acuity outcome when measured at age four years and older. J AAPOS. 2007;11(3):218–24.
Repka MX, Dean TW, Kraker RT, Li Z, Yen KG, De Alba Campomanes AG, et al. Visual acuity and ophthalmic outcomes 5 years after cataract surgery among children younger than 13 years. JAMA Ophthalmol. 2022;140(3):269–76.
Giles K, Christelle D, Yannick B, Fricke OH, Wiedemann P. Cataract surgery with intraocular lens implantation in children aged 5–15 in local anaesthesia: visual outcomes and complications. Pan Afr Med J. 2016;24:1–6.
Kishiki E, Shirima S, Lewallen S, Courtright P. Improving postoperative follow-up of children receiving surgery for congenital or developmental cataracts in Africa. J AAPOS. 2009;13(3):280–2. https://doi.org/10.1016/j.jaapos.2008.12.002.
Dawodu O. How to improve outcome of paediatric cataract surgery in Nigeria and other developing countries. Niger J Ophthalmol. 2011;19(1):1–4.
Khokhar SK, Pillay G, Dhull C, Agarwal E, Mahabir M, Aggarwal P. Pediatric cataract. Indian J Ophthalmol. 2017;65(12):1340–9.
Beck AD, Freedman SF, Lynn MJ, Bothun E, Neely DE, Lambert SR. Glaucoma-related adverse events in the Infant Aphakia Treatment Study: 1-year results. Arch Ophthalmol (Chicago, Ill 1960). 2012;130(3):300–5.
Freedman SF, Lynn MJ, Beck AD, Bothun ED, Örge FH, Lambert SR. Glaucoma-related adverse events in the first 5 years after unilateral cataract removal in the Infant Aphakia Treatment Study. JAMA Ophthalmol. 2015;133(8):907–14.
Zhang S, Wang J, Li Y, Liu Y, He L, Xia X. The role of primary intraocular lens implantation in the risk of secondary glaucoma following congenital cataract surgery: a systematic review and meta-analysis. PLoS ONE. 2019;14(4):e0214684.
Haargaard B, Andersen EW, Oudin A, Poulsen G, Wohlfahrt J, la Cour M, et al. Risk of retinal detachment after pediatric cataract surgery. Invest Ophthalmol Vis Sci. 2014;55(5):2947–51. https://doi.org/10.1167/iovs.14-13996.
Rahi JS, Dezateux C. National cross sectional study of detection of congenital and infantile cataract in the United Kingdom: role of childhood screening and surveillance. The British Congenital Cataract Interest Group. BMJ. 1999;318(7180):362–5.
Bronsard A, Geneau R, Shirima S, Courtright P, Mwende J. Why are children brought late for cataract surgery? Qualitative findings from Tanzania. Ophthalmic Epidemiol. 2008;15(6):383–8.
Vinluan ML, Olveda RM, Olveda DU, Chy D, Ross AG. Access to essential paediatric eye surgery in the developing world: a case of congenital cataracts left untreated. BMJ Case Rep. 2015;2015:10–3.
Dean WH, Buchan JC, Gichuhi S, Faal H, Mpyet C, Resnikoff S, et al. Ophthalmology training in sub-Saharan Africa: a scoping review. Eye. 2021;35(4):1066–83.
Lambert SR, Buckley EG, Plager DA, Medow NB, Wilson ME. Unilateral intraocular lens implantation during the first six months of life. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 1999;3(6):344–9.
Autrata R, Řehuřek J, Vodičková K. Visual results after primary intraocular lens implantation or contact lens correction for aphakia in the first year of age. Ophthalmol. 2005;219(2):72–9.
Lundvall A, Zetterström C. Primary intraocular lens implantation in infants: complications and visual results. J Cataract Refract Surg. 2006;32(10):1672–7. Available from: https://www.sciencedirect.com/science/article/pii/S0886335006008418.
Wilson ME, Pandey SK, Thakur J. Paediatric cataract blindness in the developing world: surgical techniques and intraocular lenses in the new millennium. Br J Ophthalmol. 2003;87(1):14–9.
Pavlovic S, Jacobi FK, Graef M, Jacobi KW. Silicone intraocular lens implantation in children: preliminary results. J Cataract Refract Surg. 2000;26(1):88–95. Available from: http://europepmc.org/abstract/MED/10646153.
Brady KM, Atkinson CS, Kilty LA, Hiles DA. Cataract surgery and intraocular lens implantation in children. Am J Ophthalmol. 1995;120(1):1–9. Available from: https://www.sciencedirect.com/science/article/pii/S0002939414737535.
Lu Y, Ji Y-H, Luo Y, Jiang Y-X, Wang M, Chen X. Visual results and complications of primary intraocular lens implantation in infants aged 6 to 12 months. Graefe’s Arch Clin Exp Ophthalmol. 2010;248(5):681–6. https://doi.org/10.1007/s00417-010-1310-4.
Vijayalakshmi P, Njambi L. Paediatric cataract: challenges and complications. Commun Eye Heal J. 2016;29(94):34–5.
Schulze Schwering M, Msukwa G, Spitzer MS, Kalua K. Pediatric cataract surgery in Malawi. Ophthalmologe. 2014;111(4):348–53.
Gupta PC, Ram J. Surgery for sight: Outcomes of congenital and developmental cataracts operated in Durban, South Africa. Vol. 30, Eye (Basingstoke). Nature Publishing Group; 2016. p. 1522–3.
Djiguimdé PW, Diomandé IA, Ahnoux-Zabsonré A, Koffi KV, Meda TA. Résultats de la chirurgie avancée de la cataracte par tunnélisation: à propos de 262 cas réalisés au CHR de Banfora (Burkina Faso). PanAfrican Med J. 2015;8688:1–9. Available from: http://www.panafrican-med-journal.com/content/article/22/366/full/.
Gradin D, Mundia D. Simultaneous bilateral cataract surgery with IOL implantation in children in Kenya. J Pediatr Ophthalmol Strabismus. 2012;49(3):139–44.
Tomkins O, Ben-Zion I, Moore DB, Helveston EE. Outcomes of pediatric cataract surgery at a tertiary care center in rural southern Ethiopia. Arch Ophthalmol. 2011;129(10):1293–7.
Olusanya BA, Baiyeroju AM, Fajola AO. Visual recovery after cataract surgery in children. Niger J Ophthalmol. 2008;14(2). Available from: https://www.ajol.info/index.php/njo/article/view/11983
Lam A, Seck C, Gueye NN, Faye M, Pintart D. Cataract surgery with posterior chamber lens implantation in Senegalese children less than 15 years old. Jounral Fr dophtalmologie. 2001;24(6):590–5.
Acknowledgements
This project was carried out in partial fulfilment of the degree of ChM in Clinical Ophthalmology at the University of Edinburgh.
Funding
No specific financial support was received for this project.
Author information
Authors and Affiliations
Contributions
Dr Mhango contributed to the review process and manuscript writing. Drs Zungu and Nkume contributed to the review process. Mr Musopole contributed to the statistical analysis of the data. Dr Mdala contributed to the manuscript writing and supervised the work on the project. All authors were involved in editing and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13643_2024_2607_MOESM1_ESM.docx
Additional file 1: Appendix 1. Search strategy. Appendix 2. PRISMA flow chart. Appendix 3. Summary of excluded studies [69,70,71,72,73,74]. Appendix 4. PRISMA checklists. Appendix 5. Post-operative complications. Appendix 6. Risk of bias assessment. Appendix 7. Summary of characteristics for comparative studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mhango, P.P., Zungu, T.L., Nkume, H.I. et al. The outcomes of paediatric cataract surgery with intraocular lens insertion in sub-Saharan Africa: a systematic review. Syst Rev 13, 204 (2024). https://doi.org/10.1186/s13643-024-02607-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13643-024-02607-z