Abstract
Background
After abandoning the zero-case strategy for COVID-19 in December 2022 in mainland China, the Hong Kong Special Administrative Region of China (HKSAR) has undergone an anticipated surge of the prevalence of COVID-19, as well as other influenzas, such as influenza A/H1N1, influenza A/H3N2, and influenza B as well. Noteworthy, people are usually concerned about the mutual influences between two families of respiratory viruses, like co-circulation or co-infection.
Methods
We fitted a unified model to weekly reported severe COVID-19 cases and the confirmed influenza A laboratory cases in HKSAR, respectively, using the R package POMP to obtain the best fitting and parameter estimates. The reconstructed transmission rates of the COVID-19 (/influenza A) versus the weekly reported influenza A (/COVID-19) confirmations between April 2022 and April 2024 were also compared.
Results
Our numerical results suggest that influenza confirmations remained either at a very low level or were absent before 2023, while starting from 2023, the influenza epidemic re-emerged as expected because of the resumption of international travels and other social communications. Besides, the peak of influenza cases in April 2023 favored the form of the peak of COVID-19 between May–June, 2023.
Additionally, during the sudden abolishment of the zero-case policy in mainland China (December 2022 to January 2023), we estimated that there were approximately 381 cases imported from mainland China into HKSAR.
Conclusions
We estimated the potential number of imported COVID-19 severe cases from mainland China to Hong Kong and revealed some potential population-level interference between the two families of respiratory viruses.
Similar content being viewed by others
1 Background
By the end of 2021, countries all over the world had gradually implemented policies to abandon strict COVID-19 control policies (e.g., the zero-case strategy), and no longer counting confirmed cases. Considering the huge socio-economic costs, high vaccination coverage, and the gradually decreased infection fatality rate, mainland China was the last region to abandon the zero-case policy after sticking to it for three years, in December 2022. The prevalence of cases has immediately surged after recovering the normal social distance. A team in the USA estimated that >90% of the population in mainland China had been infected in a month [1]. A study among self-quarantined bone marrow transplant patients reported an infection attack rate of ∼70% during the period [2]. Compared to the surge of Omicron in Hong Kong Special Administrative Region of China (HKSAR thereafter) in early 2022, it was estimated that 33.6% of the HKSAR population were infected in February–March 2022 [3]. The spreading speed of COVID-19 in mainland China was much faster than that in HKSAR and this might due to multiple reasons, among which the variant in mainland China had a substantial transmission advantage than the one in HKSAR.
Between December 8, 2022 and January 12, 2023, according to data released by the National Health Commission of China, there have been accumulated 59,938 COVID-19–related deaths (excess deaths), with 10% of people dying directly from the virus and 90% indirectly [4]. On the other hand, from 6 February 2023, full customs clearance between HKSAR and mainland China had resumed, including business, tourism and family visits. It was inevitable that the mainland’s policies and epidemic situation would certainly bring a direct and non-negligible impact on the situation in HKSAR.
Meanwhile, as expected, various types of influenza, including influenza A/H1N1, influenza A/H3N2, influenza B, have begun to strike. Generally speaking, influenza tends to be seasonal. It is relatively well-studied and well known that weather (climate) factors, school terms, population movement, and air-pollutants drove the seasonality of influenza cases. The understanding of the mechanisms behind the seasonality of influenza help inform public health policy, for instance timing of influenza vaccination and preparedness of hospital beds ahead of the influenza season. The COVID-19 (i.e., SARS-CoV-2) and influenza viruses share similarities in transmission modes and symptoms[5], often leading to concerns about simultaneous circulation and co-infection of influenza and COVID-19. Whether there will be population level of interference between the two families of respiratory viruses and whether the outbreak of one virus will support or suppress the outbreak of the other?
To assess the magnitude of the potential interference between the spread of SARS-CoV-2 and influenza, we constructed a group of models to simulate the weekly number of severe COVID-19 cases (including deaths) in HKSAR from April 2022 to April 2024. We estimated the transmission rate of SARS-CoV-2 and compared it to the weekly reported cases of influenza. Conversely, we also estimated the transmission rate of influenza A and compared it with the weekly reported severe cases of COVID-19. In this work, we aimed to model the spread of the seasonal flu and the COVID-19 in the HKSAR, China, and discuss the impacts of the sudden policy change on cross-border policies in mainland China. To our knowledge, this is the first work which addresses this issue in HKSAR in the post-pandemic era.
2 Methods
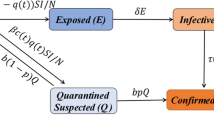
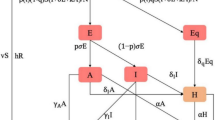
We modified the classical susceptible-exposed-infectious-recovered (SEIR) model [6] to simulate the transmission of the SARS-CoV-2 and influenza viruses, respectively. Noteworthy, various factors potentially impact their transmission, including mobility of the population, individual behavioral changes, weather conditions, evolution of viruses, and the implementation of government policies. These factors drive the transmission rate β(t) in our model. To account for these complexities, we incorporated time-varying transmission rates into our model in the form of an exponential cubic spline function of time. This approach allowed for sufficient flexibility by adjusting the number of nodes in the spline function. The greater the number of nodes, the more flexible the spline function was. We employed a partially observed Markov process [7] and an iterative filtering technique to fit the model to reported data and yield the estimate of parameters.
The public health policy in HKSAR is different from that in mainland China. HKSAR data (severe COVID-19 cases and influenza cases) reporting is considered to be relatively consistent, e.g., severe cases (including deaths) and influenza cases. Thus, HKSAR as a modern city provides a very good study object. We collected the number of COVID-19 confirmed cases in HKSAR from April 2022 to January 2023, and severe cases reported from April 2022 to April 2024 [8]. We calculated the severe cases ratio up to January 2023 and projected up to April 2024, due that the numbers of confirmed cases were not reported in HKSAR after January 2023, but severe cases reports were believed to be consistent. Meanwhile, as a comparison, we also collected influenza laboratory confirmations (severe influenza cases), including influenza A/H1N1, A/H3N2, and type B, during the same period [8].
By April, 2022, 92.1% of vaccine-eligible persons in Hong Kong had received ≥1 dose of a COVID-19 vaccine and nearly 84.7% persons had received ≥2 doses, while for the group aged 3 to 11 years old and aged 70 or above, the first dose rate was just about 60.6% and 71.9%, respectively [9]. Obviously, the daily vaccination rate has become relatively low in the post-pandemic era for both COVID-19 and influenza. For influenza, according to official statistics, about 20% [10] of the population annually were vaccinated and the effectiveness of the vaccine was about 50% generally. Thus, it was that 10% of the susceptible population were immunized. Vaccination slowed the transmission by reducing the size of the susceptible pool, presumably right before the start of the influenza season. Therefore, the annual vaccination rate of COVID-19 was relatively low, thus the effect could be ignored. Also, the effect of vaccination on both COVID-19 and influenza was synthesized in the change in transmission rate, although the mechanism of vaccination in the change of transmission rate was different. We set an initial population susceptibility at a level of 30% or 40%, considering the high vaccination coverage and a wave of Omicron in the first half of 2022 which likely infected 30–40% of the HKSAR population [11].
Our unified model reads as follows,
The population is divided into Susceptible, Exposed, Infectious, Hospitalized, Dead, and Recovered compartments, denoted as S, E, I, H, D and R, respectively. Note that only S, E, and I are involved in the transmission dynamics of the virus. The parameter β denotes transmission rate, σ denotes the infectiousness onset rate, γ denotes the infectiousness decay rate, ϕ denotes infection-hospitalization-ratio, θ denotes hospitalization-fatality-ratio, κ denotes the rate at which individuals leaving H class, and \(\kappa ^{-1}\) denotes the mean delay between loss of infectiousness and death. The mean delay from infection to death equals \(\sigma ^{-1} + \gamma ^{-1} + \kappa ^{-1}\). The infection-fatality-ratio equals the product ϕθ. In our data, we found that about half of the severe cases died. While the criterion for death is clear, the criterion for severe cases can vary widely. Given such a high severe-case-fatality ratio (∼50%), the criterion for case to be severe must be very high, thus the processes from infection to death and from infection to be severe, should be similar. Thus, we suspected that the mean duration from infection to be a severe case must be comparable to that from infection to death. Hence, for simplicity, we aggregated reported severe cases and deaths and matched the sum to weekly simulated D, i.e., we interpreted D as the total of severe cases and deaths. Considering the delay between infection and death was about two weeks and severe case report could be shorter, we considered two different set of \(\kappa ^{-1}\) at 7 days and 12 days, which reflected time delay between the infection and the report date of a severe case of COVID-19 and between the infection and the report of a death case, if we further add the infectious period. Although we could explicitly define a severe case and fit two time series simultaneously. We believed that the results must be similar since the curves of weekly severe cases and deaths were perfectly synchronized.
Similarly, for influenza A, we matched reported laboratory influenza confirmations to weekly simulated D. We presumed that the reporting delay between influenza infection and the confirmation data is short, so we used a very short \(\kappa ^{-1}\) of 2 days. Namely, H is interpreted as a delay case between the loss of infectiousness and being tested positive in laboratory.
To summarize the parameter setting, we list all parameters for COVID-19 and influenza in the following Table 1.
Here ϕθ means the infection to severe case ratio in both situations (influenza A and COVID-19). Compared to the unexpected onset of COVID-19, Hong Kong’s healthcare system has a relatively mature response system for seasonal influenza, including diagnosis, treatment, case statistics, and information disclosure. Therefore, the reporting rate of influenza cases could be higher than the reporting rate of severe COVID-19 cases (after vaccination). Consequently, we decided on reporting rates of 0.1 for COVID-19 and 0.6 for influenza. We fixed ϕ at 0.1 for COVID-19 and at 0.6 for influenza A, and we restricted ϕ in the range [0.05, 0.12] for COVID-19 and [0.1, 0.6] for influenza, the infection to severe case ratio is assumed to be in the range 0.5% and 1.2% for COVID-19 and in the range 6% and 36% for influenza A. These ranges are largely biologically reasonable. The results are insensitive to the choice of ϕ, because the H, D, and R class do not involve in the dynamics of the transmission.
We modeled the transmission rate β with an exponential-cubic-spline function of time and tested a range of nodes \((n_{\beta} ) \) in the spline (from 7 nodes to 12 nodes for COVID-19 and from 7 nodes to 14 nodes for influenza). More nodes lead to better fitting, but not necessarily an improved information criterion (e.g., the second-order Akaike Information Criterion, i.e., AICc). We used the information criterion to select the optimal number of nodes used in the β.
3 Results
We found that the influenza confirmations remained at a very low level or were absent before 2023, due to both control measures against COVID-19 and possible population interference between the two families of viruses (Fig. 1), as expected. Namely, the prevention measures taken by the government and individuals, such as, cross-border screening and quarantine of passengers, social distancing, mask wearing, etc., should have suppressed the spread of respiratory viruses. However, these preventive measures were gradually released due to increased vaccination coverage and population immunity. And these measures also became less effective due to the increased transmissibility of new variants of SARS-CoV-2. Since simultaneous infections with the two families of viruses (influenza and SARS-CoV-2) are rarely reported, it is reasonable to assume infection with one virus temporarily prevented infection with the other virus. An increase in influenza vaccination rates should suppress the spread of the virus [12], presumably to a lesser extent due to low coverage in HKSAR. Starting from 2023, the influenza epidemic re-emerged as expected, due to resumption of international travels, etc. (Fig. 1). We fitted our model to the weekly severe COVID-19 cases and reconstructed the transmission rate, \(\beta _{1}\)(t). We then compared the \(\beta _{1}\)(t) to reported influenza laboratory confirmations (Fig. 2). Conversely, we fitted the model (with parameters of flu) to influenza A laboratory confirmation(A/H1N1+A/H3N2) and obtained the transmission rate, \(\beta _{2}\)(t) of flu A, and compared it to reported severe COVID-19 cases (Fig. 3). We found slight evidence of potential population level interference, i.e., a high prevalence of one family of virus suppressed the spread of the other, which is more evident for the impact of COVID-19 on influenza. To our surprise, the peak of influenza cases in April 2023 favored the form of the peak of the COVID-19 in May–June 2023. This is consistent with previous findings in the literature. Some studies have shown that not only was there no cross-immunity between COVID-19 and influenza, but that cases already infected with COVID-19 are more likely to come in with influenza [13]. It is not ruled out that the coronavirus may cause damage to the human immune system, and the immune system cannot recover immediately even after recovery. Or if people are exposed to the flu virus, they could also be exposed to the new coronavirus.
The cases including influenza and COVID-19 severe cases (including deaths) in HKSAR China. Laboratory confirmations of influenza A/H1N1, A/ H3N2, B and the sum of the three types/subtypes; And the COVID-19 severe cases (including deaths) from April 2022 to January 2024. It can be seen that there is a surge of severe COVID-19 cases at the end of 2022 and a surge of influenza confirmations in April 2023. The influenza confirmations were low before 2023
Fit of the unified model to influenza A confirmations (left) and comparison with severe COVID-19 cases (right). The estimated high transmission rate for influenza largely overlapped with low severe COVID-19 cases. Parameter values are given Table 1
The estimated high transmission rate overlapped with the high influenza confirmations largely. High rates of influenza infection could lower the population immunity against the severe COVID-19 cases. Although double infections were rarely reported, we cannot rule out sequential infections that the COVID-19 infection in the convalescent period of influenza infection. \(\kappa =1/12 \) per day and \(S ( t =0)=0.3\). Other parameter values are given in Table 1.
We suspect that the low infections of COVID-19 increased population interaction, thus favoring the transmission of influenza. Also, this could be due to the arrival of new influenza strain with a slightly higher transmission rate after lifting of cross-border restrictions.
4 Discussion
We used the R package POMP to obtain the best fit and parameter estimates. The median simulation results of our model fit well with the number of severe COVID-19 cases reported in HKSAR (Fig. 2). Besides, in the month of the sudden abolish of zero-case policy in mainland China (from December 2022 to January 2023), the number of reported cases was significantly higher than our fitting results, which led to the conjecture that these extra cases were imported from mainland China into HKSAR. Namely, we hypothesized that this discrepancy, approximately 381 cases, was due to case importation from the mainland to HKSAR. We observed a surge in the number of severe and fatal COVID-19 cases starting in November 2022. This outbreak has left more than 4,000 people seriously ill or dead in HKSAR and the severe-fatality-ratio doubled from 0.3% before the policy change to a level of 0.75%. It then sharply declined until the end of January 2023, essentially returning to the dynamic equilibrium state before the sudden policy change. This effect lasted approximately 2.5 months.
Conversely, we applied the same approach to influenza and compared the estimate \(\beta _{2}\)(t) versus severe COVID-19 cases. We found that behind each reported flu case, there were about 16 influenza infections. It is well known that laboratory confirmation of flu is only a small proportion of the true number of influenza infections. For instance, a serological study by Steve et al. [14] concluded the Infection Attack Rate (proportion of population infected) was about 16% (13%–18%) of people overall were infected by the pandemic strain up to the end of January 2010 in HKSAR, and compared with the reported laboratory confirmations of influenza during the same period, one yielded a case to infection ratio at about 1 to 16.
Our present study may have several limitations, as sketched below. Firstly, our model only simulated the spread of one single virus, then fitted the real data and conducted the associated comparison. Although our model allowed for some time-varying transmission rates, the interferences between COVID-19 and influenza are still rather involved. Thereby, it invokes a more accurate two-virus models to admit a more explicit representation and fit the effects of population-level interference between the two families of virus. It is meaningful to fitting a large coupled two-virus model to fit the two families of viruses: COVID-19 and influenza. Nevertheless, our current work has laid the foundation for this task, which we plan to pursue in a follow-up study. Secondly, the interaction between viruses is not only affected by external physical conditions, but also the internal biochemical reactions intrinsic in one body caused by the two viruses. There remain some questions to be answered. For example, how these two viruses work in the host cell, and are they in harmony, or competitive, or even mutually exclusive; is there cross-immunity? However, it is difficult to get satisfying or affirmative answers to these questions only through mathematical modeling; instead, more convincing evidence by laboratory data are required to have these questions been well explored. Additionally, it is possible to include temperature and population movement, cross-border policy changes into the transmission rate to make it mechanized. However, previous works (e.g., Unwin et al., 2020) show that these are insufficient, and additional factors (e.g., spline function or auto-regressive process) are needed [15].
5 Conclusion
We employed a disease transmission model and a likelihood-based inference approach [16] to assess the interference between seasonal influenza and COVID-19 and the impact of mainland China’s sudden policy change on the disease dynamics in HKSAR. We estimated that in the post-pandemic, the seasonal influenza case to infection ratio was around 16%, the peak of influenza cases in early 2023 overlapped a high transmission rate of COVID-19. We estimated that approximately 381 severe and fatal cases were imported from the mainland following the sudden phase-out of zero-case policy. During the peak of COVID-19 at the end of 2023, the case-severe-case-ratio was doubled from less than 0.3% to 0.75%. In summary, we argued that through studying the relatively consistent data in HKSAR with our modelling framework, one can estimate the impact of policy change in mainland China onto HKSAR due to the close connection between two regions. The interference between COVID-19 and influenza is complex, and the peak of one virus (influenza) could help the spread of the other (SARS-CoV-2) by lowering population immunity of vulnerable population (elderly and infant). In future work, we plan to develop a two-virus models to explicitly model the effects of population level interference between the two families of virus.
Availability of data and materials
All data are publicly available in the referenced sources.
Abbreviations
- HKSAR:
-
Hong Kong Special Administrative Region of China
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- SEIR:
-
susceptible-exposed-infectious-recovered
- A/H1N1:
-
influenza A virus subtype H1N1
- A/H3N2:
-
Influenza A virus subtype H3N2
- S, E, I, H, D and R:
-
Susceptible, Exposed, Infectious, Hospitalized, Dead and Recovered compartments, respectively.
- AICc:
-
the second-order Akaike information criterion
References
Goldberg, E.E., et al.: Swift and extensive Omicron outbreak in China after sudden exit from ‘zero-COVID’policy. Nat. Commun. 14(1), 3888 (2023)
Zhang, Z., et al.: Influence of the Omicron outbreak on allo-HSCT recipients in China: a single-center short-term observational cohort study. Bone Marrow Transplant. 59, 1–3 (2024)
Zhao, J., et al.: Serum banks for serological survey of SARS-CoV-2 and emerging infectious diseases (2024)
Transcript of press conference under the Joint Prevention and Control Mechanism of the State Council on February 23, 2023. In: transcript of press conference under the Joint Prevention and Control Mechanism of the State Council, Press Conference Hall, Building 2, National Health Commission (2023)
Khorramdelazad, H., et al.: Immunopathological similarities between COVID-19 and influenza: investigating the consequences of co-infection. Microb. Pathog. 152, 104554 (2021)
He, D., et al.: Evaluation of effectiveness of global COVID-19 vaccination campaign. Emerg. Infect. Dis. 28(9), 1873 (2022)
Ionides, E.L., Bretó, C., King, A.A.: Inference for nonlinear dynamical systems. Proc. Natl. Acad. Sci. 103(49), 18438–18443 (2006)
Centre for Health Protection, D.o.H., the Government of the Hong Kong Special Administrative Region: COVID-19 and Flu Express (2024). Available from https://www.chp.gov.hk/en/resources/submenu/100148/index.html (cited 2024 20 March)
Authority, C.f.H.P.o.t.D.o.H.a.t.H.: Statistics on 5th Wave og COVID-19 2022. Available from https://www.coronavirus.gov.hk/pdf/5th_wave_statistics/5th_wave_statistics_20220401.pdf (cited 2024 20 March)
Centre for Health Protection, D.o.H., the Government of the Hong Kong Special Administrative Region: Statistics on Vaccination Programmes in the Past 3 years. Available from https://www.chp.gov.hk/en/features/102226.html
Chong, K.C., et al.: Changes in all-cause and cause-specific excess mortality before and after the Omicron outbreak of COVID-19 in Hong Kong. BMJ Glob. Health 13, 06017 (2023)
Jiang, X., et al.: Impact of the surge of COVID-19 Omicron outbreak on the intention of seasonal influenza vaccination in Hong Kong: a cross-sectional study. Vaccine 41(49), 7419–7427 (2023)
Oishi, K., et al.: The host response to influenza A virus interferes with SARS-CoV-2 replication during coinfection. J. Virol. 96(15), e00765-22 (2022)
Riley, S., et al.: Epidemiological characteristics of 2009 (H1N1) pandemic influenza based on paired sera from a longitudinal community cohort study. PLoS Med. 8(6), e1000442 (2011)
Unwin, H.J.T., et al.: State-level tracking of COVID-19 in the United States. Nat. Commun. 11(1), 6189 (2020)
He, D., et al.: Evaluation of effectiveness of global COVID-19 vaccination campaign. Emerg. Infect. Dis. 28(9), 1873–1876 (2022)
Acknowledgements
Not applicable.
Funding
The work was partially supported by grant CRF C5079-21G from the Collaborative Research Fund of the Hong Kong Special Administrative Region, China, the National Science Foundation of China (12250410241), the National Natural Science Foundation of China (82320108018) and National Key R&D Program of China (2023YFC2306004 and 2022YFC2304000).
Author information
Authors and Affiliations
Contributions
LW, YY, ZP, QL and DH conceived the study, LW, YY, QL and DH carried out the analysis, LW and DH wrote the draft, all authors revised the manuscript critically, and approved it for publishing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors report no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Wen, L., Yin, Y., Li, Q. et al. Modeling the co-circulation of influenza and COVID-19 in Hong Kong, China. Adv Cont Discr Mod 2024, 32 (2024). https://doi.org/10.1186/s13662-024-03830-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13662-024-03830-7