Abstract
Introduction
Many single-center studies and meta-analyses demonstrate that therapeutic hypothermia (TH), in which the body temperature is maintained at 32–35°C, exerts significant neuroprotection and attenuates secondary intracranial hypertension after traumatic brain injury (TBI). In 2015, two well-designed multi-center, randomized controlled trials were published that did not show favorable outcomes with the use of TH in adult patients with TBI compared to normothermia treatment (NT). Therefore, we performed an updated meta-analysis to assess the effect of TH in adult patients with TBI.
Methods
We reviewed the PubMed, EMbase, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure, and Wanfang Databases. We included randomized controlled trials that compared TH and NT in adult patients with TBI. Two reviewers assessed the quality of each study and independently collected the data. We performed the meta-analysis using the Cochrane Collaboration’s RevMan 5.3 software.
Results
We included 18 trials involving 2177 patients with TBI. There was no significant heterogeneity among the studies. TH could not decrease mortality at 3 months post-TBI (RR 0.95; 95 % CI 0.59, 1.55; z = 0.19, P = 0.85) or 6 months post-TBI (RR 0.96; 95 % CI 0.76, 1.23; z = 0.29, P = 0.77). There were no significant differences in unfavorable clinical outcomes when TH was compared to NT at 3 months post-TBI (RR 0.79; 95 % CI 0.56, 1.12; z = 1.31, P = 0.19) or 6 months post-TBI (RR 0.80; 95 % CI 0.63, 1.00; z = 1.92, P = 0.05). TH was associated with a significant increase in pneumonia (RR 1.51; 95 % CI 1.12, 2.03; z = 2.72, P = 0.006) and cardiovascular complications (RR 1.75; 95% CI 1.14, 2.70; z = 2.54, P = 0.01).
Conclusions
Therapeutic hypothermia failed to demonstrate a decrease in mortality and unfavorable clinical outcomes at 3 or 6 months post-TBI. Additionally, TH might increase the risk of developing pneumonia and cardiovascular complications.
Similar content being viewed by others
Background
Traumatic brain injury (TBI) is a major cause of death and disability in the younger population and is a great economic and social burden in modern society. Recent studies showed a 21 % increase in the incidence of TBI during the past five years (Andrews et al. 2015). However, effective strategies are few for early care of this disease. Secondary elevations in intracranial pressure (ICP) are frequent in patients with severe TBI and can cause poor outcomes. Thus, the Brain Trauma Foundation (BTF) guidelines from 2007 suggest maintaining an ICP below 20–25 mmHg (Brain Trauma Foundation et al. 2007).
Therapeutic hypothermia (TH), also termed target temperature management (TTM), is the controlled lowering of core body temperature to below 36 °C and is currently recommended by many guidelines for hypoxic ischemic encephalopathy and cardiac arrest (Michael 2013; Crossley et al. 2014). Many animal and single-center studies have demonstrated that therapeutic hypothermia, in which the body temperature is maintained at 32–35°C, exerts significant neuroprotection and attenuates secondary intracranial hypertension after TBI (Soukup et al. 2002; Oddo et al. 2009; Colbourne et al. 2003; Dietrich and Bramlett 2010; Truettner et al. 2011). The effects of TH may include a reduction in cerebral metabolic rate of oxygen (Soukup et al. 2002) and cerebral glucose demand (Soukup et al. 2002; Colbourne et al. 2003), a reduction in calcium influx into the brain cells and the release of excitotoxic amino acids (Dietrich and Bramlett 2010), and the inhibition of early molecular cascades and the stress response, thus preventing apoptosis (Truettner et al. 2011).
Two recent meta-analyses published in 2014 (Crossley et al. 2014; Li and Yang 2014) showed that TH might be effective in reducing death and unfavorable clinical outcomes. However, there were also many controversies. Conflicting results and several negative randomized controlled trials (Shiozaki et al. 1993; Clifton et al. 1993; Marion et al. 1997; Jiang et al. 2000; Clifton et al. 2001; Shiozaki et al. 2001; Yan and Tang 2001; Clifton et al. 2011) have occurred. Moreover, concerns about the potential increased risk of pneumonia following the induction of TH are evident (Sydenham et al. 2009; Woo et al. 2014).
In 2015, two well designed multi-center, randomized controlled trials were published (the Brain-Hypothermia Study, BHYPO trial (Maekawa et al. 2015); the European Study of Therapeutic Hypothermia for Intracranial Pressure Reduction after Traumatic Brain Injury, the Eurotherm3235Trial (Andrews et al. 2015)) that did not show favorable outcomes with the use of TH in patients with TBI.
In addition, a recent prospective study (Mtaweh et al. 2014) indicated that the energy metabolism rate of children is lower than that of adults, which might make the feasibility and efficacy of TH different for adult patients. Therefore, in the present meta-analysis, we aimed to reassess the effect of TH on mortality, unfavorable clinical outcomes (defined as death, a persistent vegetative state, or severe disability) and complications in adult patients with TBI compared to normothermia treatment (NT).
Methods
Data sources and search strategy
We reviewed studies published in the Pubmed, EMbase, Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure and the Wanfang databases. To avoid missing trials, we also searched the references from relevant articles. The keywords and MeSH and Emtree terms used in different combinations for the searches, with limitations set to randomized controlled trials, were “hypothermia”, “target temperature management”, “moderate hypothermia”, “moderate temperature”, “adult”, “traumatic brain injur*”, “head injur*”, “brain injuries”[MeSH]; “traumatic brain injury”[Emtree]; “moderate hypothermia, induced”[MeSH]; and “hypothermia”[MeSH/Emtree]. No limits for language, sample size, gender or the location of the original study were entered for the search.
Study selection
We determined the publications that were suitable for the meta-analysis using selection criteria as follows: (1) Randomized controlled trial (RCT); (2) Population: hospitalized adult patients with TBI (as in a previous study (Crossley et al. 2014), we defined adult as being the legal age for consent in the country where the trial was conducted); (3) Comparison between therapeutic hypothermia (32–35°C) and normothermia; and (4) Evaluation of mortality or unfavorable clinical outcomes at 3 or 6 months post-TBI. Unfavorable clinical outcomes included death, persistent vegetative state or severe disability that was classified by the Glasgow Outcome Scale. Additionally, variables were compared as follows: incidence of new pneumonia, cardiovascular complications and bleeding complications. All analyses were based on previously published studies; thus, ethical approval and patient consent were not required.
Data extraction and quality assessment
Two independent reviewers (Rui Zhang and Haiyan Yin) screened the titles and abstracts using a structured data abstraction form, which resulted in high and satisfactory inter-observer agreement. Any disagreement was resolved by consensus or by consulting a third author (Jianrui Wei). We extracted the authors’ names, title of the article, journal in which the study was published, country and year of the study, methodological variables and clinical outcomes. The modified Jadad score was used to evaluate the quality of the included trials. Two independent reviewers (Youfeng Zhu and Xiaoling Ye) assessed the bias of the included studies according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Marion et al. 1993). The parameters were assessed as follows: random sequence generation, blinding of participants and personnel, allocation concealment, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. According to the Cochrane Handbook, other sources of bias were related to the specific trial design used or the early termination of the study due to an extreme baseline imbalance in the selected patients. Because of the nature of these trials, the blind method could not be implemented.
Statistical analysis
The Cochrane Collaboration’s Review Manager Software 5.3 (RevMan 5.3) was used for the meta-analysis. The results were obtained by direct extraction or by indirect calculation. The risk ratios (RR) and 95 % confidence intervals (CI) were calculated for the binary data, and the standardized mean differences (SMD) and 95% CI were calculated for the continuous data variables. Heterogeneity between trials was tested using the Chi square test, with P < 0.05 and I 2 greater than 50 % indicating significant heterogeneity (Mtaweh et al. 2014). The random effects model was used to calculate the outcomes of both the binary and continuous variables, regardless of statistical heterogeneity. Forest plots were used to graphically display the results. A funnel plot was used to uncover potential publication bias.
Results
Figure 1 shows the selection process for the eligible trials. Initially, 3345 records were identified. After removing duplicate records, animal studies, case reports, review articles, comments, or studies that were not randomized controlled trials, 22 studies remained for assessment. Three studies were preliminary reports of subsequent studies (Liu et al. 2006; Flynn et al. 2015; Marion et al. 1993) and were excluded to avoid duplication. One study did not report the length of the follow-up period and incidence of complications, and was excluded (Yan and Tang 2001). Finally, 18 studies were included in the present meta-analysis (Andrews et al. 2015; Shiozaki et al. 1993; Clifton et al. 1993; Marion et al. 1997; Jiang et al. 2000; Clifton et al. 2001; Shiozaki et al. 2001; Clifton et al. 2011; Maekawa et al. 2015; Gal et al. 2002; Zhi et al. 2003; Qiu et al. 2005, 2006, 2007; Hashiguchi et al. 2003; Lee et al. 2010; Shiozaki et al. 1999; Zhao et al. 2011). The qualities of the included RCTs are shown in Table 1.
A total of 2177 patients with TBIs were included in the present meta-analysis. Of these cases, 1122 patients were randomly assigned to a TH group, and 1055 patients were assigned to an NT group. The characteristics and basic demographic parameters of all patients are shown in Table 2.
Risk of bias in the included studies We used a tool from the Cochrane Collaboration to assess the risk of bias for each study and presented the details of the results in Fig. 2.
Effects of mortality
All but one of the included studies (Gal et al. 2002) reported the mortality at the end of the follow-up period. Due to variations in trial protocol, the length of the long-term follow-up period usually varied between 3 and 6 months. Of these included studies, 4 studies reported mortality at 3 months after TBI, 10 studies reported mortality at 6 months after TBI, 3 studies reported mortality at 1 year after TBI, and 1 study reported mortality at 2 years after TBI; the length of the follow-up period was unclear in 1 studies (Lee et al. 2010). We analysed mortality at 3 and 6 months post-TBI.
Mortality at 3 months post-TBI
For the analysis of mortality at 3 months post-TBI, 4 trials involving 300 patients were included. When the results of the 4 studies were statistically aggregated, no significant heterogeneity was observed (Chi2 = 1.98, df = 3, P = 0.58; I 2 = 0 %) among the studies and therapeutic hypothermia was not associated with a significant reduction in mortality (RR 0.95; 95 % CI 0.59, 1.55; z = 0.19, P = 0.85, Fig. 3).
Mortality at 6 months post-TBI
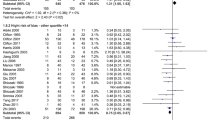
For the analysis of mortality at 6 months post-TBI, 10 trials involving 1621 patients were included. When the results of the 10 studies were statistically aggregated, no significant heterogeneity was observed (Chi2 = 15.52, df = 8, P = 0.05; I 2 = 48 %) among the studies and therapeutic hypothermia was not associated with a significant reduction in mortality (RR 0.96; 95 % CI 0.76, 1.23; z = 0.29, P = 0.77, Fig. 4).
Mortality in trials with a lower risk of bias
For mortality at the final follow-up in trials assessed as having a lower risk of bias (modified Jadad score >3), 5 trials involving 781 patients were included in this sub-analysis. When the results of the 5 studies were statistically aggregated, no significant heterogeneity was observed (Chi2 = 3.97, df = 4, P = 0.41; I 2 = 0 %) among the studies and therapeutic hypothermia was not associated with a significant reduction in mortality (RR 1.22; 95 % CI 0.97, 1.54; z = 1.69, P = 0.09, Fig. 5).
Effects of unfavorable clinical outcomes
All of the included studies reported unfavorable clinical outcomes at the end of the follow-up period. Due to variations in trial protocol, the length of the long-term follow-up period usually varied between 3 and 6 months. Of these included studies, 4 studies reported unfavorable clinical outcomes at 3 months after TBI, 11 studies reported unfavorable clinical outcomes at 6 months after TBI, 2 studies reported unfavorable clinical outcomes at 1 year after TBI, and 1 study reported unfavorable clinical outcomes at 2 years after TBI; the length of the follow-up period was unclear in 1 study (Lee et al. 2010). We analysed unfavorable clinical outcomes at 3 and 6 months post-TBI.
Unfavorable clinical outcomes at 3 months post-TBI
For the analysis of unfavorable clinical outcomes at 3 months after TBI, 4 trials involving 300 patients were included. When the results of the 4 studies were statistically aggregated, significant heterogeneity was observed (Chi2 = 7.09, df = 3, P = 0.07; I 2 = 58 %) among the studies and no significant difference between the TH and NT groups was observed (RR 0.79; 95 % CI 0.56, 1.12; z = 1.31, P = 0.19, Fig. 6).
Unfavorable clinical outcomes at 6 months post-TBI
For the analysis of unfavorable clinical outcomes at 6 months post-TBI, 11 trials involving 1651 patients were included. When the results of the 11 studies were statistically aggregated, significant heterogeneity was observed (Chi2 = 44.59, df = 10, P < 0.001; I 2 = 78 %) among the studies and no significant difference between the TH and NT groups was observed (RR 0.80; 95 % CI 0.63, 1.00; z = 1.92, P = 0.05, Fig. 7).
Unfavorable clinical outcomes in trials with a lower risk of bias
For the analysis of unfavorable clinical outcomes at the final follow-up in trials assessed as a lower risk of bias (modified Jadad score >3), 5 trials involving 781 patients were included in this sub-analysis. When the results of the 5 studies were statistically aggregated, significant heterogeneity was observed (Chi2 = 16.78, df = 4, P = 0.002; I 2 = 76 %) among the studies and no significant difference was observed between the TH and NT groups (RR 0.84; 95 % CI 0.62, 1.15; z = 1.07, P = 0.29, Fig. 8).
Pneumonia complications
A total of 13 RCTs were included involving 844 patients who reported pneumonia complications. Significant heterogeneity was observed (Chi2 = 26.67, df = 12, P = 0.009; I 2 = 55 %) among the 13 trials. In the random effects model, the TH group was associated with a higher risk of developing pneumonia than the NT group (RR 1.51; 95 % CI 1.12, 2.03; z = 2.72, P = 0.006, Fig. 9).
Cardiovascular complications
A total of 11 included studies involving 1346 patients reported cardiovascular complications. No significant heterogeneity was observed (Chi2 = 10.96, df = 10, P = 0.36; I 2 = 9 %) among the 11 trials. In the random effects model, the TH group was associated with a higher risk of developing cardiovascular complications than the NT group (RR 1.75; 95 % CI 1.14, 2.70; z = 2.54, P = 0.01, Fig. 10).
Bleeding complications
A total of 3 RCTs were included involving 522 patients who reported bleeding complications. No significant heterogeneity was observed (Chi2 = 1.95, df = 2, P = 0.38; I 2 = 0 %) among the 3 trials. In the random effects model, no significant difference in bleeding complications between the TH and NT groups was observed (RR 1.28; 95 % CI 0.40, 4.15; z = 0.42, P = 0.68, Fig. 11).
No publication bias was observed based on a visual inspection of the funnel plot (Fig. 12).
Discussion
Many previous studies and meta-analyses (Crossley et al. 2014; Li and Yang 2014) have assessed the effect of TH compared to NT in TBI patients, and there were contradictory results. This meta-analysis involved 18 studies including 2177 adult patients with TBI (1122 in the TH group and 1055 in the NT group) to further investigate the effectiveness of TH for the treatment of TBI.
The two meta-analyses published in 2014 (Crossley et al. 2014; Li and Yang 2014) showed that TH might be effective in the treatment of TBI, could decrease mortality and could be associated with a reduction in unfavorable clinical outcomes compared to NT. No significant increases were observed in the development of pneumonia complications in TH compared to NT (Crossley et al. 2014). The results of present study were different from previous studies (Crossley et al. 2014; Li and Yang 2014).
The present meta-analysis indicated that TH could not decrease the mortality at 3 months post-TBI (RR 0.95; 95 % CI 0.59, 1.55; z = 0.19, P = 0.85) or the mortality at 6 months post-TBI (RR 0.96; 95 % CI 0.76, 1.23; z = 0.29, P = 0.77) in adult patients with TBI. Additionally, There were no significant differences in unfavorable clinical outcomes at 3 months post-TBI (RR 0.79; 95 % CI 0.56, 1.12; z = 1.31, P = 0.19) or 6 months post-TBI (RR 0.80; 95 % CI 0.63, 1.00; z = 1.92, P = 0.05) when TH was compared to NT. Furthermore, TH was associated with a significant increase in pneumonia complications (RR 1.51; 95 % CI 1.12, 2.03; z = 2.72, P = 0.006) and cardiovascular complications (RR 1.75; 95 % CI 1.14, 2.70; z = 2.54, P = 0.01). The findings suggesting possible harm of hypothermia could be due to a biologic effect of hypothermia or due to the harms or benefits of the other therapies used differentially in the two groups (Andrews et al. 2015). The results of the present study might lead to further understanding of TH in adult patients with TBI and should be interpreted with great caution.
Furthermore, there are still many debates regarding the recent two RCTs. The BHYPO trial was stopped early before the scheduled sample size (300 cases) was reached because of a concern about a shortage of TBI patients (95 cases). The actual sample size was far below the intended target, which might produce bias. For the Eurotherm3235 trial, there were many more debates. Kiwon Lee considered that hypothermia might be helpful only in those patients with truly severe TBI (Lee 2015). Patients in the Eurotherm3235 trial, including all TBI patients with ICP greater than 20 mmHg for 5 min after stage 1, might not be the right population to support the value of hypothermia. As a matter of fact, some controversial therapies may be effective only in more critically ill patients. Therefore, it was not surprising to observe that some patients did well no matter what therapy was used. The Eurotherm3235 trial did not compare the combination of TH and standard therapy to standard therapy alone. In the TH group, mannitol and hypertonic saline were not given unless hypothermia failed to control ICP, which differed from the practice of many other medical centers where TH was used synchronously with standard therapy. In the Eurotherm3235 trial, hypothermia alone was compared to the combination of mannitol and hypertonic saline. Additionally, many independent variables that might affect the long-term clinical outcomes, such as nutrition and advanced rehabilitation capabilities, that might affect the outcome rather significantly. Control of these factors was difficult after patients were discharged from the hospital. Additionally, more patients in the NT group of the Eurotherm3235 trial, though there was no statistical difference, had decompressive craniectomies, which might decrease intracranial hypertension more effectively and influence the outcomes between the TH and NT groups.
Several limitations were present in our meta-analysis. First, the majority of involved RCTs were single-center studies that were assessed to have a high risk of bias, which might confound the effects of TH. Additional high quality and better-designed multi-center studies are needed in the future. Second, the starting time and duration of TH and the protocol of rewarming were different among the involved studies, which increased the risk of bias. Third, significant heterogeneities were detected among the studies involved in the present meta-analysis when we analysed unfavorable clinical outcomes at 3 and 6 months post-TBI, which might confound the results, as heterogeneity was one of the major concerns in the meta-analysis for validity.
Conclusions
Our meta-analysis demonstrated that therapeutic hypothermia failed to decrease mortality and unfavorable clinical outcomes at 3 months post-TBI or 6 months post-TBI, and might increase the risk of developing pneumonia and cardiovascular complications.
Abbreviations
- TH:
-
therapeutic hypothermia
- NT:
-
normothermia treatment
- RCT:
-
randomized controlled trial
- TBI:
-
traumatic brain injury
- TTM:
-
target temperature management
- BTF:
-
the Brain Trauma Foundation
References
Andrews PJ, Sinclair HL, Rodriguez A, Harris BA, Battison CG, Rhodes JK (2015) Hypothermia for intracranial hypertension after traumatic brain injury. N Engl J Med 373:2403–2412
Brain Trauma Foundation, American Association of Neurological S, Congress of Neurological S (2007) Guidelines for the management of severe traumatic brain injury. J Neurotrauma 24:S1–S106
Clifton GL, Allen S, Barrodale P, Plenger P, Berry J, Koch S (1993) A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma 10:263–271
Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR (2001) Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med 344:556–563
Clifton GL, Valadka A, Zygun D, Coffey CS, Drever P, Fourwinds S (2011) Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol 10:131–139
Colbourne F, Grooms SY, Zukin RS, Buchan AM, Bennett MV (2003) Hypothermia rescues hippocampal CA1 neurons and attenuates down-regulation of the AMPA receptor GluR2 subunit after forebrain ischemia. Proc Natl Acad Sci USA 100:2906–2910
Crossley S, Reid J, McLatchie R, Hayton J, Clark C, MacDougall M (2014) A systematic review of therapeutic hypothermia for adult patients following traumatic brain injury. Crit Care 18:R75
Dietrich WD, Bramlett HM (2010) The evidence for hypothermia as a neuroprotectant in traumatic brain injury. Neurotherapeutics 7:43–50
Flynn LM, Rhodes J, Andrews PJ (2015) Therapeutic hypothermia reduces intracranial pressure and partial brain oxygen tension in patients with severe traumatic brain injury: preliminary data from the Eurotherm3235 trial. Ther Hypothermia Temp Manag 5:143–151
Gal R, Cundrle I, Zimova I, Smrcka M (2002) Mild hypothermia therapy for patients with severe brain injury. Clin Neurol Neurosurg 104:318–321
Hashiguchi N, Shiozaki T, Ogura H, Tanaka H, Koh T, Noborio M (2003) Mild hypothermia reduces expression of heat shock protein 60 in leukocytes from severely head-injured patients. J Trauma 55:1054–1060
Jiang J, Yu M, Zhu C (2000) Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg 93:546–549
Lee Kiwon (2015) Therapeutic hypothermia for elevated intracranial pressure in traumatic brain injury: does it do more harm than good? Korean J Anesthesiol 68:523–524
Lee H-C, Chuang H-C, Cho D-Y, Cheng K-F, Lin P-H, Chen C-C (2010) Applying cerebral hypothermia and brain oxygen monitoring in treating severe traumatic brain injury. World Neurosurgery 74:654–660
Li P, Yang C (2014) Moderate hypothermia treatment in adult patients with severe traumatic brain injury: a meta-analysis. Brain Inj 28:1036–1041
Liu WG, Qiu WS, Zhang Y, Wang WM, Lu F, Yang XF (2006) Effects of selective brain cooling in patients with severe traumatic brain injury: a preliminary study. J Int Med Res 34:58–64
Maekawa T, Yamashita S, Nagao S, Hayashi N, Ohashi Y (2015) Prolonged mild therapeutic hypothermia versus fever control with tight hemodynamic monitoring and slow rewarming in patients with severe traumatic brain injury: a randomized controlled trial. J Neurotrauma 32:422–429
Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM (1993) The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg 79:354–362
Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM (1997) Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 336:540–546
Michael H (2013) Therapeutic hypothermia following cardiac arrest. Best Pract Res Clin Anaesthesiol 27:335–346
Mtaweh H, Smith R, Kochanek PM, Wisniewski SR, Fabio A, Vavilala MS (2014) Energy expenditure in children after severe traumatic brain injury. Pediatr Crit Care Med 15:242–249
Oddo M, Frangos S, Milby A, Chen I, Maloney-Wilensky E, Murtrie EM (2009) Induced normothermia attenuates cerebral metabolic distress in patients with aneurismal subarachnoid hemorrhage and refractory fever. Stroke 40:1913–1916
Qiu WS, Liu WG, Shen H, Wang WM, Zhang ZL, Zhang Y (2005) Therapeutic effect of mild hypothermia on severe traumatic head injury. Chin J Traumatol 8:27–32
Qiu W, Shen H, Zhang Y, Wang WM, Liu WG, Jiang QZ (2006) Noninvasive selective brain cooling by head and neck cooling is protective in severe traumatic brain injury. J Clin Neurosci 13:995–1000
Qiu W, Zhang Y, Sheng H, Zhang J, Wang W, Liu W (2007) Effects of therapeutic mild hypothermia on patients with severe traumatic brain injury after craniotomy. J Crit Care 22:229–336
Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T (1993) Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg 79:363–368
Shiozaki T, Kato A, Taneda M, Hayakata T, Hashiguchi N, Tanaka H (1999) Little benefit from mild hypothermia therapy for severely head injured patients with low intracranial pressure. J Neurosurg 91:185–191
Shiozaki T, Hayakata T, Taneda M, Nakajima Y, Hashiguchi N, Fujimi S (2001) A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. J Neurosurg 94:50–54
Soukup J, Zauner A, Doppenberg EM, Menzel M, Gilman C, Bullock R (2002) Relationship between brain temperature, brain chemistry and oxygen delivery after severe human head injury: the effect of mild hypothermia. Neurol Res 24:161–168
Sydenham E, Roberts I, Alderson P (2009) Hypothermia for traumatic head injury. Cochrane Database Syst Rev 1:CD001048
Truettner JS, Alonso OF, Bramlett HM, Dietrich WD (2011) Therapeutic hypothermia alters microRNA responses to traumatic brain injury in rats. J Cereb Blood Flow Metab 31:1897–1907
Woo JH, Lim YS, Yang HJ, Park WB, Cho JS, Kim JJ, Hyun SY, Lee G (2014) Factors associated with pneumonia in post-cardiac arrest patients receiving therapeutic hypothermia. Am J Emerg Med 32:150–155
Yan Y, Tang W (2001) Changes of evoked potentials and evaluation of mild hypothermia for treatment of severe brain injury. Chin J Traumatol 4:8–13
Zhao QJ, Zhang XG, Wang LX (2011) Mild hypothermia therapy reduces blood glucose and lactate and improves neurologic outcomes in patients with severe traumatic brain injury. J Crit Care 26:311–315
Zhi D, Zhang S, Lin X (2003) Study on therapeutic mechanism and clinical effect of mild hypothermia in patients with severe head injury. Surg Neurol 59:381–385
Authors’ contributions
All authors conceived the study and contributed to the study design. HYY and RZ collected data and helped to extract data. YFZ performed the analyses. JRW and XLY performed the literature review. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge all the people who contributed to this study.
Competing interests
The authors declare that they have no competing interests.
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhu, Y., Yin, H., Zhang, R. et al. Therapeutic hypothermia versus normothermia in adult patients with traumatic brain injury: a meta-analysis. SpringerPlus 5, 801 (2016). https://doi.org/10.1186/s40064-016-2391-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40064-016-2391-2