Abstract
Objective
Tafamidis-associated adverse events (AEs) were investigated retrospectively by data mining the US Food and Drug Administration Adverse Event Reporting System (FAERS) to inform clinical safety.
Methods
Data were gathered from the FAERS database, which spans the second quarter of 2019 to the fourth quarter of 2023. A total number of 8532 reports of Tafamidis-related adverse events were detected after evaluating 8,432,351 data. Disproportionality analyses were used to quantify the signal and assess the significance of Tafamidis-associated AEs using four algorithms, including the reporting odds ratio (ROR), the proportional reporting ratio (PRR), the multi-item gamma Poisson shrinker (MGPS) and the Bayesian confidence propagation neural network (BCPNN).
Results
Among the 8532 reports of AEs with Tafamidis as the primary suspected drug, Tafamidis-induced AEs were identified as occurring in 27 system organ classes (SOC). A total of 207 Tafamidis-induced AEs were detected which simultaneously complied with the four algorithms. Our analysis also identified new adverse reactions including Hypoacusis, Deafness, and Essential hypertension. The median onset of adverse reactions associated with Tafamidis was 180 days (interquartile range [IQR] 51–419 days).
Conclusion
Tafamidis is a drug that has shown favorable safety and tolerability results in clinical trials. However, a number of adverse reactions associated with Tafamidis have been identified through analysis of the FAERS database. In clinical applications, it is recommended to closely monitor patients’ hearing while using Tafamidis. In addition, it is hoped that further experimental and clinical studies will be conducted in the future to understand the mechanism of occurrence between Tafamidis and adverse reactions such as primary hypertension, hyperlipidemia, and height reduction.
Similar content being viewed by others
Introduction
Transthyretin amyloidosis (ATTR) is a systemic disease caused by misfolding of transthyretin (TTR), which causes TTR to become unstable and deposited. ATTR often causes progressive organ dysfunction, and the disease can affect multiple systems and key organs, with the heart and nerves being the main organs affected [1]. The two most common types of ATTR are transthyretin amyloid cardiomyopathy (ATTR-CM) and transthyretin amyloid polyneuropathy (ATTR-PN). Prior to Tafamidis was developed, the primary therapies available for ATTR were liver and/or heart transplantation or symptomatic therapy. Tafamidis, a first-of-its-kind kinetic stabilizer of TTR that improves prognosis, represents a significant step forward in the treatment of ATTR. As an orally available small molecule drug, Tafamidis selectively binds to TTR and kinetically inhibits the dissociation of TTR tetramers into monomers, thereby inhibiting the formation of TTR amyloid deposits [2]. Tafamidis offers patients a more effective treatment alternative, improves the disease’s prognosis, and improves their quality of life. In 2019, the US Food and Drug Administration (FDA) approved Tafamidis for the treatment of the cardiomyopathy of wild-type or hereditary ATTR-CM in adults [3]. Tafamidis is the first medicine licensed for the therapy of wild-type and hereditary ATTR-CM [4]. Currently, Tafamidis has been approved for the therapy of ATTR-CM in nearly 50 countries [3], and for the therapy of ATTR-PN in over 40 countries around the world [5]. Tafamidis has a promising application in the field of ATTR therapy, and its safety deserves to be focused on.
In the quest for drug efficacy, attention to AEs is crucial. And most of Tafamidis’ past research has been on topics such as literature analysis and clinical trials, with few publications focusing on the latest real-world research. In the case of Tafamidis, adverse reactions are not systematically described in its specification. In the 30-month Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR-ACT) study, the incidence and type of adverse events were similar to those in the placebo group [6]. However, long-term extension (LTE) and clinical data from Tafamidis showed the following adverse events: acute heart failure, peripheral edema, pleural effusion, Upper abdominal pain, diarrhea, dyspnea, Pain in extremities, gout, falls, increased prothrombin time, urinary tract infection, and others [5, 7, 8]. Although clinical trials and exploratory investigations have provided some evidence regarding the safety of Tafamidis, due to differences in the conditions of clinical trials and actual application scenarios, they may not correctly reflect the incidence of adverse reactions that arise in actual clinical applications. Therefore, more in-depth exploration of the safety of Tafamidis in actual clinical applications is still needed.
To fill this gap, this study collected and analyzed post-marketing adverse drug reactions to Tafamidis based on real-world data from the largest sample, aiming to provide a reference for rational clinical drug use. FAERS, one of the largest pharmacovigilance databases in the world, contains reports of adverse events and medication errors submitted to the FDA [9]. This study collected and analyzed post-marketing adverse reactions to Tafamidis based on the largest sample of real-world data. These data encompass a broader population and a longer observation period, providing more comprehensive and realistic information compared to clinical trials. This is essential for assessing the safety of Tafamidis in clinical applications. In this study, the adverse event signals of Tafamidis were mined and analyzed through the FAERS database, with the aim of providing a reference for the rational use of the drug in the clinic. This will allow physicians to better weigh the potential risk of adverse effects against the benefits to the patient and to more effectively select the appropriate treatment regimen.
Materials and methods
Data source and processing
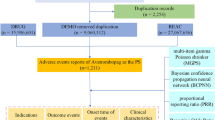
In conjunction with the time of drug launch, AEs reports with Tafamidis as the primary suspected drug (PS) were obtained by collecting safety data related to Tafamidis for a total of 19 quarters from the second quarter of 2019 to the fourth quarter of 2023 via the FAERS database using the subject terms “tafamidis,” “tafamidis meglumine,” “Vyndaqel,” and “Vyndamax” after eliminating duplicated data and irrelevant reports. Reports of tafamidis-related AEs were summarized and analyzed using SOC as a category of risk signals and preferred term (PT) as a standard name for risk signals within the Medical Dictionary for Regulatory Activities (Med DRA version 26.1).
Data is imported into R.4.3.2 and processed for analysis. Only the latest report on the basis of date is kept for data with the identical caseID in the demographic and administrative information (DEMO) table. Clinical characteristics were described in detail in the reports, including gender, age, reporting country, indication, outcome, and reporter occupation. Notably, serious outcomes include death, life-threatening, hospitalization, disability, need for intervention to prevent permanent injury/damage, and other serious consequences. However, the total number of serious consequences may exceed the total number of reports, as some cases listed more than one serious consequence. The flowchart for data processing and refining is displayed in Fig. 1.
Statistical analysis
In this study, we used the disproportionality analysis in pharmacovigilance to determine the potential association between tafamidis and AEs. In pharmacovigilance databases, disproportionality analysis is now a validated and effective method for drug safety research and monitoring [10].
Based on the disproportionality analysis, we simultaneously applied four algorithms to quantify the signals of tafamidis-related AEs, respectively reporting odds ratio (ROR) [11], the proportional reporting ratio (PRR) [12], the multi-item gamma Poisson shrinker (MGPS) [13], the Bayesian confidence propagation neural network (BCPNN) [14]. ROR have the advantage of allowing relative risk to be assessed and focusing the study on who should be included or who should be excluded from the control sequence and can correct for bias due to low numbers of reports for certain events. The advantages of MGPS are more comprehensive algorithms, effective reduction of false alarms, and the ability to mine signals from rare events. BCPNN specializes in integrating data from multiple sources and performing cross-validation, is suitable for dealing with complex association structures, and is able to identify potential associations between drugs and AEs in the data. In this study, the joint application of four algorithms, ROR, PRR, MGPS, and BCPNN, is used to utilize the advantages of each of the four algorithms to validate the analysis results from different perspectives in order to reduce the errors and produce a more fully reliable safety signal from the comprehensive assessment. In this study, positive signals for drug-related AEs were considered when at least one algorithm met the criteria. When all four algorithms met the criteria, it suggested a strong correlation between the AEs, which helped to minimize the likelihood of false-positive signals. Higher values obtained indicate a stronger signal strength, which implies a stronger link between the target medicine and the adverse reaction [15]. Data were statistically analyzed using Microsoft Excel 2021 software. All the algorithms as well as detailed formulas and thresholds are shown in Tables 1 and 2.
Results
Annual distribution of Tafamidis-related AEs reports
There have been 8532 AEs reports for Tafamidis between May 2019 and December 2023, according to the FAERS database. As a whole, the number of AEs reports is increasing every year. Taking 2019 (446 reports) and 2023 (3442 reports) as an example, the number of reports shows a minimum and a maximum. Notably, the number of reports has increased significantly in the last two years, with 40.34% of the total number of reports in 2023 alone. Figure 2 shows more specific information on annual allocations.
General characteristics
From the second quarter of 2019 through the fourth quarter of 2023, a total of 8,432,351 adverse event reports were obtained from the FAERS database for this study. After rigorous data screening, 8532 reports identifying Tafamidis as the primary suspect drug for AEs were identified, and the data were subsequently further analyzed. In adverse event reports involving Tafamidis, there were significantly more male patients than female patients (72.95% vs. 17.80%). Regarding age, the 65 to 85 age group had the highest number of elderly patients, accounting for 58.97% of the total. The bulk of reports, totaling 51.80% of all reports, were notably submitted by patients rather than by medical personnel. With 72.14% of the total reports, the United States accounted for the vast majority of the reports. Japan (6.97%), France (6.21%), Canada (4.47%), and Germany (2.00%) were the next most frequently reported countries. In terms of serious clinical outcomes, death was the most commonly reported serious outcome with a total of 2922 (28.37%) reported. Other serious outcomes and hospitalization rates were 2758 (26.77%) and 1806 (17.53%), respectively. Detailed information is shown in Table 3.
Signal of system organ class
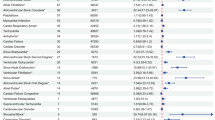
In total, our statistical analysis identified 27 organ systems associated with Tafamidis-induced AEs at the SOC level. Cardiac disorders (n = 2636, ROR 6.81, PRR 6.11, IC 2.60, EBGM 6.08), and Ear and labyrinth disorders (n = 402, ROR 4.50, PRR 4.44, IC 2.15, EBGM 4.42) were the SOC that matched all four criteria at the same time and demonstrated a significant connection with Tafamidis AEs. In addition, other important SOC that were positive in the ROR and MGPS algorithms include General disorders and administration site conditions (n = 5430, ROR 1.53, PRR 1.40, IC 0.49, EBGM 1.40), Nervous system disorders (n = 1861, ROR 1.17, PRR 1.15, IC 0.21, EBGM 1.15), Respiratory, thoracic and mediastinal disorders (n = 1234, ROR 1.25, PRR 1.24, IC 0.31, EBGM 1.24), Metabolism and nutrition disorders (n = 618, ROR 1.47, PRR 1.45, IC 0.54, EBGM 1.45), Surgical and medical procedures (n = 390, ROR 1.25, PRR 1.25, IC 0.33, EBGM 1.25). Of these, Cardiac disorders (n = 2636, ROR 6.81, PRR 6.11, IC 2.60, EBGM 6.08,) were consistent with the characterization of tafamidis as a treatment for ATTR-CM. Ear and labyrinth disorders (n = 402, ROR 4.50, PRR 4.44, IC 2.15, EBGM 4.42) are not mentioned in the drug inserts as an adverse reaction that satisfies all four algorithms simultaneously and deserves focused attention and study. Table 4 shows the signal strength and amount of reports of Tafamidis at the SOC level.
Signal of preferred terms
At the PT level, four algorithms were used in this study to analyze AEs and assess their compliance with various screening criteria. A total of 207 Tafamidis-induced AEs were detected which simultaneously complied with the four algorithms covering 22 SOC at the PT level. See Supplementary Table S1 for details. To avoid unclear presentations [16], we excluded PT associated with Tafamidis indications (Cardiac amyloidosis, Hereditary neuropathic amyloidosis, Acquired attr amyloidosis, Familial amyloidosis, Amyloidosis). Patients with more than 20 cases of AEs (a > 20) were selected, and a total of 49 AEs meeting the screening criteria were selected for analysis and grouped according to SOC, the results are shown in Table 5. In our study, Oedema peripheral (n = 99, ROR 3.55, PRR 3.54, IC 1.82, EBGM 3.53), Cardiac failure (n = 532, ROR 20.62, PRR 20.14, IC 4.30, EBGM 19.77), Atrial fibrillation (n = 251, ROR 7.69, PRR 7.62, IC 2.92, EBGM 7.57), Cardiac failure congestive (n = 199, ROR 15.31 PRR 15.18, IC 3.90, EBGM 14.97), Cardiac failure acute (n = 29, ROR 11.85, PRR 11.84, IC 3.55, EBGM 11.71), Ventricular tachycardia (n = 28, ROR 6.05, PRR 6.05, IC 2.59, EBGM 6.02), Dysphagia (n = 176, ROR 6.18, PRR 6.14, IC 2.61, EBGM 6.11), Pleural effusion (n = 71, ROR 3.95, PRR 3.94, IC 1.97, EBGM 3.93), Dizziness postural (n = 20, ROR 5.80, PRR 5.79, IC 2.53, EBGM), 5.76), Gout (n = 42, ROR 7.11, PRR 7.09, IC 2.82, EBGM 7.05) etc. are consistent with the results once seen in clinical trials [5, 7, 8]. These results show that our analysis is consistent with known clinical data and also highlight the importance of new potential safety issues.
In the analysis of Tafamidis, several new AEs worthy of further study were identified in the table, including but not limited to the following PT: Essential hypertension (n = 31, ROR 41.95, PRR 41.90, IC 5.33, EBGM 40.26), Hypoacusis (n = 319, ROR 15.90, PRR 15.68, IC 3.95, EBGM 15.46), Deafness (n = 39, ROR 4.17, PRR 4.16, IC 2.05, EBGM 4.15), Body height decreased (n = 89, ROR 28.89, PRR 28.78, IC 4.81, EBGM 28.01), Hyperlipidaemia (n = 29, ROR 13.29, PRR 13.28, IC 3.71, EBGM 13.12). It is worth noting that both Hypoacusis (n = 319, ROR 15.90, PRR 15.68, IC 3.95, EBGM 15.46) and Deafness (n = 39, ROR 4.17, PRR 4.16, IC 2.05, EBGM 4.15) are associated with ear and labyrinthine disorders, which are not mentioned in the drug insert or in the clinical trials associated with Tafamidis, and which deserve to be explored further.
Onset time of events
The onset time of Tafamidis-related adverse reactions was collected from the FAERS database. With a median onset time of 180 days (interquartile range [IQR] 51–419 days), 868 cases overall with reported onset times were excluded from reports of unreported or unknown onset times. As Fig. 3 illustrates, the results showed that Tafamidis cases varied in timing, sometimes spanning more than a year. Within the first month after initiation of treatment with Tafamidis (n = 151, 17.40%), it was the month with the most cases of all months. In the first quarter (n = 284, 32.72%), second quarter (n = 149, 17.17%), third quarter (n = 105, 12.12%), and fourth quarter (n = 66, 7.60%), there was a general trend of decreasing number of cases quarter by quarter. Notably, as shown in our data, adverse reactions may still occur one year after the initiation of Tafamidis, in the second year of treatment (n = 157, 18.09%), and even after the second year of treatment, the incidence is still 12.33%.
Discussion
Our study revealed a trend of significant annual increases in reported adverse events (AEs) associated with Tafamidis, with male patients accounting for the vast majority of these reports (72.95%). Signals that satisfy all four algorithmic criteria simultaneously at the SOC level are Cardiac disorders, Ear and labyrinth disorders. At the PT level, death (n = 2359) was the most frequent adverse reaction we found. Of note, Ear and labyrinth disorders were found to be significant adverse events, which have not been previously documented in clinical trials or drug inserts and require further investigation. These findings emphasize the importance of continuous monitoring and vigilance in the management of adverse events associated with Tafamidis to ensure patient safety.
Our study found that the number of reported adverse events associated with Tafamidis increased each year beginning in 2019, with an even more significant increase in the number of reports since 2022. This upward trend not only demonstrates the significant therapeutic efficacy of Tafamidis, which has led to an increase in its use across a wide range of indications and patients, but also serves as a reminder of the importance of analyzing these adverse reactions.
Among the AEs regarding Tafamidis, there were more males (72.95%) than females (17.80%). This may be due to the fact that there are more male patients, thus increasing their chances of taking the drug. Data from THAOS suggest that in the United States, the majority of patients with ATTR are older men with a predominantly cardiac-dominant phenotype [17]. ATTR-CM is categorized into the wild type ATTR (ATTRwt) and mutant ATTR (ATTRm) type [18]. ATTRm also known as hereditary ATTR (hATTR) or the variant ATTR (ATTRv) type. There are more male than female patients of both types in clinical reports [19, 20]. Of these, ATTR-CM (ATTRwt) has traditionally been recognized as a distinctly sex-specific disease that primarily affects the heart alone and occurs most often in men over the age of 60 [21, 22]. This gender difference may be due to the fact that some biological features associated with females prevent myocardial involvement in ATTRv amyloidosis [19]. In contrast, androgens promote the hepatic production of TTR and may be a risk factor for ATTR amyloidosis development [23]. Therefore, with the widespread clinical use of Tafamidis, it is important for clinicians to remain vigilant regarding adverse reactions associated with Tafamidis, especially in elderly male patients.
Based on the disproportionality analysis, we find that the signals that satisfy all four algorithmic criteria simultaneously at the SOC level are Cardiac disorders (n = 2636, ROR 6.81, PRR 6.11, EBGM 6.08, IC 2.60), Ear and labyrinth disorders (n = 402, ROR 4.5, PRR 4.44, EBGM 4.42, IC 2.15). One of the most important signals, Cardiac disorders, is not documented in the Tafamidis drug insert, which may be related to the patient’s own disease progression rather than a direct correlation with the drug itself. ATTR-CM, also known as Transthyretin cardiac amyloidosis (ATTR-CA), usually presents with heart failure and arrhythmias [17, 24]. ATTR-CA is the leading cause of restrictive cardiomyopathy and Heart Failure with Preserved Ejection Fraction (HFpEF) [17, 25]. The resulting heart failure syndrome is a product of biventricular involvement and usually includes symptoms of both left and right heart failure, including fatigue, hypotension, exertional dyspnea, telangiectasia, paroxysmal nocturnal dyspnea, hepatomegaly, ascites, early satiety, nausea, and lower extremity edema [26]. With further deposition of TTR, echocardiography may show a decreased left ventricular ejection fraction (LVEF). In addition, the patient’s heart conduction system is often compromised, which can lead to arrhythmias, including atrial fibrillation (AF) and conduction block [27]. Unfortunately, due to the heterogeneous nature of the disease and its multisystem involvement, there is often overlap with other diseases, and it is usually diagnosed only in the presence of significant myocardial amyloid deposition and advanced restrictive cardiomyopathy, which is already at an advanced stage of the disease [28]. Tafamidis does not reverse the amyloid deposits that have formed but only slows the disease progression of cardiomyopathy and peripheral neuropathy [29]. Tafamidis is primarily indicated in the early stages of the disease and has been shown in clinical trials to be more effective in patients with mild to moderate symptoms [8]. The results may be less than ideal for patients who already have severe lesions or are in the advanced stages of the disease. So it may not be reasonable to judge whether the onset and progression of Cardiac disorder and some of the associated clinical manifestations are caused by Tafamidis based on AEs signals. For this situation, we should strengthen the identification, screening, and evaluation of high-risk groups, and establish a standardized diagnosis and treatment process to ensure that ATTR-CA patients can be diagnosed and treated as early as possible.
At the PT level, Death (n = 2359, ROR 8.57, PRR 7.76, IC 2.95, EBGM 7.71) was the adverse reaction with the highest frequency of occurrence that we detected. This is because, as a systemic disease that can involve multiple systems, ATTR seriously affects the quality of life and life expectancy of patients, and the prognosis is worse especially when the heart is involved. ATTR-CA is a rapidly progressive, inevitably progressive, and ultimately fatal cardiomyopathy. ATTR-CA patients have poor quality of life and low survival rates, with the median survival of ATTRwt patients ranging from 43 to 57 months after diagnosis [25, 30], and that of ATTRm patients depending on the mutant gene, of which the median survival of Val122Ile mutant patients is only 31 months after diagnosis [30]. Deaths in patients with ATTR-CA are usually due to cardiac causes, mainly including sudden death and heart failure [31]. Studies have shown that Tafamidis is effective in reducing all-cause mortality and cardiovascular mortality in patients with ATTR-CA, which excludes heart transplant patients and patients with mechanical cardiac assist devices [32]. Meanwhile, a systematic review from Singh et al. also supports the possibility that Tafamidis may halt disease progression, thereby reducing cardiovascular mortality and all-cause mortality. Meanwhile, a systematic review from Singh et al. also supports that Tafamidis slows disease progression and reduces cardiovascular mortality and all-cause mortality [33]. The high frequency of deaths at the PT level may be based on the high mortality rate that characterizes the disease itself rather than the adverse effects of Tafamidis. Therefore, we need to be careful in assessing the safety of drugs and not judge their safety and efficacy solely on the basis of the frequency of deaths.
In analyzing the adverse effects of the drug, we found some unexpected safety signals, especially involving ear and labyrinthine disorders, including Hypoacusis (n = 319, ROR 15.90, PRR 15.68, IC 3.95, EBGM 15.46), Deafness (n = 39, ROR 4.17, PRR 4.16, IC 2.05, EBGM 4.15). Molecular studies in animal models suggest that TTR may play a role in the ear. Studies have already recorded the presence of transcripts of this protein in the inner ear of mice [34]. A study on ATTR and hearing loss showed that hearing loss is prevalent and more severe in patients with ATTRv amyloidosis, but is mostly overlooked in clinical practice. Simultaneous studies suggest that amyloid deposits can penetrate various anatomical structures in the inner and mild ear [35]. Although Tafamidis works by reducing amyloid formation, hearing may be affected if the medication is not effective or if amyloid is deposited in the ear structure due to some unknown factor. Data from the ATTR-PN long-term follow-up and clinical practice settings show the presence of reduced thyrotropin, lower serum thyroxine levels in laboratory outliers [36]. Studies have shown that abnormal thyroid function can affect the ear’s hearing function, especially as a risk factor for low-frequency descending sudden deafness [37, 38]. Therefore, this may be one of the reasons why Tafamidis causes adverse reactions to ear and labyrinthine disorders. It is worth noting that these reasons are based on pharmacologic and physiologic assumptions, and the reality may be more complex. Adverse drug reactions are usually caused by a mixture of factors, including the pharmacologic nature of the drug, individual differences, and drug metabolic pathways. Therefore, further experimental studies and clinical observations are needed to reveal that Tafamidis causes Ear and labyrinth disorders to better understand the mechanisms underlying it. In addition, we found no relevant literature reports on other important new signals (primary hypertension, hyperlipidemia, height reduction). In this regard, more clinical studies are needed to understand the pathogenesis of these adverse events.
Our findings showed that the median time to onset was 180 days (interquartile range [IQR] 51–419 days), with the highest incidence within the 1st month after initiation of Tafamidis treatment (n = 151, 17.40%). The incidence rate showed a decreasing trend quarter by quarter over time as follows: first quarter (n = 284, 32.72%), second quarter (n = 149, 17.17%), third quarter (n = 105, 12.12%), and fourth quarter (n = 66, 7.60%). The incidence rate showed a decreasing trend quarter by quarter over time as follows: first quarter (n = 284, 32.72%), second quarter (n = 149, 17.17%), third quarter (n = 105, 12.12%), and fourth quarter (n = 66, 7.60%). It is worth noting that adverse reactions may still occur even after one year of Tafamidis treatment, with a prevalence of (n = 157, 18.09%), and even after the second year of treatment, the prevalence was still as high as 12.33%. There have been relatively few detailed analyses of the timing of adverse reactions to Tafamidis in prior studies. Most studies focus on the efficacy and safety of the drug and do not specifically characterize a particular time point for adverse effects. The data from our study suggest that attention should be paid to the occurrence of adverse reactions associated with Tafamidis within the first month of initiation of treatment with Tafamidis, as well as long-term monitoring of the occurrence of adverse reactions. Early detection of Tafamidis treatment-induced adverse events may reduce patient suffering and improve patient quality of life.
In this study, the adverse reaction signals of Tafamidis were mined and analyzed based on the FAERS database. FAERS is an essential database of adverse events and medication error reports submitted to FDA and plays an invaluable role in the timely identification of potential drug safety problems. Disproportionality analysis, which was employed in the study to detect signals in pharmacovigilance databases, is a widely used method [39, 40]. In this study, the joint application of four algorithms is used to validate the analysis results from different perspectives in order to minimize the errors and produce a more fully reliable safety signal from the comprehensive assessment. In the FAERS database, data mining techniques have sufficient statistical power to identify drug safety problems more quickly than traditional methods [41]. However, it is worth noting that these data have some limitations. First, the FAERS database is a spontaneous reporting system, and consumers can also submit adverse reaction reports to the database. Nevertheless, generally, consumers’ medical expertise is limited, which may lead to certain misunderstandings or errors in the report. In addition, it is important to emphasize that this study is a retrospective study that only observes associations and does not establish causality [42]. Despite the numerous benefits provided by the data mining approaches utilized in this work, there remain limits in the identification and analysis of adverse drug reaction signals through spontaneous reporting systems, which cannot be entirely addressed. As a result, our findings should be interpreted as a piece of advice to doctors and pharmacists to remain cautious in preventing potential adverse reactions. In summary, although this study utilized data mining techniques to analyze the adverse effects of Tafamidis, based on the limitations of the data, we recommend caution in interpreting the results of the study and encourage further research investigations to validate and extend our observations.
In conjunction with the above discussion, we believe that with the widespread use of Tafamidis in clinical applications, clinicians should exercise caution, especially in older male patients. We should establish a standardized diagnostic and treatment process to ensure that patients with ATTR can be diagnosed and treated as early as possible. We emphasize the need for special attention to ear and labyrinthine disorders and recommend that patients’ ear hearing be closely monitored during the use of Tafamidis. Such adverse events should be emphasized in the clinical use of the drug, and further studies and clinical observations are expected in the future to refine the mechanisms behind them. To address the timing of Tafamidis adverse reactions, special attention should be paid to reactions within the first month of treatment initiation and monitored over time to improve the chances of early detection of adverse reactions and intervention to improve patient quality of life.
Conclusion
In summary, through the comprehensive and systematic mining and analysis of FAERS data, this study provides a strong scientific basis for the safety evaluation of Tafamidis. Tafamidis is a drug that has shown favorable safety and tolerability results in clinical trials. However, in our study, we emphasized the need for special attention to ear and labyrinthine disorders. This is an important clinical warning and it is recommended that patients’ hearing in the ear be closely monitored while using Tafamidis. Clinicians should be on high alert for these potential adverse effects. In addition, we look forward to more experimental and clinical studies in the future to understand the mechanism of occurrence between Tafamidis and adverse effects such as essential hypertension, hyperlipidemia, and height reduction. This study provides additional insight into the safety of Tafamidis and will hopefully help clinicians make informed decisions.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- FDA:
-
US Food and Drug Administration
- FAERS:
-
US Food and Drug Administration Adverse Event Reporting System
- AEs:
-
Adverse events
- ROR:
-
Reporting odds ratio
- PRR:
-
Proportional reporting ratio
- MGPS:
-
Multi-item gamma Poisson shrinker
- BCPNN:
-
Bayesian confidence propagation neural network
- SOC:
-
System organ classes
- IQR:
-
Interquartile range
- ATTR:
-
Transthyretin amyloidosis
- TTR:
-
Transthyretin
- ATTR-CM:
-
Transthyretin amyloid cardiomyopathy
- ATTR-PN:
-
Transthyretin amyloid polyneuropathy
- ATTR-ACT:
-
Transthyretin Amyloidosis Cardiomyopathy Clinical Trial
- LTE:
-
Long-term extension
- PS:
-
Primary suspected drug
- PT:
-
Preferred terms
- DEMO:
-
Demographic and administrative information
- ATTRwt:
-
Wild type ATTR
- ATTRm:
-
Mutant ATTR
- hATTR:
-
Hereditary ATTR
- ATTRv:
-
Variant ATTR
- ATTR-CA:
-
Transthyretin cardiac amyloidosis
- HFpEF:
-
Heart Failure with Preserved Ejection Fraction
- (LVEF):
-
Left ventricular ejection fraction
- AF:
-
Atrial fibrillation
References
Muchtar E, Dispenzieri A, Magen H, Grogan M, Mauermann M, McPhail ED, et al. Systemic amyloidosis from a (aa) to t (attr): a review. J Intern Med. 2021;289(3):268–92. https://doi.org/10.1111/joim.13169.
Coelho T, Merlini G, Bulawa CE, Fleming JA, Judge DP, Kelly JW, et al. Mechanism of action and clinical application of tafamidis in hereditary transthyretin amyloidosis. Neurol Ther. 2016;5(1):1–25. https://doi.org/10.1007/s40120-016-0040-x.
Burton A, Castaño A, Bruno M, Riley S, Schumacher J, Sultan MB, et al. Drug discovery and development in rare diseases: taking a closer look at the tafamidis story. Drug Des Devel Ther. 2021;15:1225–43. https://doi.org/10.2147/DDDT.S289772.
Ney S, Gertz RJ, Pennig L, Nies RJ, Holtick U, Völker LA, et al. Multiparametric monitoring of disease progression in contemporary patients with wild-type transthyretin amyloid cardiomyopathy initiating tafamidis treatment. J Clin Med. 2024;13(1). https://doi.org/10.3390/jcm13010284.
Lamb YN, Deeks ED. Tafamidis: a review in transthyretin amyloidosis with polyneuropathy. Drugs. 2019;79(8):863–74. https://doi.org/10.1007/s40265-019-01129-6.
Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379(11):1007–16. https://doi.org/10.1056/NEJMoa1805689.
Huber P, Flynn A, Sultan MB, Li H, Rill D, Ebede B, et al. A comprehensive safety profile of tafamidis in patients with transthyretin amyloid polyneuropathy. Amyloid. 2019;26(4):203–09. https://doi.org/10.1080/13506129.2019.1643714.
Elliott P, Drachman BM, Gottlieb SS, Hoffman JE, Hummel SL, Lenihan DJ, et al. Long-term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail. 2022;15(1):e008193. https://doi.org/10.1161/CIRCHEARTFAILURE.120.008193.
Vogel U, van Stekelenborg J, Dreyfus B, Garg A, Habib M, Hosain R, et al. Investigating overlap in signals from evdas, faers, and vigibase(®). Drug Saf. 2020;43(4):351–62. https://doi.org/10.1007/s40264-019-00899-y.
Montastruc J, Sommet A, Bagheri H, Lapeyre-Mestre M. Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol. 2011;72(6):905–08. https://doi.org/10.1111/j.1365-2125.2011.04037.x.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23.
Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (prrs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001;10(6):483–86.
Dumouchel W. Bayesian data mining in large frequency tables, with an application to the fda spontaneous reporting system. Am Stat. 1999;53(3):177–90. https://doi.org/10.1080/00031305.1999.10474456.
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–21.
Jiang Y, Zhou L, Shen Y, Zhou Q, Ji Y, Zhu H. Safety assessment of brexpiprazole: real-world adverse event analysis from the faers database. J Affect Disord. 2024;346:223–29. https://doi.org/10.1016/j.jad.2023.11.025.
Tang S, Wu Z, Xu L, Wen Q, Zhang X. Adverse reaction signals mining and hemorrhagic signals comparison of ticagrelor and clopidogrel: a pharmacovigilance study based on faers. Front Pharmacol. 2022;13:970066. https://doi.org/10.3389/fphar.2022.970066.
Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: thaos (transthyretin amyloid outcome survey). J Am Coll Cardiol. 2016;68(2):161–72. https://doi.org/10.1016/j.jacc.2016.03.596.
Porcari A, Fontana M, Gillmore JD. Transthyretin cardiac amyloidosis. Cardiovasc Res. 2023;118(18):3517–35. https://doi.org/10.1093/cvr/cvac119.
Caponetti AG, Rapezzi C, Gagliardi C, Milandri A, Dispenzieri A, Kristen AV, et al. Sex-related risk of cardiac involvement in hereditary transthyretin amyloidosis: insights from thaos. Jacc Heart Fail. 2021;9(10):736–46. https://doi.org/10.1016/j.jchf.2021.05.005.
Campbell CM, LoRusso S, Dispenzieri A, Kristen AV, Maurer MS, Rapezzi C, et al. Sex differences in wild-type transthyretin amyloidosis: an analysis from the transthyretin amyloidosis outcomes survey (thaos). Cardiol Ther. 2022;11(3):393–405. https://doi.org/10.1007/s40119-022-00265-7.
Gertz MA, Benson MD, Dyck PJ, Grogan M, Coelho T, Cruz M, et al. Diagnosis, prognosis, and therapy of transthyretin amyloidosis. J Am Coll Cardiol. 2015;66(21):2451–66. https://doi.org/10.1016/j.jacc.2015.09.075.
García-Pavía P, Tomé-Esteban MT, Rapezzi C. [Amyloidosis. Also a heart disease]. Rev Esp Cardiol. 2011;64(9):797–808. https://doi.org/10.1016/j.recesp.2011.05.003.
Gonçalves I, Alves CH, Quintela T, Baltazar G, Socorro S, Saraiva MJ, et al. Transthyretin is up-regulated by sex hormones in mice liver. Mol Cell Biochem. 2008;317(1–2):137–42. https://doi.org/10.1007/s11010-008-9841-2.
Damy T, Costes B, Hagège AA, Donal E, Eicher J, Slama M, et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J. 2016;37(23):1826–34. https://doi.org/10.1093/eurheartj/ehv583.
Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, et al. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68(10):1014–20. https://doi.org/10.1016/j.jacc.2016.06.033.
Dharmarajan K, Maurer MS. Transthyretin cardiac amyloidoses in older North americans. J Am Geriatr Soc. 2012;60(4):765–74. https://doi.org/10.1111/j.1532-5415.2011.03868.x.
Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, et al. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation. 2016;133(3):282–90. https://doi.org/10.1161/CIRCULATIONAHA.115.018852.
Givens RC, Russo C, Green P, Maurer MS. Comparison of cardiac amyloidosis due to wild-type and v122i transthyretin in older adults referred to an academic medical center. Aging Health. 2013;9(2):229–35.
Nawarskas JJ, Shephard EA. Tafamidis: a novel treatment for transthyretin amyloid cardiomyopathy. Cardiol Rev. 2020;28(3):156–60. https://doi.org/10.1097/CRD.0000000000000306.
Lane T, Fontana M, Martinez-Naharro A, Quarta CC, Whelan CJ, Petrie A, et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. https://doi.org/10.1161/CIRCULATIONAHA.118.038169.
Ruberg FL, Berk JL. Transthyretin (ttr) cardiac amyloidosis. Circulation. 2012;126(10):1286–300. https://doi.org/10.1161/CIRCULATIONAHA.111.078915.
Sukaina M, Rehman S, Waheed M, Shehryar M, Rasool R, Ahmed N, et al. Efficacy of tafamidis in transthyretin amyloid cardiomyopathy: a systematic review and meta-analysis. Annals Med Surg (2012). 2024;86(1):433–38. https://doi.org/10.1097/MS9.0000000000001482.
Singh BM, Bohara N, Gautam K, Basnet M, Kc S, Kc B, et al. A systematic review of tafamidis in patients with transthyretin amyloid cardiomyopathy. Cureus. 2021;13(9):e18221. https://doi.org/10.7759/cureus.18221.
Klockars T, Perheentupa T, Dahl HM. In silico analyses of mouse inner-ear transcripts. J Assoc Res Otolaryngology. 2003;4(1):24–40.
Bartier S, Bodez D, Kharoubi M, Guellich A, Canouï-Poitrine F, Chatelin V, et al. Association between hearing loss and hereditary attr amyloidosis. Amyloid. 2019;26(4):234–42. https://doi.org/10.1080/13506129.2019.1663814.
Barroso FA, Judge DP, Ebede B, Li H, Stewart M, Amass L, et al. Long-term safety and efficacy of tafamidis for the treatment of hereditary transthyretin amyloid polyneuropathy: results up to 6 years. Amyloid. 2017;24(3):194–204. https://doi.org/10.1080/13506129.2017.1357545.
Melse-Boonstra A, Mackenzie I. Iodine deficiency, thyroid function and hearing deficit: a review. Nutr Res Rev. 2013;26(2):110–17. https://doi.org/10.1017/S0954422413000061.
Chen L, Wang YJ, Sun X, Zhang N, Li YN, Fan ZM, et al. [Analysis of prognostic factors of low-frequency type of sudden sensorineural hearing loss]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi = Chin J Otorhinolaryngol Head Neck Surg. 2020;55(7):652–57. https://doi.org/10.3760/cma.j.cn115330-20191212-00756.
Coloma PM, Trifirò G, Patadia V, Sturkenboom M. Postmarketing safety surveillance: where does signal detection using electronic healthcare records fit into the big picture? Drug Saf. 2013;36(3):183–97. https://doi.org/10.1007/s40264-013-0018-x.
Vilar S, Friedman C, Hripcsak G. Detection of drug-drug interactions through data mining studies using clinical sources, scientific literature and social media. Brief Bioinform. 2018;19(5):863–77. https://doi.org/10.1093/bib/bbx010.
Shi X, Cheng Q, Zhao Y, Zou S, Sun M. A real-world pharmacovigilance study of abaloparatide based on the fda adverse event reporting system (faers). Osteoporos Int. 2023;34(12):2047-58. https://doi.org/10.1007/s00198-023-06877-6
Zhu H, Qu Y, Du Z, Zhou Q, Shen Y, Jiang Y, et al. Mining and analysis of adverse event signals of cariprazine based on the real-world data of faers database. J Affect Disord. 2024;347:45–50. https://doi.org/10.1016/j.jad.2023.11.076.
Acknowledgements
We extend our sincere gratitude to the FAERS for providing high-quality data for researchers.
Funding
This study was supported by Shandong Traditional Chinese Medicine Science and Technology Program (No. Z-2023035T).
Author information
Authors and Affiliations
Contributions
YL: Formal Analysis, Writing—original draft, Visualization, Writing—Review & Editing. SS: Software, Data curation, Investigation. HW: Validation, Project administration. LZ: Conceptualization, Methodology, Supervision, Writing—Review & Editing. WP: Resources, Supervision, Funding acquisition. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable. This study was deemed non-human subject related research.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Li, Y., Sun, S., Wu, H. et al. Safety assessment of Tafamidis: a real-world pharmacovigilance study of FDA adverse event reporting system (FAERS) events. BMC Pharmacol Toxicol 25, 71 (2024). https://doi.org/10.1186/s40360-024-00790-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40360-024-00790-2