Abstract
Dissolving microneedles (DMNs) represent an innovative advancement in drug delivery and skincare technologies, offering significant advantages compared to traditional needles. This paper presents an overview of the historical evolution of microneedles and the rise of dissolving types, exploring their definition, concept, and diverse clinical applications such as vaccinations, drug delivery, and skincare treatments. Design and manufacturing considerations cover the materials employed, fabrication techniques, and methods for characterizing DMNs, focusing on aspects like mechanical strength, dissolution rate, and delivery efficiency. The mechanism of action section examines skin penetration mechanics, the process of microneedle dissolution, controlled release of active compounds, and considerations of biocompatibility and safety. Recent developments in DMNs encompass technological advancements, improved delivery systems, and updates on clinical trials and studies. Challenges and opportunities in scaling up production, overcoming market adoption barriers, and future research directions are discussed, aiming to address unmet medical needs and expand applications. In summary, DMNs have the potential to transform drug delivery and skincare treatments, with ongoing advancements aimed at tackling current challenges and unlocking new opportunities for enhanced healthcare outcomes.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
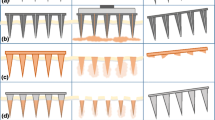
To overcome the drawbacks of oral administration and hypodermic injections, arrays of microscopic needles have been designed to pierce through the outermost layer of the skin, called the stratum corneum, without inducing pain. The objective is to deliver medications with the effectiveness of a traditional needle injection while offering the ease of use of a transdermal patch. This method has proven to improve transdermal delivery of various substances, including small molecules, proteins, DNA, and vaccines [1]. Microneedles consist of clusters of extremely tiny needles, usually measuring in the micrometer scale (less than 1000 µm in length). These needles create pores, allowing for the localized delivery of medications either within the skin or through the skin into the bloodstream [2]. Moreover, microneedles (MNs) could also serve for the purpose of collecting bodily fluids, such as monitoring glucose levels in the management of diabetes. ALZA Corporation is credited as the first entity to conceptualize MNs, as outlined in a patent from 1976 [3]. Microneedle technology has a rich developmental history spanning over four decades. The United States Patent and Trademark Office granted a patent application by Gerstel and Place in 1976, which is when microscale needles first came into existence. Progress in the microfabrication industry has enabled more accurate and controlled manufacturing of microneedles. This has led to the creation of different types of microneedles as shown in Fig. 1, including solid, hollow, coated and dissolving [4]. Each type has distinct characteristics, advantages, and applications. Solid microneedles penetrate the stratum corneum for enhanced drug delivery, particularly suitable for vaccines. Hollow microneedles store and deliver larger drug volumes, while coated microneedles offer rapid drug delivery. DMNs, introduced in 2005, enable quick release of macromolecules and simplified drug administration, though they require complete insertion and may experience delayed dissolution [5].
Numerous innovations have occurred in recent decades in the field of microneedle technologies, offering novel perspectives on fluid sampling, biosensors, microanalysis and related areas. MNs have also been combined with electroporation and iontophoresis methods to boost drug delivery rates and enhance the transportation of large molecular weight (LMW) macromolecules from the skin into the bloodstream [6].
In this review, we’ll explore the design strategies, significant hurdles and prospects linked with DMNs, encompassing aspects like stability, scalability, manufacturing intricacies, regulatory factors, and their translation into clinical practice. Additionally, we’ll delve into how DMNs could potentially revolutionize patient care by improving treatment outcomes, increasing drug effectiveness, enabling self-administration, and aiding in worldwide vaccination efforts. By consolidating the latest advancements and future possibilities in DMNs, our aim is to stimulate ongoing research endeavors and nurture innovation in this dynamic and swiftly evolving domain.
Design and fabrication of MNs
Types of polymer used in DMNs
Various types of polymers with diverse characteristics have been employed in the production of polymeric microneedles, as detailed in Table 1. Based on their in vivo performance, polymeric microneedles can be categorized into dissolvable microneedles, degradable microneedles, and swellable microneedles. Their characteristics, pros and cons are described in Table 2. Drugs are encapsulated in the polymeric matrix, which protects them from biological or physical disruption. Microneedles dissolve, swell, or break down when inserted into the skin, releasing the drugs inside [7].
Polymer selection is critical in microneedle design, impacting strength, penetration, and drug release. Unlike non-dissolving materials like silicon or copper, water-soluble polymers typically exhibit lower mechanical strength, which may be further compromised by drug encapsulation. Mechanical properties like flexural modulus and fracture resistance are crucial for insertion capability. Researchers explore combining polymers and materials for enhanced performance. Consideration of target tissue (transdermal or non-transdermal) is vital, balancing strength and flexibility, especially for delicate tissues. Environmental moisture affects polymer resistance, emphasizing the importance of polymer choice in MN fabrication [11].
Fabrication methods of DMNs
Numerous physical and chemical variables can affect the stability and efficacy of drugs contained in DMNs during preparation and storage. Therefore, selecting an appropriate preparation method is crucial. Common methods for preparing DMNs include the micromolding/solvent casting method, droplet-born air-blowing method, centrifugal lithography and drawing lithography method. We will describe the specific steps involved in these production methods in the following section [12].
Drawing lithography
Drawing lithography relies on the critical point of the viscous polymer during the glass transition process to achieve the manufacturing capabilities of a 3D microstructure [13]. Drawing lithography makes use of the viscosity of a polymer during the glass transition stage for production. Polymer materials undergo elastic deformation during this phase, and 3D microneedle structures are subsequently fabricated through extensional deformation, which involves stretching [2]. The process involves coating a baseplate with molten polymer, which is then brought into contact with drawing pillars. Afterward, the plate and pillars are vertically separated, inducing the polymers to stretch into needle-shaped formations. Simultaneously, the material’s temperature is carefully controlled to facilitate curing and isolate the microneedle structures, as illustrated in Fig. 2 [14]. For example, maltose, which is dissolved by the hydrolytic cleavage of maltose-glucoamylase in the skin, is frequently employed as a safe polymer for encasing biomolecules. Its controlled viscosity during the glass transition process makes it a building block for DMNs. Drawing lithography techniques can manipulate maltose's viscosity to create various microneedle shapes, but precise control is necessary to avoid structural defects. A stepwise controlled drawing technique was developed to produce sharp-conical microneedles, offering flexibility in length adjustment for targeted drug delivery. This innovative approach overcomes limitations of traditional methods by enabling rapid manufacturing without molding [15]. Lee et al. developed microneedle cuff (MNC) devices for drug delivery to the tunica adventitia and media of blood vessels. These cuffs, designed to wrap around vessels, have an inner surface with drug-coated microneedles. When applied, the microneedles penetrate the target tissue layers and gradually release the drug to prevent intimal hyperplasia (IH), offering higher delivery efficiency compared to other perivascular devices [16].
Micromolding method (solvent casting)
DMNs are typically manufactured by pouring a liquid formulation onto a pre-made MN mold [17]. Since the late 1980s, laser machining has been a prominent alternative or complementary tool to lithography-based microfabrication techniques. CO2 lasers, in particular, are commonly used for rapid prototyping and cost-effective microfabrication. Microchannel engraving has proven to be an efficient method for fabricating microfluidic devices. Additionally, laser drilling, known as ablation, is a traditional technique used to create arrays of microstructures, such as concavities or through-holes, for various industrial and research applications [18]. This method is efficient, simple, and time-saving, making it suitable for mass production and more cost-effective compared to alternative technologies. It is particularly well-suited for sugar and polymeric microneedles [19]. Figure 3 demonstrates the fabrication of dissolving microneedle by solvent casting method. However, mold-filling remains a significant challenge in micromolding. At the microscale, surface tension becomes predominant, impeding the materials from adequately filling the mold cavity solely under the influence of gravity. Therefore, mold-filling strategies are essential to overcome surface tension. Various approaches have been devised by researchers, including vacuum-assisted methods, centrifugation, spinning coating, imprinting, infiltration and atomized spray, to tackle this mold filling challenge. Despite these efforts, micromolding has its limitations. It struggles to produce intricate structures such as adhesive structures with barbs or hollow structures. Additionally, demolding stress can potentially damage final structures, particularly for soft and fragile materials like hydrogels. Moreover, materials such as metal, silicon, and glass are unsuitable for processing via this method [20].
Droplet-born air blowing method
This innovative method allows for the production of polymeric microneedles without relying on preformed silicon molds [21]. Using air blowing, this technique creates microneedles from polymer droplets as depicted in Fig. 4, providing a gentle process that avoids the need for harsh external conditions like UV irradiation or heat [22]. In the droplet-born air blowing method, polymer solution drops are arranged in an array pattern on two plates. These plates are then brought into contact and moved at a controlled rate. As the plates reach their final distance from each other, the stretched polymer is solidified using air blowing, resulting in the formation of microneedles. Recently, this method has been enhanced by incorporating a cyclic contact and drying process on pillars (CCDP process), allowing for the production of dissolvable microneedle patches. These patches offer the advantage of quick separation of microneedles from their backing film [23]. Moreover, employing a sole polymer drop per microneedle enables precise management of droplet size and concentration, facilitating controlled drug loading without any loss of the drug. Usually, this procedure requires approximately 10 min and has been successfully utilized in producing DMNs loaded with drug [24].
Centrifugal lithography
Centrifugal lithography utilizes centrifugal force to shape the polymer into pillar-shaped needles and is applicable for creating multi-layered needle structures as depicted in Fig. 5 [25]. The initial layer was formed by applying a viscous hyaluronic acid (HA) droplet onto a solidified HA layer and then subjecting the droplet to centrifugal force, aided by a non-adhesive parafilm. Subsequently, another HA droplet was applied onto the first layer and centrifuged again [26]. The complex needles in the shape of wine glasses with a narrow neck at the backing layer and a raindrop-shaped tip are mechanically strong and are able to be produced consistently. However, the requirement for materials with specific mechanical properties limits the range of materials and active pharmaceutical ingredients (APIs) that can be effectively integrated and delivered using this technique. Furthermore, material loss occurs on the outer plate, resulting in API wastage. Although centrifugal lithography offers a novel one-step fabrication approach, its suitability for industrial production is limited, and its current applications are restricted [25].
Characterization techniques
The characterization of microneedles is crucial in their development to ensure their safety, efficacy, and reliability. Various fundamental methods are commonly employed for microneedle characterization, as outlined below.
Scanning electron microscopy (SEM)
The morphological properties of microneedles, such as needle height, tip and base diameter, needle spacing, and base dimensions, are evaluated using scanning electron microscopy (SEM). The microneedle patch is put on a stage, and to improve visibility, a gold solution is used for sputter coating [6].
Mechanical strength
Microneedles must possess enough mechanical strength to penetrate into the skin without bending or breaking down. Factors influencing their strength include polymer composition, fabrication conditions, and curing methods. Researchers have developed innovative microneedle designs like Separable micropillar integrated dissolving microneedles (SPDMN) and micro-pillar integrated dissolving microneedles (PDMN) to enhance strength. Various testing devices, such as the Texture Analyzer and micro-mechanical test machine, evaluate mechanical properties. Studies compare microneedle height changes after compression to gauge strength. These evaluations enable comparisons between different materials and assess factors like humidity's impact on mechanical properties. Overall, microneedle strength is crucial for their effectiveness in transdermal applications [12].
Insertion test
The insertion test holds greater significance and provides more accurate measurements compared to the axial force test. Various skin subjects, including pigs, rats, and humans, were targeted in this test. One advantage of MNs is their capability to load and deliver drugs to the skin. Despite several mechanical tests simulating needle fracture force, validation with actual skin is crucial. Lee et al. embedded a pyramidal MN in full-thickness cadaver pork skin, observing the MN imprints under a microscope. Donnelly et al. used a digital microscope to examine the skin surface of a dead piglet after inserting an MN connected to a movable cylindrical probe into its skin. Jun et al. used a material testing machine to measure the transverse compression load. Using a texture analyzer, Khan et al. applied various MN forces to the skin of newborn pigs to investigate insertion depth. Davis et al. used a texture analyzer to perform insertion tests on three Caucasian male skin samples. Additionally, optical coherence tomography (OCT) technology was utilized to scan the depth of MN insertion in human skin in another study [5].
Microneedle dissolution testing
The in vitro dissolution of microneedles involves assessing how effectively they dissolve and release the drug when exposed to simulated physiological conditions outside the body. Zhang et al. utilized microneedle arrays on ex vivo chicken skin sourced from a local slaughterhouse, with a 3% (w/v) agarose gel acting as a skin mimic. A 500 g weight was positioned atop the arrays, and microneedles were extracted at predefined intervals of 15, 45, and 120 s. Both pre- and post-dissolution images of the microneedles were captured using a microscope camera [27]. The visual representation of dissolution kinetics of microneedle is shown in Fig. 6.
Determination of drug content
To determine the drug content of the patch’s needles, microneedle (MN) patches were applied to mice’s depilated dorsal skin and removed once the needles had completely dissolved. After that, the baseplates were dissolved in deionized water to release the remaining drug. An ultraviolet–visible (UV–Vis) spectrophotometer was used to measure the absorbance of the obtained solution at the drug’s characteristic wavelength of 302 nm. A standard curve was used to determine the drug’s concentration. Lastly, the drug content in the needles was determined by deducting the original amount in the entire MN patch from the drug content still present in the baseplate [28].
In vitro drug diffusion study
To assess MNs potential for transdermal drug delivery, an in vitro diffusion study is conducted. With the stratum corneum (the side of the MNs) facing up, prepared arrays of MNs are placed inside shaved animal skin and placed on the opening of a Franz diffusion cell. At 37 °C, the recipient medium, which is usually phosphate buffer saline with a pH of 7.4, is continuously stirred. To determine how much drug has penetrated the skin, samples are taken from the recipient medium and replaced with new medium at predetermined intervals [29].
Understanding the mechanism of DMNs
The effectiveness of topical drug administration relies on the mechanism of diffusion. In microneedle drug delivery systems, the skin experiences momentary disruption. A microneedle device comprises thousands of microneedles arranged on a small patch, similar to a standard commercially available transdermal patch. This design aims to deliver adequate quantities of a drug to achieve the required therapeutic outcome [30]. Initially, microneedles puncture the skin and dissolve, forming channels through which drugs can penetrate into the skin and interstitial fluid can exit the skin. While drug diffusion may occur through a dry backing layer, the backing layer will be hydrated with interstitial fluid from the skin, which is expected to enhance drug diffusion [1]. Researchers are exploring polymeric microneedles to enhance drug release, focusing on sustained release properties. Unlike rapid dissolution, these microneedles gradually release drugs, potentially improving therapy by reducing side effects and administration frequency. Various types have been developed, including slow-dissolving, degradable, and bioresponsive microneedles, each offering unique mechanisms for controlled drug delivery [31]. Biocompatibility is crucial for the safety of DMNs, ensuring no adverse reactions occur in the skin due to the materials used. Safety studies evaluate different materials, with many polymers showing promise. However, long-term effects of repeated skin penetration remain unclear. Materials should be biodegradable or excreted from the body to prevent accumulation. Concerns arise regarding potential tissue accumulation or organ deposition with repeated use, especially over time. Varying the application site may help mitigate these risks to some extent [32].
Recent developments in MN research
Manufacturing technology plays a crucial role in the development and application of MN transdermal drug delivery systems. Different manufacturing processes are selected based on substrate material and drug characteristics. MN production methods include mold-based and mold-free techniques, with additive manufacturing, such as 3D printing, emerging as a prominent option for polymer MN fabrication. Three-dimensional printing offers advantages in fabricating MNs with complex shapes and multifunctional capabilities, but challenges remain in achieving precision and mechanical performance. Researchers have developed and optimized various fabrication methods, often requiring a combination of techniques to produce MNs tailored to specific applications [33]. Hydrogel microneedles, introduced in 2012, offer excellent biocompatibility and mechanical flexibility for biomedical applications. They enable drug delivery with high loading capacity and tunable release rates. A recent development includes a segmented microneedle patch for colorimetric skin tattoo biosensing, allowing sensitive and reversible detection of pH, uric acid, glucose and temperature. This innovation showcases the diagnostic potential of hydrogel microneedles for long-term and multiplexed health monitoring [34]. Excipients such as hyaluronic acid (HA), polyvinylpyrrolidone (PVP), and polyvinyl alcohol (PVA) are normally water-soluble polymers used in nano/microparticle-loaded DMNs. Drugs are encapsulated in slow-release systems, like microspheres and nanoparticles, in the MN matrix, composed of materials like liposomes and metal nanoparticles, enabling sustained drug release. For instance, Ito et al. demonstrated the hypoglycaemic effect of MNs loaded with insulin-absorbed porous silicate particles in mice. Wu et al. achieved sustained delivery of ovalbumin to ocular scleral tissue using bilayer-dissolving MNs loaded with PLGA nanoparticles. Zhang et al. created MNs that contained silica microparticles loaded with cytokines and PLGA nanoparticles loaded with tetracycline for the purpose of regeneration of periodontal tissue. Tekko et al. delivered methotrexate to the skin for 72 h using PVP/PVA dissolving MNs loaded with methotrexate nanocrystals, while Peng et al. localized amphotericin B inside the skin for a week using microparticle-loaded MNs. Drug bioavailability and stability are increased when microparticles like liposomes are combined with MN delivery systems, as the treatment of alopecia shows [35].
Clinical trials and scientific investigations
A clinical study (NCT02438423) on DMN patches for influenza vaccination was carried out by Emory University in 2015. One hundred participants were split into four groups for the study: two groups received the influenza vaccine intramuscularly or through MN patches, one group received a placebo MN patch, and the final group self-administered the vaccine through MN patches. The MN patches were composed of a water-soluble polymeric platform with an array of 100 MNs, each 650 μm long, mounted to an adhesive backing. The patches contained 18 μg of three seasonal influenza vaccine strains. According to the study's findings, there were no significant adverse events documented, and the MN patches were well tolerated and produced antibody responses comparable to those of intramuscular injections [36]. Hirobe et al. conducted a clinical study on a new microneedle patch (new-MH) for human skin. They found that the microneedles successfully penetrated human skin, dissolved within 6 h, and induced immune responses comparable to longer application times in animal experiments. The 6-h application period was deemed necessary for optimal antigen delivery and recovery of skin barrier function. Assessment of local and systemic adversities in 20 human subjects revealed only slight erythema in some individuals, which disappeared after 30 days, and no serious adverse systemic events were noted. Thus, the new-MH device was deemed safe for application on human skin [37]. Arya et al. (2017) used 100 conical microneedles with a height of 650 µm and a base diameter of 200 µm to test DMNs in 15 healthy volunteers between the ages of 18 and 57. There was only mild erythema seen, and the microneedles were well tolerated with no pain or swelling. In a study by [38], ten healthy individuals between the ages of 20 and 60 participated in a microneedle system. The microneedles were made of polyglycolic acid and Nylon-6 and had a patch area of 0.785 cm2. Human skin was safely treated with the microneedles, causing no breakage or severe irritation [39]. Rouphael et al. carried out a groundbreaking human clinical investigation demonstrating the efficacy, safety, and acceptability of influenza vaccination using microneedle patches. The study revealed that microneedle patches were well tolerated, induced robust immune responses, and were strongly preferred over traditional intramuscular injection by participants. Microneedle patch vaccination could potentially increase vaccination rates and reduce healthcare costs. However, the study acknowledges limitations and suggests further research to confirm its findings and optimize microneedle patch formulations [40]. In this split-face study by Yang et al., the effects and adverse reactions of nano-antiaging fluid (NAFL) and hyaluronic acid dissolving microneedles (HA-DMNs) on infraorbital wrinkles were compared. Both treatments showed similar improvements in wrinkle numbers and skin assessment scores. However, NAFL appeared to provide longer-lasting anti-wrinkle effects compared to HA-DMNs. The adverse effects were more severe on the NAFL side, including pain, erythema, and edema. HA-DMNs had milder adverse effects but were still more prevalent compared to previous studies. The study highlights the attractiveness of HA-DMNs for aesthetic treatments but suggests the need for further investigation into treatment protocols, cost, and the mechanism of action [41].
Current challenges and perspectives
The translation of microneedles (MNs) from research labs to industries poses exciting yet challenging tasks. To achieve this, important questions and challenges must be addressed promptly. This includes ensuring feasibility in relevant markets and overcoming obstacles. Active strategies are needed to tackle these difficulties, which will ultimately shape the future of MNs and their commercial applications [42]. Challenges encountered with DMNs is their inability to fully penetrate the skin, resulting in wasted medication. Additionally, there are notable variations in skin penetration among DMNs made from different biomaterials. For instance, pure silk fibroin microneedle patches tend to break easily at their base, whereas microneedles made of pure PVA exhibits insufficient mechanical strength necessary to pierce the outer layer of the skin [2]. Polymeric microneedles, while potentially biocompatible, may pose hepatic and immune system risks if materials accumulate in the body. Strengthening their insertion capabilities to prevent breakage and bending is crucial. Combining multiple polymeric materials can enhance structural integrity and flexibility, particularly important for delicate tissues. Future advancements in polymeric microneedle technology aim to improve processing methods and manufacturing techniques. Despite current transdermal product applications, there's significant room for development and commercialization, addressing manufacturing challenges and cost-effectiveness. Polymeric microneedle devices are seen as transformative in smart product development, promising to elevate living standards [43]. Currently, the licensing process for MN products is done on a case-by-case basis due to the absence of standardized production and implementation criteria, hindering their translation and commercialization. The establishment of universal MN requirements based on classification is urgently needed. Regulatory standards, such as those set by the FDA, determine quality control acceptance criteria and influence mass production investments. The classification of MNs whether as consumer products, drug delivery systems or medical devices, poses a key question. Recent FDA guidance suggests MNs may be categorized as medical devices, especially considering their design and technological features impacting skin penetration. However, there's ambiguity regarding whether MNs should be treated similarly to traditional injections, potentially requiring sterilization, which could affect drug stability. Establishing regulatory standards for MNs, aligning with Good Manufacturing Practices (GMP), is crucial for their scalability and commercialization. Proposed quality specifications for MNs encompass various tests, including dissolution, disintegration, and mechanical characteristics, to ensure safety and efficacy for human use [44].
The future of DMNs is filled with exciting possibilities and ongoing trends that will shape their path in healthcare and skincare applications. The optimization of drug delivery efficiency, kinetics of dissolution, and mechanical strength of DMNs is a significant feature that contributes to their overall performance and therapeutic area applicability. It is anticipated that developments in material science and fabrication methods will spur innovations in DMN technology, opening the door to the creation of microneedle platforms that are more biocompatible, scalable, and economical. Furthermore, there is a great deal of promise to change the way healthcare is delivered through the integration of DMNs with cutting-edge technologies like biosensors, wearable technology, and personalized medicine. By playing a crucial role in targeted drug delivery modalities, continuous monitoring systems, and point-of-care diagnostics, DMNs have the potential to completely transform patient care and treatment results. Furthermore, the growing emphasis on patient-centric strategies and self-administration choices emphasizes how crucial it is to create accessible, easy-to-use, and convenient DMN systems. To address current issues, promote innovation, and integrate DMN research into clinical practice, cooperation between academic institutions, business, and regulatory agencies is essential. All things considered, the future of DMNs is one of constant evolution, propelled by the convergence of medical needs, technology innovations, and scientific discoveries that open up new avenues for better therapeutic interventions and patient outcomes.
Conclusion
DMNs represent a significant advancement in drug delivery and skincare technologies, providing notable benefits over traditional needles. This paper has traced the historical evolution of microneedles, highlighting the emergence and definition of dissolving microneedles (DMNs). We explored their broad range of clinical applications, including vaccinations, drug delivery, and skincare treatments. Our discussion on design and manufacturing covered crucial materials, fabrication techniques, and characterization methods, focusing on key factors such as mechanical strength, dissolution rate, and delivery efficiency. The detailed examination of the mechanism of action provided insights into skin penetration mechanics, microneedle dissolution, controlled release of active compounds, and biocompatibility and safety considerations. Recent advancements in DMNs were reviewed, showcasing technological progress, enhanced delivery systems, and updates from clinical trials and studies.
Despite the promising potential of DMNs, challenges remain in scaling up production and overcoming market adoption barriers. Future research directions must address these challenges to expand applications and meet unmet medical needs. The continued innovation and development of DMNs hold the promise of transforming drug delivery and skincare treatments, ultimately leading to improved healthcare outcomes. The ongoing advancements in this field are poised to overcome current challenges and unlock new opportunities, paving the way for enhanced and more efficient healthcare solutions.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- DMNs:
-
Dissolving microneedles
- MNs:
-
Microneedles
- HA:
-
Hyaluronic acid
- PVP:
-
Polyvinylpyrrolidone
- PVA:
-
Polyvinyl alcohol
References
Lee JW, Park J-H, Prausnitz MR (2008) Dissolving microneedles for transdermal drug delivery. Biomaterials 29(13):2113–2124. https://doi.org/10.1016/j.biomaterials.2007.12.048
Ita K (2017) Dissolving microneedles for transdermal drug delivery: advances and challenges. Biomed Pharmacother 93:1116–1127. https://doi.org/10.1016/j.biopha.2017.07.019
Donnelly RF, Singh TRR, Woolfson AD (2010) Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv 17(4):187–207. https://doi.org/10.3109/10717541003667798
Nguyen HX, Nguyen CN (2023) Microneedle-mediated transdermal delivery of biopharmaceuticals. Pharmaceutics 15(1):277. https://doi.org/10.3390/pharmaceutics15010277
Aldawood FK, Andar A, Desai S (2021) A comprehensive review of microneedles: types, materials, processes, characterizations and applications. Polymers 13(16):2815. https://doi.org/10.3390/polym13162815
Dalvi M, Kharat P, Thakor P, Bhavana V, Singh SB, Mehra NK (2021) Panorama of dissolving microneedles for transdermal drug delivery. Life Sci 284. https://doi.org/10.1016/j.lfs.2021.119877
Liu T, Luo G, Xing M (2020) Biomedical applications of polymeric microneedles for transdermal therapeutic delivery and diagnosis: current status and future perspectives. Adv Ther 3(9):1900140. https://doi.org/10.1002/adtp.201900140
Kim Y-C, Park J-H, Prausnitz MR (2012) Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev 64(14):1547–1568. https://doi.org/10.1016/j.addr.2012.04.005
Turner JG, White LR, Estrela P, Leese HS (2020) Hydrogel-forming microneedles: current advancements and future trends. Macromol Biosci 21(2):2000307. https://doi.org/10.1002/mabi.202000307
Park J-H, Allen MG, Prausnitz MR (2005) Biodegradable polymer microneedles: fabrication, mechanics and transdermal drug delivery. J Control Release 104(1):51–66. https://doi.org/10.1016/j.jconrel.2005.02.002
Yadav PR, Munni MN, Campbell L, Mostofa G, Dobson L, Shittu M, Pattanayek SK, Uddin MdJ, Das DB (2021) Translation of polymeric microneedles for treatment of human diseases: recent trends, progress, and challenges. Pharmaceutics 13(8):1132. https://doi.org/10.3390/pharmaceutics13081132
Zhang L, Guo R, Wang S, Yang X, Ling G, Zhang P (2021) Fabrication, evaluation and applications of dissolving microneedles. Int J Pharm 604. https://doi.org/10.1016/j.ijpharm.2021.120749
Xu J, Xu D, Xuan X, He H (2021) Advances of microneedles in biomedical applications. Molecules 26(19):5912. https://doi.org/10.3390/molecules26195912
Yang J, Zhang H, Hu T, Xu C, Jiang L, Shrike Zhang Y, Xie M (2021) Recent advances of microneedles used towards stimuli-responsive drug delivery, disease theranostics, and bioinspired applications. Chem Eng J 426. https://doi.org/10.1016/j.cej.2021.130561
Lee K, Jung H (2012) Drawing lithography for microneedles: a review of fundamentals and biomedical applications. Biomaterials 33(30):7309–7326. https://doi.org/10.1016/j.biomaterials.2012.06.065
Lee KJ, Park SH, Lee JY, Joo HC, Jang EH, Youn Y-N, Ryu W (2014) Perivascular biodegradable microneedle cuff for reduction of neointima formation after vascular injury. J Control Release 192:174–181. https://doi.org/10.1016/j.jconrel.2014.07.007
Tucak A, Sirbubalo M, Hindija L, Rahić O, Hadžiabdić J, Muhamedagić K, Čekić A, Vranić E (2020) Microneedles: characteristics, materials, production methods and commercial development. Micromachines 11(11):961. https://doi.org/10.3390/mi11110961
Chen YW, Chen MC, Wu KW, Tu TY (2020) A facile approach for rapid prototyping of microneedle molds, microwells and micro-through-holes in various substrate materials using CO2 laser drilling. Biomedicines 8(10):427. https://doi.org/10.3390/biomedicines8100427
Gupta J, Gupta R, Vanshita (2020) Microneedle technology: an insight into recent advancements and future trends in drug and vaccine delivery. ASSAY Drug Dev Technol. https://doi.org/10.1089/adt.2020.1022
Lyu S, Dong Z, Xu X, Bei H-P, Yuen H-Y, James Cheung C-W, Wong M-S, He Y, Zhao X (2023) Going below and beyond the surface: microneedle structure, materials, drugs, fabrication, and applications for wound healing and tissue regeneration. Bioactive Mater 27:303–326. https://doi.org/10.1016/j.bioactmat.2023.04.003
Dharadhar S, Majumdar A, Dhoble S, Patravale V (2018) Microneedles for transdermal drug delivery: a systematic review. Drug Dev Ind Pharm 45(2):188–201. https://doi.org/10.1080/03639045.2018.1539497
Nagarkar R, Singh M, Nguyen HX, Jonnalagadda S (2020) A review of recent advances in microneedle technology for transdermal drug delivery. J Drug Deliv Sci Technol 59. https://doi.org/10.1016/j.jddst.2020.101923
Jamaledin R, Di Natale C, Onesto V, Taraghdari Z, Zare E, Makvandi P, Vecchione R, Netti P (2020) Progress in microneedle-mediated protein delivery. J Clin Med 9(2):542. https://doi.org/10.3390/jcm9020542
Ogundele M, Okafor H (2017) Transdermal drug delivery: microneedles, their fabrication and current trends in delivery methods. J Pharm Res Int 18(5):1–14. https://doi.org/10.9734/jpri/2017/36164
Moore LE, Vucen S, Moore AC (2022) Trends in drug- and vaccine-based dissolvable microneedle materials and methods of fabrication. Eur J Pharm Biopharm 173:54–72. https://doi.org/10.1016/j.ejpb.2022.02.013
Chang H, Zheng M, Chew SWT, Xu C (2020) Advances in the formulations of microneedles for manifold biomedical applications. Adv Mater Technol 5(4):1900552. https://doi.org/10.1002/admt.201900552
Zhang D, Das DB, Rielly CD (2014) Microneedle assisted micro-particle delivery from gene guns: experiments using skin-mimicking agarose gel. J Pharm Sci 103(2):613–627. https://doi.org/10.1002/jps.23835
Du H, Liu P, Zhu J, Lan J, Li Y, Zhang L, Zhu J, Tao J (2019) Hyaluronic acid-based dissolving microneedle patch loaded with methotrexate for improved treatment of psoriasis. ACS Appl Mater Interfaces 11(46):43588–43598. https://doi.org/10.1021/acsami.9b15668
Asfour MH (2020) Advanced trends in protein and peptide drug delivery: a special emphasis on aquasomes and microneedles techniques. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-020-00746-z
Damiri F, Kommineni N, Ebhodaghe SO, Bulusu R, Jyothi VGSS, Sayed AA, Awaji AA, Germoush MO, Al-malky HS, Nasrullah MZ, Rahman MdH, Abdel-Daim MM, Berrada M (2022) Microneedle-based natural polysaccharide for drug delivery systems (DDS): progress and challenges. Pharmaceuticals 15(2):190. https://doi.org/10.3390/ph15020190
Du G, Sun X (2020) Current advances in sustained release microneedles. Pharm Fronts 02(01):e11–e22. https://doi.org/10.1055/s-0040-1701435
Quinn HL, Larrañeta E, Donnelly RF (2016) Dissolving microneedles: safety considerations and future perspectives. Ther Deliv 7(5):283–285. https://doi.org/10.4155/tde-2016-0015
Zhang W, Zhang W, Li C, Zhang J, Qin L, Lai Y (2022) Recent advances of microneedles and their application in disease treatment. Int J Mol Sci 23(5):2401. https://doi.org/10.3390/ijms23052401
Hu Y, Chatzilakou E, Pan Z, Traverso G, Yetisen AK (2024) Microneedle sensors for point-of-care diagnostics. Adv Sci. https://doi.org/10.1002/advs.202306560
Meng F, Qiao X, Xin C, Ju X, He M (2024) Recent progress of polymeric microneedle-assisted long-acting transdermal drug delivery. J Pharm Pharm Sci. https://doi.org/10.3389/jpps.2024.12434
Zhang J, Li H, Albakr L, Zhang Y, Lu A, Chen W, Shao T, Zhu L, Yuan H, Yang G, Wheate NJ, Kang L, Wu C (2023) Microneedle-enabled therapeutics delivery and biosensing in clinical trials. J Control Release 360:687–704. https://doi.org/10.1016/j.jconrel.2023.07.023
Hirobe S, Azukizawa H, Matsuo K, Zhai Y, Quan Y, Kamiyama F, Suzuki H, Katayama I, Okada N, Nakagawa S (2013) Development and clinical study of a self-dissolving microneedle patch for transcutaneous immunization device. Pharm Re 30(10):2664–2674. https://doi.org/10.1007/s11095-013-1092-6
Ono A, Azukizawa H, Ito S, et al (2017) Development of novel double-decker microneedle patches for transcutaneous vaccine delivery. Int J Pharm 532:374–383. https://doi.org/10.1016/j.ijpharm.2017.08.110
Jung JH, Jin SG (2021) Microneedle for transdermal drug delivery: current trends and fabrication. J Pharm Investig. https://doi.org/10.1007/s40005-021-00512-4
Rouphael NG, Paine M, Mosley R, Henry S, McAllister DV, Kalluri H, Pewin W, Frew PM, Yu T, Thornburg NJ, Kabbani S, Lai L, Vassilieva EV, Skountzou I, Compans RW, Mulligan MJ, Prausnitz MR, Beck A, Edupuganti S, Heeke S (2017) The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet 390(10095):649–658. https://doi.org/10.1016/s0140-6736(17)30575-5
Yang Y, Yan J, Shi L, Zhang L, Liu X, Long F, Wang P, Wang X (2023) Hyaluronic acid dissolving microneedles and nonablative fractional laser for infraorbital wrinkles: a prospective, randomized. Split-Face Study Dermatol Ther 2023:1–7. https://doi.org/10.1155/2023/2087120
Avcil M, Çelik A (2021) Microneedles in drug delivery: progress and challenges. Micromachines 12(11):1321. https://doi.org/10.3390/mi12111321
Gera AK, Burra RK (2022) The rise of polymeric microneedles: recent developments, advances, challenges, and applications with regard to transdermal drug delivery. J Funct Biomater 13(2):81. https://doi.org/10.3390/jfb13020081
Zhao J, Xu G, Yao X, Zhou H, Lyu B, Pei S, Wen P (2021) Microneedle-based insulin transdermal delivery system: current status and translation challenges. Drug Deliv Transl Res. https://doi.org/10.1007/s13346-021-01077-3
Acknowledgements
The authors warmly acknowledge support and facilities provided by AETs St. John Institute of Pharmacy and Research, Palghar, Maharashtra, India.
Funding
No specific grant from any funding agency has been received.
Author information
Authors and Affiliations
Contributions
R.D.: Data collection and analysis, manuscript writing, editing. S.S.: Manuscript structure, supervision. N.K.: Manuscript structure, conceptualization, administration, supervision. A.B.: Manuscript writing, editing, coordination.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dave, R., Shinde, S., Kalayil, N. et al. Engineering microscopic delivery systems: a review of dissolving microneedle design, fabrication, and function. Micro and Nano Syst Lett 12, 14 (2024). https://doi.org/10.1186/s40486-024-00204-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40486-024-00204-2