Abstract
Ginger nut, AGA soil, and shell lime are the primary building limes used in traditional Chinese architectural sites. They have been widely researched and developed for restoring rock and soil heritage over the last decade. Previous studies have shown that these materials are compatible with weathered rock in terms of mechanical properties and environmental adaptability. In this study, metakaolinite was added to Chinese hydraulic limes to improve the mortar abilities. The basic properties and weather abilities of the mortars were evaluated. The characteristics of carbonation and hydration were analyzed over 900 days. The results indicated that the early strength improved and the contracting rate reduced when metakaolinite was added. The shell lime mortar was improved considerably compared with the modified ginger nut and AGA soil. The lime mortar content was determined using the X-ray diffraction results. The carbonation and hydration characteristics revealed that the metakaolinite aided the generation of hydraulic products (Ca2Al2SiO7·nH2O and β-CaSiO3·nH2O), particularly in the early stage. The microstructures were observed by scanning electron microscopy, which revealed more uniform and consolidated structures when metakaolinite was added.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Hydraulic lime is more durable and environmentally friendly than cement and air-setting lime. Hence, it has been widely used in construction and building materials in ancient and modern architecture [1,2,3,4,5,6,7,8]. Natural hydraulic limes have been researched and implemented in European countries, including modification and application in the conservation of historical buildings [1, 3, 8,9,10,11,12,13,14]. In China, hydraulic limes were not researched until recent decades. With the discovery of several hydraulic limes in ancient architectural ruins, Chinese hydraulic limes achieved a breakthrough. Ginger nut, discovered as ground material in the Dadiwan site in Qin’an County, Gansu Province, China [8, 12, 15,16,17], is a type of calcite concretion in the Quaternary sedimentary ore deposit, primarily comprising calcite and clay minerals [18]. However, the original ginger nut is not a proper construction material because no cementitious components are present. This material is considered the first evidence of natural hydraulic lime in China. Another Chinese hydraulic lime, the aga tu, a unique building material of the Tibetan ethnic group, abbreviated as AGA soil [16], is a calcite concretion formed in a semiarid plateau area in south Tibet [19]. Shell lime is another hydraulic lime calcined from shells, and it was widely used in ancient buildings in southeast China [20]. These three hydraulic limes are found mainly in ancient Chinese buildings and have been researched to restore rock heritages in recent years [21].
However, higher requirements for restoring mortars have been proposed because stone relics are in complex and changing environments (Fig. 1). These stone relics require urgent rescue. The shrinkage and early strength of Chinese hydraulic lime mortars have not yet reached the demands of grouting when dealing with uncertain conservations, leading to poor grouting compactness and secondary cracks. However, previous research has suggested that cement and lime mixed with metakaolinite have better strength, higher weather resistance abilities, and less shrinkage [22,23,24,25,26,27]. The metakaolinite was transformed from kaolinite under a sintering process (600 °C–900 °C), which primarily comprised amorphous SiO2 and Al2O3. This is not surprising because SiO2 and Al2O3 in metakaolinite react with Ca(OH)2 to generate calcium aluminosilicate, improving the mortar strength [28]. A growing number of studies have focused on the modification effect of metakaolinite on concrete in terms of structure, mortar working ability, and durability [29,30,31,32,33,34,35]. However, no information on the improvement mechanism of the metakaolinite and Chinese hydraulic limes mentioned above is available. This information could guide potential applications in mortar design that may lead to a better understanding of Chinese hydraulic limes.
Typical stone relics requiring restoration intervention in China: a interpenetrated crack of stone tablet in Yunju Temple, Beijing; b dehiscence of Sumeru in Mountain Resort, Chengde; c cracks of baluster in Mountain Resort, Chengde; and d structural fracture of cliff carvings in Yuanjue Cave, Sichuan Province
Therefore, to figure out how metakaolinite modified Chinese hydraulic lime mortars, we conducted tests on Chinese hydraulic lime mortar mixed with metakaolinite. The mortar working abilities including setting time, fluidity, and stone body behavior, such as contracting rate, porosity, and mechanical properties were also tested. In addition, we conducted weathering resistance ability tests on the specimens prepared using a new ingredient. Moreover, the characteristics of carbonation and hydration were analyzed over 900 days using X-ray diffraction (XRD).
Materials and methods
Binders and aggregates
The ginger nut primarily comprised calcite and clay minerals (Fig. 2a). The AGA soil was found as a tomb-building material in south Tibet and is now widely used in houses and temple buildings (Fig. 2b). Shell lime is another hydraulic lime calcined from shells (Fig. 2c) and can be traced to the Spring and Autumn Period and the Warring States Period. Currently, this material is widely used to maintain ancient buildings in the southwest coastal area of China. The shell calcined in this study was a type of oyster sampled from Zhejiang province, southeast China. The leading Chinese hydraulic lime currently used comprises ginger nut, AGA soil, and shell lime (Table 1). The calcium carbonate and silica composition can produce hydraulic lime through calcination. Table 2 lists the chemical compositions of the calcinated materials. The Chinese hydraulic limes are composed mainly of air-setting (CaO) and hydraulic (β-CaSiO3 and Ca2Al2SiO7) components. The aggregate was quartz sand (2 mm) in equal proportion.
Mixture ratio
The mortar performance and consolidation properties largely depend on the water binder ratio and aggregates. Generally, several criteria, including the water binder ratio and aggregates, should be considered before hydraulic lime is applied to heritage conservation. The water binder ratio and mortar setting time should be neither too large nor too small, considering handleability and fluidity. Therefore, some criteria must be satisfied before it can be considered: (1) the water binder ratio should not be too small because of grouting fluidity; (2) the setting time should be proper for mortar mixing and grouting; (3) the final setting time should be short because early strength is necessary for grouting; (4) the strength due to consolidation should be high enough for reinforcement. Different mixture and water binder ratios were used to determine the optimal mixture ratio of these three materials. According to the design of the masonry mortar [36], the optimal mixture and water binder ratios were determined based on the evaluation of mortar fluidity, setting times, consolidation shrinkage, and age strengths. Table 3 lists the mortar composition and proportion by weight.
Experimental procedure
The slurry fluidity and setting times were determined according to the mixture ratio in Table 3. The consolidation age strengths of 3, 7, 14, and 28 days were tested, including the compressive and flexural strengths. After curing for 50 days, weather tests were conducted under different weathering destruction conditions [37], and the weather resistance performance was evaluated from the compressive and flexural strength variations according to weather ability test standards [38].
This study examined how materials carbonate and hydrate in a moist environment. After curing for 28 days, the consolidations were placed in Feilaifeng Cliffside Sculptures in Hangzhou, Zhejiang Province (Fig. 3). The annual average rainfall and relative humidity in the meteorological environment (Fig. 3a) are approximately 1100–1600 mm and 70%, respectively. The carbonation and hydration of the consolidations were monitored by XRD after curing for 5, 300, 600, and 900 days.
Freeze–thaw resistance test: The samples were frozen (40 × 40 × 160 mm) at − 30 °C for 12 h and thawed at 25 °C and a relative humidity of 70% for 12 h. Dry–wet resistance test: The samples (40 × 40 × 160 mm) were dried at 100 °C for 12 h and cooled to 25 °C at a relative humidity of 70% for 12 h. Salt erosion resistance test: The samples (40 × 40 × 160 mm) were immersed in a saturated Na2SO4 solution for 20 h and dried at 105 °C for 4 h. Alkalinity resistance test: The samples (40 × 40 × 160 mm) were immersed in a NaOH solution (2%) for 12 h and dried at 105 °C for 4 h. Water stability test: The water stability of the specimens mixed with metakaolinite was determined by immersing the samples (40 × 40 × 160 mm) in water (25 °C) for 24 h, followed by natural air drying; these were called the water-immersed dry samples. Uniaxial compression test: The uniaxial compression test was conducted using the suggested methods for determining the uniaxial compressive strength and deformability of rock materials [39]. Six samples were tested under the same experimental conditions. Mercury intrusion test: Sample porosity tests were performed using an AutoPore 9500 high-performance automatic mercury porosimeter. The samples were cut into cubes and dried in an oven. Each undisturbed specimen was divided into two equal parts. Multiple cube specimens smaller than 1 cm3 were taken for testing in each part.
Results
Basic properties of mortars
The mortars were mixed and evaluated in terms of mortar setting times and shrinkage (Table 4). The fluidity of the mortars did not change considerably when metakaolinite was added. However, the initial and final setting times were shortened after mixing with metakaolinite, particularly the initial setting time of HP. Hence, metakaolinite would improve the early strength of the mortar. In addition, the porosity of the consolidation mixed with metakaolinite increased and shrinkage decreased. Therefore, the mortar behavior of the restoration functions was improved in terms of water and air permeability. The shrinkage of the consolidations was decreased so that it would barely produce secondary cracks during the grouting process.
Figure 4 shows the 3-, 7-, 14-, and 28-day age strengths of the mortar specimens. The age strengths, especially the early strengths, improved when metakaolinite was added to the mortar. The 3-day compression strength increased by 47.8% (LP), 165.2% (AP), and 302.5% (HP). The strengths of the shell lime specimens improved considerably when metakaolinite was added. The final compression and flexural strength of the shell lime mortar increased approximately 9 and 6 times, respectively. However, the strength of shell lime mixed with metakaolinite (HP) appeared to continue to increase in the later period. Metakaolinite first reacts with CaO in shell lime and produce Ca2Al2SiO7 in the carbonation process. Ca2Al2SiO7 is then transformed into Ca2Al2SiO7·nH2O during the hydration process. Hence, the consolidation process will last longer than those mortars without metakaolinite.
Weather resistance abilities
Environmental changes are important factors in the deterioration of cultural relics. Hence, evaluating the weathering resistance ability is necessary to restore materials. After curing for 50 days at 25 °C and relative humidity of 70%, strength tests were conducted on the specimens under different environmental destruction conditions to evaluate their weathering resistance ability. These evaluations include freeze–thaw resistance ability, dry–wet resistance stability, water stability, salt erosion resistance ability, and alkalinity resistance ability. The evolution of the specimen strength is shown in Fig. 5.
Freeze–thaw resistance ability: Freeze–thaw and soluble salt are the most significant erosion agents for construction materials [40], and performance degradation caused by freeze–thaw cycles and salt erosion was more conspicuous for the lime materials. Therefore, after 18 freeze–thaw cycles, we conducted compression strength test. The compressive strength of all specimens decreased after 18 freeze–thaw cycles (Fig. 5). The compression strength of the modified ginger nut specimens (L) decreased only by 17.40%, while that of the specimens mixed with metakaolinite (LP) decreased by 15.7%. Specimen A decreased by 17.16%, but AP decreased by only 8.62%. For the H and HP specimens, the compression strength did not change considerably. Thus, mortars mixed with metakaolinite have superior freeze–thaw resistance ability to those without metakaolinite.
Dry–wet resistance ability: After 18 dry–wet cycles, we conducted compression strength test. The results are shown in Fig. 5. The compression strength of L increased from 8.6 MPa to 18.3 MPa after the tests. The strengths of A and H were similar, which could be explained by the promotion of carbonation during dry–wet cycles [41]. However, the strength of the mortars mixed with metakaolinite changed slightly after 18 cycles. Therefore, mortars with metakaolinite were more adapted to the alternate wetting and drying environments. Indeed, many studies have shown that the frost resistance of cement could be improved by metakaolinite by reducing the pore size [42, 43].
Salt erosion resistance ability: Compressive strength tests were conducted after five cycles. The compression strengths of all specimens improved after five cycles (Fig. 5). This may be due to the possible recrystallization of Na2SO4 at high temperatures, which filled the pore spaces. Comparing these six kinds of materials, the shell lime mixed with metakaolinite has better salt erosion resistance ability than the others.
Alkalinity resistance ability: Water resistance is a crucial indicator for mortars because they will be exposed to an alkaline environment. Compressive strength tests were conducted after drying. The compression strength of specimens without metakaolinite changed slightly after the tests (Fig. 5). However, the mortars mixed with metakaolinte showed poor alkalinity resistance compared to those without metakaolinite. This was attributed to the higher porosity and larger pore size after adding metakaolinite to the mortars. The alkaline environment may eventually trigger a dedolomitization process inside the aggregate grains [44].
Water stability: To ascertain the degree to which the different mortar mixes permitted better water resistance ability, compressive strength tests were conducted before and after water immersion. Figure 5 shows that mortars mixed with metakaolinite have better mechanical strength than those without metakaolinite.
Carbonation and hydration characteristic analysis
The strength of the hydraulic mortar increased due to concretion, carbonation, and hydration. The carbonation and hydration of hydraulic lime are affected by many factors, such as temperature, relative humidity, and CO2 content [46,47,48]. To monitor the carbonation and hydration of the consolidations, we placed these mortars in Feilaifeng Cliffside Sculptures in Hangzhou, Zhejiang Province, and analyzed using XRD after curing for 5, 300, 600, and 900 days.
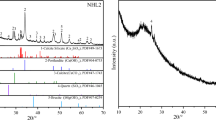
Figure 6 shows the XRD patterns of the specimens after curing. The original composition of the modified ginger nut was CaO (39.20%), β-CaSiO3 (26.70%), and Ca2Al2SiO7 (18.90%). After curing for 5 days, XRD revealed Ca(OH)2 (22.70%), CaCO3 (32.60%), β-CaSiO3· nH2O (22.27%), and Ca2Al2SiO7·nH2O (14.48%). The chemical reactions can be summarized as Eqs. (1), (2), (3), and (4), respectively. After 300 days, the Ca2Al2SiO7·nH2O content increased to 19.02% during the hydration process, and the CaCO3 content was 38.46% (Fig. 6). However, the CaO content was reduced to 7.78% due to carbonation, which decreased to 1.06% after 900 days. Regarding the modified ginger nut mortar with metakaolinite, the β-CaSiO3·nH2O and Ca2Al2SiO7·nH2O contents were higher than those of mortar L after 900 days. Correspondingly, the CaO, β-CaSiO3, and Ca2Al2SiO7 contents were much lower than those of the mortar without metakaolinite. The increased Ca2Al2SiO7 contents can be explained by the reaction (formula (5)) of Al2O3·SiO2 in metakaolinite and Ca(OH)2 produced by the CaO and water reaction in the early stage.
Carbonation and hydration results of the specimens by XRD: a ginger nut without metakaolinite, b ginger nut with metakaolinite, c AGA without metakaolinite, d AGA with metakaolinite, e shell lime without metakaolinite, and f shell lime with metakaolinite. (Peak 1-CaCO3. 2-CaO. 3-Ca(OH)2. 4-βCaSiO3. 5-Ca2Al2SiO7. 6-βCaSiO3·H2O. 7-Ca2Al2SiO7·H2O)
The modified AGA clay mortars showed similar behavior to the modified ginger nut in 900 days, but the Ca2Al2SiO7·nH2O contents increased more slowly than the modified ginger nut mortars. The modified AGA clay mortars mixed with metakaolinite produced more β-CaSiO3·nH2O than those without metakaolinite (formula (3)). This is a cause of a higher early strength increase of mortars when adding metakaolinite.
The carbonation and hydration of shell lime mortar (Fig. 6e, f) were modified when metakaolinite was added. In the first 5 days, the mortar without metakaolinite generated 48.14% CaCO3, and the CaO was retained at 27.60% due to carbonation. The β-CaSiO3·nH2O content increased from 0 to 5.26% due to hydration. However, the mortar mixed with metakaolinite (Fig. 6f) produced 5.25% Ca2Al2SiO7·nH2O, which was not found in the mortar without metakaolinite (Fig. 6e). Therefore, Ca2Al2SiO7 is generated by the action of active metakaolinite and the CaO of shell lime. Accordingly, the sections of mortars with metakaolinite showed higher degrees of carbonation (thickness of the carbonized layer: approximately 5 mm on the surface) than those without metakaolinite (thickness of the carbonized layer: approximately 2 mm on the surface), as shown in Fig. 7. After 900 days, the mortar mixed with metakaolinite produced more Ca2Al2SiO7·nH2O and β-CaSiO3·nH2O than the shell lime mortars. As a result, the CaCO3 contents decreased in shell lime mortars mixed with metakaolinite because it generated more hydration products.
The variations of the relative percentages of the contents during the carbonation and hydration process were examined by XRD to determine how the components transformed in the mortars. Figure 8 shows the changes in the relative components. The CaCO3, β-CaSiO3·nH2O, and Ca2Al2SiO7·nH2O contents increased rapidly in the first 5 days, while the CaO, β-CaSiO3, and Ca2Al2SiO7 contents decreased because of consumption during the carbonation and hydration processes. After 900 days, the carbonation and hydration product increased at a slower rate than in the early stage, and the consumption of CaO, β-CaSiO3, and Ca2Al2SiO7 decreased.
The mortar-modified ginger nut mixed with metakaolinite (LP) produced more Ca2Al2SiO7·nH2O than the mortar without metakaolinite (L), and the modified AGA clay mortar (A and AP) showed results similar to those of the modified ginger nut and modified AGA clay. However, the mortar shell lime mixed with metakaolinite (HP) produced less CaCO3 but more β-CaSiO3·nH2O and Ca2Al2SiO7·nH2O than the mortar shell lime without metakaolinite (H), and the rate of CaO consumption was higher. This could be explained by the reaction of Al2O3·SiO2 and Ca(OH)2 (formula (5)). In addition, the relative percentage of Ca2Al2SiO7 initially increased and then decreased. CaO consumption in the shell lime mortar was lower than in the modified ginger nut and AGA clay, which was likely due to the higher CaO contents in the original shell lime than in the other two limes.
The energy dispersive spectrometry (EDS) results (Fig. 9) showed that the weight percentage (Wt) of Ca increased from 4.39% to 61.06% when modified using metakaolinite (HP), while the percentage of Si decreased from 80.39% to 24.31%. This further proves that the lime mortars produced more carbonation with the modification of metakaolinite, which could also be inferred from the SEM results (Fig. 10).
Conclusion
In this study, we tried to modify Chinese hydraulic limes using metakaolinite. The following conclusions were drawn concerning the mechanical properties and weather resistance of lime combined with metaklinite. The physical properties of the Chinese hydraulic lime mortars, such as shrinkage, porosity, mechanical strength, and weather resistance, were considerably improved. The mortar fluidity and initial setting time decreased because metaklinite can replace some of the lime. The porosity increased because active SiO2 in metaklinite promoted the hydration and carbonation processes, resulting in more pores during consolidation. Adding metakaolin resulted in additional hydrate phases that contributed to the mechanical strength and weather resistance ability. XRD and SEM indicated that more carbonation and hydration products were produced after mixing with metakaolinite, and more uniform and consolidated structures were formed from the effects of metakaolinite. The shell lime mortars were considerably modified when adding metakaolinite compared to ginger nut and AGA soil. The mortars modified in this study provided a selection for crack grouting or repair works. The application issue and evaluation of the restoration results will be examined in future work.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
References
Curulli A, Montesperelli G, Ronca S, Cavalagli N, Ubertini F, Padeletti G, et al. A multidisciplinary approach to the mortars characterization from the Town Walls of Gubbio (Perugia, Italy). J Therm Anal Calorim. 2020;142(1):1721–37. https://doi.org/10.1007/s10973-020-09937-9.
Fort R, Varas-Muriel MJ, Ergen D, Cassar J, Anastasi M, Vella NC. The technology of ancient lime mortars from the Żejtun roman villa (malta). Archaeol Anthropol Sci. 2023;15:15. https://doi.org/10.1007/s12520-022-01710-3.
Yildizlar B, Sayin B, Akcay C. A case study on the restoration of a historical masonry building based on field studies and laboratory analyses. Int J Arch Herit. 2020;14(9):1341–59. https://doi.org/10.1080/15583058.2019.1607625.
Fournari R, Kyriakou L, Ioannou I. On the effect of poor-quality aggregates on the physico-mechanical performance of repair lime-based mortars. In: Bokan Bosiljkov, V, Padovnik, A, Turk, T (eds) Conservation and Restoration of Historic Mortars and Masonry Structures. HMC 2022. RILEM Bookseries. 2023; 42: 416–425. https://doi.org/10.1007/978-3-031-31472-8_33.
Dai SB. Building limes for cultural heritage conservation in China. Heritage Sci. 2013;1:25. https://doi.org/10.1186/2050-7445-1-25.
Dettmering T. Modernised traditional lime plasters for modern historic living of built heritage: Case Studies from Germany and Reflection for China. Built Heritage. 2019;3(1):26–36. https://doi.org/10.1186/BF03545733.
Dettmering T, Dai SB. Types of lime binders in mortars used for the construction of the Ming Great Wall of China and their importance for the development of a conservation strategy. Built Heritage. 2022;6:1. https://doi.org/10.1186/s43238-022-00047-z.
Santhanam K, Ramadoss R. Sustainability development and performance evaluation of natural hydraulic lime mortar for restoration. Environ Sci Pollut Res. 2022;29(52):79634–48. https://doi.org/10.1007/s11356-022-21019-x.
Torres I, Matias G, Pinho N. Characterization of old mortars for the formulation of replacement mortars. In: Bokan Bosiljkov, V, Padovnik, A, Turk, T (eds) Conservation and Restoration of Historic Mortars and Masonry Structures. HMC 2022. RILEM Bookseries.2023; 42: 97–107. https://doi.org/10.1007/978-3-031-31472-8_8.
Cristofaro M T, D’Ambrisi A, De Stefano M, Tanganelli M. Mechanical properties of mortars for structural restoration of historic masonry buildings. In: Calabrò, F, Della Spina, L, Piñeira Mantiñán, M J (eds) New Metropolitan Perspectives. NMP 2022. Lecture Notes in Networks and Systems. 2022; 482: 2213–2222. https://doi.org/10.1007/978-3-031-06825-6_213.
Hosseini M, Karapanagiotis I. Advanced Materials for the Conservation of Stone. In: Hosseini, M, Karapanagiotis, I (eds) Advanced Materials for the Conservation of Stone. Springer, Cham. 2018; Chapter 2: 27–55. https://doi.org/10.1007/978-3-319-72260-3_2.
Chen WC, Li L, Li ZX, Zhao LY, Shao MS, Afolagboye LO. Modification of traditional Chinese ginger nut and its mechanical behavior. Constr Build Mater. 2017;144:138–46. https://doi.org/10.1016/j.conbuildmat.2017.03.164.
Isabel T, Gina M, Paulina F. Natural hydraulic lime mortars - The effect of ceramic residues on physical and mechanical behaviour. J Building Eng. 2020;32: 101747. https://doi.org/10.1016/j.jobe.2020.101747.
Francesca S, Nicola P, Costanzo DP, Francesca T. Experimental evaluation of natural hydraulic lime renders with nanoclay and nanolime to protect raw earth building surfaces. Case Stud Construction Mater. 2022;17: e01564. https://doi.org/10.1016/j.cscm.2022.e01564.
Zeng XC, Yu H, Wu CY. An overview of study on basic magnesium sulfate cement and concrete in China (2012–2019). KSCE J Civ Eng. 2019;23(10):4445–53. https://doi.org/10.1007/s12205-019-0199-7.
Li JJ, Zhang BJ. Why ancient Chinese people like to use organic-inorganic composite mortars? - Application history and reasons of organic-inorganic mortars in ancient Chinese buildings. J Archaeol Method Theory. 2018;26:502–36. https://doi.org/10.1007/s10816-018-9380-4.
Li L, Zhang ZJ, Shao MS. Shell lime and its properties used on China’ s ancient buildings. China Cultural Heritage Sci Res. 2015;1:91–6. https://doi.org/10.3969/j.issn.1674-9677.2015.01.024.
Ma QL, Chen GL, Lu YL, Li ZX. The enforcement material for the wall-painting plaster under high humidity condition. Dunhuang Research. 2005;93(5):66–70. https://doi.org/10.3969/j.issn.1000-4106.2005.05.014.
Brooks JJ, Megat Johari MA. Effect of metakaolin on creep and shrinkage of concrete. Cement Concr Compos. 2001;23(6):495–502. https://doi.org/10.1016/S0958-9465(00)00095-0.
Li L, Zhao LY. Study on the lime materials in ancient China. Beijing: Cultural Relics Publishing House; 2015. p. 109.
Li ZX, Zhao LY, Li L. Light weight concrete of Yangshao Period of China: The earliest concrete in the world. Science China Technol Sci. 2012;55(3):629–39. https://doi.org/10.1007/s11431-011-4725-1.
Li ZX, Zhao LY, Li L, Wang J. Research on the modification of two traditional building materials in ancient China. Heritage Sci. 2013;1:27. https://doi.org/10.1186/2050-7445-1-27.
Talero R. Performance of metakaolin and Portland cements in ettringite formation as determined by ASTM C 452–68: kinetic and morphological differences. Cem Concr Res. 2005;35(7):1269–84. https://doi.org/10.1016/j.cemconres.2004.10.002.
Zeng JJ, Shui ZH, Wang GM. The early hydration and strength development of high-strength precast concrete with cement /metakaolin systems. J Wuhan Univ Technol-Mater Sci. 2010;25(4):712–6. https://doi.org/10.1007/s11595-010-0077-0.
Guneyisi E, Gesoglu M, Karaoglu S, Mermerdaş K. Strength, permeability and shrinkage cracking of silica fume and metakaolin concretes. Constr Build Mater. 2012;34:120–30. https://doi.org/10.1016/j.conbuildmat.2012.02.017.
Abbasi SM, Ahmadi H, Khalaj G, Ghasemi B. Microstructure and mechanical properties of a metakaolinite-based gopolymer nanocomposite reinforced with carbon nanotubes. Ceram Int. 2016;42(14):15171–6. https://doi.org/10.1016/j.ceramint.2016.06.080.
Zhang S, Fan YF, Huang JD, Surendra PS. Effect of nano-metakaolinite clay on the performance of cement-based materials at early curing age. Constr Build Mater. 2021;291: 123107. https://doi.org/10.1016/j.conbuildmat.2021.123107.
Jordan VSM, Kaze CR, Deutou JG, Venyite P, Nana A, Kamseu E, et al. Evaluation of performances of volcanic-ash-laterite based blended geopolymer concretes: Mechanical properties and durability. J Build Eng. 2021;34: 101935. https://doi.org/10.1016/j.jobe.2020.101935.
The People’s Republic of China. JGJ 98–2000. Specification for mix proportion design of masonry mortar. 2001. p. 10–13.
Razak HA, Wong HS. Strength estimation model for high-strength concrete incorporating metakaolin and silica fume. Cem Concr Res. 2005;35(4):688–95. https://doi.org/10.1016/j.cemconres.2004.05.040.
Love CA, Richardson IG, Brough AR. Composition and structure of C-S-H in white Portland cement-20% metakaolin pastes hydrated at 25°C. Cem Concr Res. 2007;37(2):109–17. https://doi.org/10.1016/j.cemconres.2006.11.012.
Li XM, Wu D, Yin S, Ren KB, Lu GY. Natural hydraulic lime versus lime-metakaolin modified silt in earthen heritages. Mater Struct. 2022;55:197. https://doi.org/10.1617/s11527-022-02034-3.
Rashad AM. Metakaolin as cementitious material: history, scours, production and composition-A comprehensive overview. Constr Build Mater. 2013;41:303–18. https://doi.org/10.1016/j.conbuildmat.2012.12.001.
Rakhimov RZ, Kamalova ZA, Rmilova EY. Blended portland cement based on thermally activated clays and carbonate additives. Inorganic Mater Appl Res. 2018;9:578–83. https://doi.org/10.1134/S2075113318040329.
Paul V, Charles ME, Cyriaque KR, Achile N, Juvenal G, Deutou N, et al. Effect of combined metakaolin and basalt powder additions to laterite-based geopolymers activated by rice husk ash (RHA)/NaOH solution. SILICON. 2022;14(4):1643–62. https://doi.org/10.1007/s12633-021-00950-7.
The People’s Republic of China. GB/T 50082–2009.Standard for test methods of long-term performance and durability of ordinary concrete. 2009. p. 3–46.
The People’s Republic of China. T0506–2005. Examining methods for ISO cementation sand strength. 2006. p. 3–14.
Sabbioni C, Bonaza A, Zappia G. Damage on hydraulic mortars: the Venice Arsenal. J Cult Herit. 2002;3(1):83–8. https://doi.org/10.1016/S1296-2074(02)01163-9.
International Society for Rock Mechanics. Suggested methods for determining the uniaxial compressive strength and deformability of rock materials. 1979. p. 137–140.
Wang SW, Wang SL, Liu Z, Meng ZB, Li BB, Zhao N. Revamp of the sticky rice-lime binder with metakaolin and natural fiber for restoration: Properties and characteristics. J Cult Herit. 2022;57:1–15. https://doi.org/10.1016/j.culher.2022.07.003.
El-Turki A, Ball RJ, Holmes S, Allen WL, Allen GC. Environmental cycling and laboratory testing to evaluate the significance of moisture control for lime mortars. Constr Build Mater. 2010;24(8):1392–7. https://doi.org/10.1016/j.conbuildmat.2010.01.019.
NežErka V, Slížková Z, Tesárek P, Plachý T, Frankeová D, Petráňová V. Comprehensive study on mechanical properties of lime-based pastes with additions of metakaolin and brick dust. Cem Concr Res. 2014;64:17–29. https://doi.org/10.1016/j.cemconres.2014.06.006.
Sepulcre-Aguilar A, Hernández-Olivares F. Assessment of phase formation in lime-based mortars with added metakaolin, Portland cement and sepiolite, for grouting of historic masonry. Cem Concr Res. 2010;40(1):66–76. https://doi.org/10.1016/j.cemconres.2009.08.028.
Petra S, Violeta BB, Marjan M. Alkali-dolomite reaction in air lime mortar-implications for increased strength and water resistance. J Cult Herit. 2020;45:160–8. https://doi.org/10.1016/j.culher.2020.02.007.
The People’s Republic of China. GB/T 41060–2021. Test Method for Frost Resistance of Cement Mortar.
Van Balen K. Carbonation reaction of lime, kinetics at ambient temperature. Cem Concr Res. 2005;35(4):647–57. https://doi.org/10.1016/j.cemconres.2004.06.020.
Pesce C, Godina MC, Henry A, Pesce G. Towards a better understanding of hot-mixed mortars for the conservation of historic buildings: the role of water temperature and steam during lime slaking. Heritage Sci. 2021;9:72. https://doi.org/10.1186/s40494-021-00546-9.
Linda MS, Janille M, Paolo S, Michel DT, James CW, Admir M. Hot mixing: Mechanistic insights into the durability of ancient Roman concrete. Sci Adv. 2023;9:eadd1602. https://doi.org/10.1126/sciadv.add1602.
Acknowledgements
This research was funded by the National Key R & D plan, Grant Number 2019YFC1520600 and National Natural Science Foundation of China, Grant Number 41902285, National High-Level Talent Special Support Plan (2023), and Fundamental Research Funds of China Academy of Cultural Heritage (2024).
Funding
The National key R & D plan, Grant Number 2019YFC1520600. National Natural Science Foundation of China, Grant Number 41902285. National High-Level Talent Special Support Plan (2023). Fundamental Research Funds of China Academy of Cultural Heritage (2024).
Author information
Authors and Affiliations
Contributions
“Methodology, Li.Li. and Weichang Chen.; validation, Li.Li. and Weichang Chen.; formal analysis, Weichang Chen.; investigation, Li.Li. and Weichang Chen.; resources, Li.Li. and Weichang Chen.; data curation, Li.Li. and Weichang Chen.; writing—original draft preparation, Li.Li. and Weichang Chen.; writing—review and editing, Li.Li. and Weichang Chen.; supervision, Li.Li.; project administration, Li.Li. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approved the final manuscript and the submission to this journal.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Chen, W., Li, L. A new ingredient to improve Chinese traditional hydraulic lime and its assessment on stone heritage conservation. Herit Sci 12, 111 (2024). https://doi.org/10.1186/s40494-024-01228-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-024-01228-y