Abstract
Rationale
Intensive care unit-acquired weakness (ICUAW) is common in critically ill patients, characterized by muscle weakness and physical function loss. Determining risk factors for ICUAW poses challenges due to variations in assessment methods and limited generalizability of results from specific populations, the existing literature on these risk factors lacks a clear and comprehensive synthesis.
Objective
This overview aimed to synthesize risk factors for ICUAW, categorizing its modifiable and nonmodifiable factors.
Methods
An overview of systematic reviews was conducted. Six relevant databases were searched for systematic reviews. Two pairs of reviewers selected reviews following predefined criteria, where bias was evaluated. Results were qualitatively summarized and an overlap analysis was performed for meta-analyses.
Results
Eighteen systematic reviews were included, comprising 24 risk factors for ICUAW. Meta-analyses were performed for 15 factors, while remaining reviews provided qualitative syntheses. Twelve reviews had low risk of bias, 4 reviews were unclear, and 2 reviews exhibited high risk of bias. The extent of overlap ranged from 0 to 23% for the corrected covered area index. Nonmodifiable factors, including advanced age, female gender, and multiple organ failure, were consistently associated with ICUAW. Modifiable factors, including neuromuscular blocking agents, hyperglycemia, and corticosteroids, yielded conflicting results. Aminoglycosides, renal replacement therapy, and norepinephrine were associated with ICUAW but with high heterogeneity.
Conclusions
Multiple risk factors associated with ICUAW were identified, warranting consideration in prevention and treatment strategies. Some risk factors have produced conflicting results, and several remain underexplored, emphasizing the ongoing need for personalized studies encompassing all potential contributors to ICUAW development.
Similar content being viewed by others
Introduction
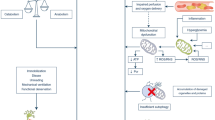
Intensive care unit-acquired weakness (ICUAW), coined in 1984 [1], is defined as a neuromuscular condition developing during extended intensive care unit stays or as clinically detected weakness explained by critical illness as the main reason [2, 3]. The neuromuscular dysfunction of ICUAW has no clear etiology related to critical illness or the associated treatments [2]. The reported incidence of ICUAW varies widely, with studies indicating rates from 25% to as high as 100%. This variability can be attributed to factors such as the characteristics of the patient population under study and the timing of evaluations [2, 4, 5]. The diagnosis of ICUAW is based on clinical findings (Medical Research Council score, MRC), electrophysiological assessments, radiological techniques (muscle ultrasound, magnetic resonance), and, if necessary, muscle biopsies (histological and molecular analysis) [6, 7]. The signs of ICUAW include loss of muscle mass and short- and long-term physical deterioration [8]. The disorder presents with generalized, symmetrical muscle weakness, affecting the muscles of the limbs (mainly proximal) and respiratory muscles, while facial, and ocular muscles usually remain unscathed [2, 3]. The long-term effects of ICUAW can be significant and can impact a patient's quality of life, including persistent fatigue and a reduced ability to perform daily activities [6, 9].
Determining risk factors for ICUAW and neuropathies in critically ill patients poses challenges due to variations in assessment methods and limited generalizability of results from specific populations [10, 11]. The existing literature on these risk factors lacks a clear and comprehensive synthesis. The objective of this overview was to comprehensively identify and synthesize all the reported risk factors for ICUAW or similar conditions in critically ill patients.
Methods
An overview of systematic reviews was conducted. The study protocol was registered in PROSPERO (CRD42020207863). Guidelines set forth by the JBI Collaboration [12, 13] and the PRIOR statement [14] were followed.
Search strategy
A systematic, sensitive, and reproducible search strategy was conducted up to August 2023. The following electronic databases were searched: MEDLINE, EMBASE, CINAHL, Cochrane Library, Google Scholar, and Epistemonikos (Supplementary Table 1).
Selection criteria and study selection
Systematic reviews with or without meta-analyses were included. Only reviews with critical methodological components, such as comprehensive search strategies and risk of bias assessment [14], were integrated into the analysis. This meticulous approach aimed to minimize selection and interpretation biases, as comprehensive searches spanned multiple databases and were unrestricted by language or publication date, enhancing the inclusivity of the study selection.
The target population consisted of adult patients hospitalized in intensive care units (ICU). Risk factors, defined as any condition or attribute that increases the likelihood of ICUAW.
As for the event of interest (outcome), systematic reviews that addressed ICUAW or similar diagnoses, such as critical illness polyneuromyopathy (CIPNM), critical illness polyneuropathy (CIP), and critical illness myopathy (CIM), were considered. Systematic reviews also eligible if they addressed any outcome related to muscular weakness. This inclusive criterion ensure that our review captured the complete spectrum of factors cited in previous systematic reviews, including biological, pre-existing and illness-associated factors.
For this overview, studies related to social or economic risk factors, or external factors such as the infrastructure of the facility, access, and availability of resources, were not considered and were excluded. This exclusion criterion was applied to maintain a focused analysis on the clinical and physiological aspects directly associated with the patient's condition and treatment within the ICU setting, thereby delineating the scope of our review to factors that are inherently linked to the patient's immediate medical care and biological responses.
The screening and review selection was independently conducted by two pairs of collaborators (RGA, RTC, GMN, FGS) using COVIDENCE® [15]. Discrepancies were resolved by a third reviewer (RFA) and a senior researcher (PS). The selection process and reasons for exclusion are presented in accordance with the PRISMA 2020 flow diagram [16].
Data extraction, and data analysis
Data extraction carried out by the lead author (RFA) was verified by two coauthors (RGA, GMN) using a standardized data collection form. The data extracted from the selected systematic review included: year of publication, author(s), title, type of study (systematic review with or without meta-analyses), characteristics of the study population, study designs, number of primary studies included, the range of participants covered in the reviews, and the diagnostic tool used.
Both quantitative and descriptive information concerning risk factors from each systematic review was meticulously extracted. Risk factors are categorized as modifiable and nonmodifiable to underscore their potential for clinical intervention. This classification approach was derived from definitions found in the literature, particularly a narrative review led by experts in ICUAW [3]. The findings were clearly delineated, presenting each risk factor alongside its respective association outcome with ICUAW. Meta-analysis results are described only when they were conducted in the included systematic reviews. These results are detailed in the text for each meta-analysis, specifying whether or not there is an association of each risk factor with ICUAW. Additionally, the results of the heterogeneity analysis presented in the original articles are detailed. The numerical data from these analyses are also presented in the Summary Tables. For systematic reviews without meta-analyses, outcomes were described exactly as reported in the original studies, ensuring no interpretations, selections, or omissions were made. All data are comprehensively displayed in the text, tables, and figures.
A systematic review of systematic reviews should assess and report the degree of overlap of primary studies in the conducted meta-analyses [17].
In this overview, such an analysis was performed for systematic reviews with meta-analyses reporting the same risk factor. The "Corrected Coverage Area Index" (CCA) was calculated for these overlap analyses, and a heatmap was created to visualize the overlap results using the "ccaR package" [18]. The results of the overlaps for each risk factor are detailed in the text and categorized as Slight (CCA: 0–5), Moderate (CCA: 6–10), High (CCA: 11–15), or Very High (> 15), following the CCA interpretation guidelines by Pieper et al. [17]. The level of overlap is reported exclusively in the results section; it is described for informational purposes without specific strategies to address it. Further details on data extraction and analysis are provided in the supplementary material.
Risk of bias assessment
The risk of bias was assessed using ROBIS tool [19]. Two reviewers (GMN, RGA) independently assessed bias risk, while disagreements were resolved by the senior (PS) and principal researchers (RFA). Risk of bias didn´t influence study eligibility or exclusion in this overview. A graphical representation of the bias risks was created using the templates from the "resources for ROBIS tool" at the University of Bristol [20].
Results
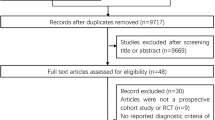
Out of 9090 titles found, screening yielded 122 reviews, of which 18 were included after full text review. (Fig. 1, PRISMA flow diagram). Of them, ten included meta-analyses [21,22,23,24,25,26,27,28,29,30] while eight were qualitative reviews of primary studies [31,32,33,34,35,36, 38, 39] (Table 1 and Supplemental material Table 1 complementary).
Risk of bias analysis
The risk of bias analysis revealed that 12 systematic reviews had a "low risk of bias" [21,22,23,24,25,26,27,28,29,30, 35, 39], 4 reviews had an "unclear risk of bias" [32, 33, 36, 38], and 2 reviews had a “high risk of bias” [31, 34]. The reviews were classified as “unclear risk of bias” or “high risk of bias” due to limitations in methodology because the language was restricted (Domain 1), the searches were limited across various databases (Domain 2), the risk of bias assessments in the primary studies was inadequate (Domain 3), or the reviews failed to address the biases in primary studies (Domain 4). Most reviews with a higher or uncertain risk of bias were published before 2012. Table 2 shows the ROBIS assessments, and Fig. 2 shows the overall risk of bias ratings.
Risk factors for ICUAW
Twenty-four risk factors for ICUAW were identified, predominantly nonmodifiable, including sex, age, and severity of pathology upon ICU admission, among others. Modifiable factors that were identified include hyperglycemia, use of neuromuscular blocking agents (NMBAs), corticosteroid treatment, aminoglycoside use, renal replacement therapy (RRT), and norepinephrine (NA) use. All factors identified across the systematic reviews are detailed and schematized in Figs. 3 and 4. Tables 3 and 4 describe the magnitude of the association of each risk factor with ICUAW.
Nonmodifiable factors
Three systematic reviews assessed inherent and pre-existing patient characteristics [23, 29, 39], including biological sex, age, presence of comorbidities (Table 3). The association of ICUAW with biological sex was analyzed in three systematic reviews, including two meta-analyses [29, 39]. The meta-analyses demonstrated association between female biological sex and ICUAW with low heterogeneity. The CCA index for the two reviews was 12.50, indicating high overlap (Fig. 5A). A systematic review showed that the female biological sex was associated with a higher risk of developing ICUAW [23].
The association of age with ICUAW was described in three systematic reviews. A meta-analysis showed a significant association between older age and ICUAW with moderate heterogeneity [29]. Annoni et al. reported a positive association in 4 out of 19 studies analyzed, and their meta-analyses revealed association of age with ICUAW with low heterogeneity [39]. Yang et al. found no association between age and ICUAW with high heterogeneity [23].
One review addressed the presence of pre-existing comorbidities. In the review by Annoni et al. no association between a history of diabetes and ICUAW was detected in the meta-analyses or in the seven other single studies analyzed in the review [39]. No other systematic reviews addressed comorbidities as a risk factor for ICUAW.
Three reviews with meta-analyses detected association between illness severity (APACHE II) and ICUAW but with high heterogeneity between primary studies [23, 29, 39]. APACHE II scores predicted CIPNM in mechanically ventilated patients in another study [36].
No meta-analyses of systematic reviews addressed the association of systemic inflammatory response syndrome (SIRS) with ICUAW. However, Yang et al. [23] described two independent studies showing association between SIRS and ICUAW wherein prolonged SIRS was a risk factor for ICUAW. The review by Hohl et al. [36] concluded that SIRS was a significant predictor of CIPNM based on a primary study.
Four reviews reported results concerning the association between sepsis and ICUAW. One review detected a positive association with low heterogeneity [39]. A more recent review showed an association but was not significant and the heterogeneity was high with no changes after sensitivity analysis [29]. Another review with high heterogeneity showed no association between sepsis and ICUAW [22]. Finally, one review expressed a positive association between sepsis and the duration of sepsis and ICUAW based on a primary study [23]. No overlap was detected (Fig. 5D).
Organ failure, assessed by the sequential organ failure assessment (SOFA) or MOF, was investigated in four reviews with three meta-analyses. Yang et al. [29] and Annoni et al. [39] detected association between SOFA and ICUAW. Although Yang et al. did not detect association between SOFA and ICUAW in their meta-analyses, the results of individual studies suggests that a SOFA score of > 7 and a total SOFA score of > 45 during the 1st week were independent risk factors for ICUAW. Yang et al. also indicated that the duration of dysfunction in two organs and neurological failure were associated with ICUAW in individual studies [23]. The overlap analysis between the systematic reviews was slight (CCA = 0.17) (Fig. 5C).
The association between shock and ICUAW was analyzed in one systematic review. Although meta-analysis was not conducted, the primary study results indicated an association between shock and ICUAW [23]. Infectious diseases were analyzed in one review with a meta-analysis, showing association between infectious diseases and ICUAW with low heterogeneity [29]. Neurological condition or failure (Glasgow coma scale score < 10) was evaluated in a single review without meta-analysis; one primary study detected association between neurological failure and ICUAW [23].
The relationship between MV and ICUAW was analyzed in ten systematic reviews, including four meta-analyses. Yang et al. detected a significant association between MV and ICUAW, but with high heterogeneity [29]. Medrinal et al. detected an association between muscle weakness and a longer duration of MV and ICU stay [28]. Yang et al. reported association between MV with the use of corticosteroids and ICUAW [22]. Annoni et al. detected a positive association between ICUAW and the duration of MV but with high heterogeneity [39]. Hohl et al. indicated that the likelihood of developing CIPNM within 30 days of MV ranged from 8% in the low-risk group to 72% in the high-risk group [36]. Finally, De Jonghe et al. reported that 76% of patients ventilated for > 5 days developed electrophysiological abnormalities, a longer duration of MV, and a twofold increase in mortality [38].
The association between the duration of ICU stay and ICUAW was analyzed in four systematic reviews, including one meta-analysis. The meta-analysis demonstrated a positive association between ICU length of stay and ICUAW, but the heterogeneity between primary studies finding was high even after sensitivity analysis [29].
Only one systematic review [23] analyzed high lactate levels, hyperosmolarity, and electrolyte imbalances, all of which were associated with higher odds of ICUAW in independent studies.
The relationship between severe burns and polyneuropathy was analyzed by McKittrick et al. The occurrence of critical polyneuropathy was detected in 4.4% of the entire burn patient population. And these patients were more likely to have a prolonged ICU stay, which increased the risk of developing CIP [34].
Respiratory muscle dysfunction was analyzed by Prentice et al. [33], respiratory muscle dysfunction was less severe compared to peripheral muscles. However, patients with low maximal inspiratory pressure (MIP) and maximal expiratory pressure (MEP) had lower MRC scores and delayed extubation.
Modifiable factors
Seven modifiable factors related to therapeutic or pharmacological measures used in the treatment of critical illness were evaluated for their association with the development of ICUAW or similar conditions (Table 4).
In the meta-analysis conducted by Yang et al., it was found that blood glucose levels were correlated with ICUAW. Notably, this association was observed without heterogeneity in the results after large-scale studies were excluded from the analysis [29]. No association between glucose and ICUAW was observed by Annoni et al., although the meta-analyses exhibited high heterogeneity [39]. Four reviews described independent studies; one highlighted the association of hyperglycemia with higher ICUAW risk [23]. Another systematic review described a reduction in CIPNM in patients with glycemic control using insulin in combination with early mobilization [32]. Other reviews detected a reduction in the risk and incidence of CIPNM with intensive glycemic control therapy and strict glucose control (< 6.1 mmol/L) [31, 36].
The relationship between NMBAs and ICUAW was the focus of most meta-analyses but the results were contradictory. Five meta-analyses showed no association between NMBAs and ICUAW [24,25,26, 29, 39]. Only one meta-analysis detected association between NMBAs and ICUAW; although the analysis exhibited low heterogeneity, possible bias due to small studies with significant associations could have influenced [27]. In the other meta-analyses, high heterogeneity was notable no conclusive associations [23, 30]. The overlap was high (CCA = 14.9%, Fig. 5I).
Contradictory relationships between corticosteroid treatment and ICUAW were reported in five systematic reviews. One meta-analyses of 18 studies demonstrated a significant association between corticosteroids and a higher risk of ICUAW, with a 67.2% of heterogeneity. Subgroup analyses also yielded conflicting results, based on clinical evaluation results, an association between corticosteroid treatment and ICUAW was detected; however, no association was detected based on electrophysiological analysis [22]. Annoni et al. in a meta-analyses of 3 studies showed a positive association with ICUAW with moderate heterogeneity [39]. Others meta-analyses found no significant association with significant heterogeneity [23, 29]. The CCA for the meta-analyses was very high, 19% (Fig. 5F). The other review did not provide definite findings [32].
Analyses of the relationship between aminoglycosides and ICUAW yielded mixed results. The most recent systematic review by Zi Yang et al. [29] detected association between aminoglycosides and ICUAW with no heterogeneity, whereas two reviews by Tao Yang et al. found association between aminoglycosides and ICUAW with significant heterogeneity [21, 23]. It is important to highlight that when examining patient subgroups, including clinical and electrophysiological evaluations, it was found that aminoglycosides showed no significant association with ICUAW in the electrophysiology subgroup, unlike the clinically evaluated subgroup [21]. The CCA for the meta-analyses was very high (CCA = 23.10%) (Fig. 5G). Finally, Hohl et al. [36] detected association between aminoglycoside use and ICUAW in only one prospective study in patients with SIRS.
Two meta-analyses were conducted on the association between RRT and ICUAW. The systematic review by Zi Yang et al. included four studies and showed association between RRT and ICUAW with good consistency in findings [29]. However, the meta-analysis by Tao Yang et al. reported no association between RRT and ICUAW, with high heterogeneity [22]. No overlap was detected (Fig. 5H).
Only one review focused on the association of norepinephrine use and ICUAW. The meta-analysis with low heterogeneity showed no association between norepinephrine use and ICUAW. However, in the same review, an individual study revealed that norepinephrine treatment was associated with ICUAW in a multivariable analysis [23].
Only two reviews analyzed the association between nutritional support and ICUAW. Yang et al. reported one study showing a significant association between nutritional support and ICUAW based on a multivariable analysis [22]. Lambell et al. [35] evaluated the effects of energy and/or protein delivery in six studies using various methodologies to assess skeletal muscle mass or the cross-sectional area of the biceps. In this review muscle loss ranged from 0 to 22.5% during the first 2 weeks of ICU admission and no association was detected between the delivery of energy and proteins and these changes in skeletal muscle mass.
Discussion
The risk factors identified were scattered across various systematic reviews, none of which comprehensively covered all the risk factors or employed a definitive categorization. In this context, categorizing the risk factors as modifiable and nonmodifiable is based on established definitions in the literature, as highlighted in a narrative review by experts in ICUAW [3]. This categorization not only enhances the analysis but also amplifies the clinical utility of the findings. Distinguishing between factors that clinicians can modify and those that are immutable allows for more targeted and effective patient management in the ICU.
Of the nonmodifiable factors, age, female biological sex, and MOF, which were included in most of the reviews, were consistently associated with a higher risk of developing ICUAW. Advanced age may be related to decreased physiological reserve and increased vulnerability to complications [40, 41]. However, the review by Rooij et al. concluded that the risk of loss of functionality during an ICU stay is not solely dependent on advanced age but is also influenced by the patient's prior state, both cognitively and functionally [37]. One study focused on skeletal muscle metabolism in the context of ICUAW detected sex-specific differences in muscle strength, insulin sensitivity, muscle metabolites, protein degradation pathways, and the cross-sectional area of myocytes. These findings complement the analysis showing that females may be at a disadvantage in the context of ICUAW [42]. MOF, indicating more severe and prolonged illness, was identified as a risk factor for ICUAW in others reviews too [2, 3, 43]. Although MV, disease severity upon ICU admission, and sepsis were the most studied factors, they showed greater heterogeneity in the meta-analyses. This may be due to the diverse pathologies and the severity and type of illness, each with different recovery times, in patients admitted to the ICU [44].
The ICU length of stay, infectious diseases, and the presence of comorbidities may be associated with ICUAW, but these associations cannot be confirmed due to the high heterogeneity between primary studies in reviews, despite the meta-analysis showing an association. No meta-analyses were available for SIRS, neurological failure, shock, high lactate levels, hyperosmolarity, severe burns, or respiratory muscle dysfunction. Conclusions could not be drawn based on primary study descriptions only. However, the potential risk of high lactate levels cannot be overlooked. Lactate is the main metabolite of anaerobic glycolysis induced by hypoperfusion and tissue hypoxia. Hypoperfusion and hypoxia can cause muscle damage and mitochondrial dysfunction, contributing to the onset of ICUAW. Lactate can also act as an inflammatory and oxidative mediator that can contribute to ICUAW [45]. However, more specific studies are needed.
We identified a significant gap concerning the relationship between intrinsic and pre-existing characteristics of critically ill patients and ICUAW. Among these factors, high BMIs or obesity [46,47,48,49,50], prior frailty [40, 41, 51], comorbidities concurrent with the baseline condition or specific pathologies that triggered admission to the ICU including previous strokes, kidney dysfunction, decreased cardiac function, chronic pulmonary disease [44], cardiac surgery [52, 53], severe COVID-19 [54, 55], may play an important role as additional risk factors for ICUAW.
Of note, we did not find systematic reviews that specifically analyze the relationship between obesity and ICUAW. Whether obesity is a risk or protective factor is still under debate. An “obesity paradigm” has been proposed, hypothesizing that obese patients might be able to metabolize their excessive adipose reserves as a predominant energy source and preserve muscle mass during critical illness [56]. However, a study in critically ill patients suggests that obese and nonobese individuals experience muscle mass loss in a similar fashion [46]. Additionally, “sarcopenic obesity” has been proposed, in which fat accumulation and muscle mass loss mutually influence each other, resulting in muscles with excess fat [47]. Obesity also affects calcium signaling and proteins like adiponectin and actinin, influencing muscle contraction [48]. Furthermore, obesity may cause low-grade chronic inflammation, characterized by elevated levels of proinflammatory cytokines and adipokines during critical illness (i.e., an exacerbated inflammatory response in obese patients), which could increase the risk of muscular complications, including ICUAW. Zhao et al. [49] and Hogue et al. [50] investigated the relationship between mortality, MV, and hospital stay in critically ill obese patients but did not address functional outcomes. Both investigations showed that obesity did not increase mortality but did prolong MV, which may impact the incidence of ICUAW. Both reviews highlight the need for further research.
Frailty is a multidimensional syndrome characterized by a decrease in physiological and adaptive reserves, increasing vulnerability to adverse events. Frailty may be an important risk factor for the development of ICUAW. Preliminary epidemiological data suggest a high prevalence of frailty among critically ill patients, which may increase due to the demographic transition of the population [51]. In a systematic review and meta-analysis, Muscedere et al. [41] showed that frailty at the time of ICU admission impacts in hospital and long-term mortality. Additionally, frail patients are less likely to be discharged to return to their homes. Although Muscedere et al. did not address outcomes associated with physical function, this review highlights the potential use of frailty as an independent prognostic predictor in critically ill patients. However, a current systematic review aimed at assessing the impact of age, frailty, and comorbidities on ICU outcomes concluded that these variables were not evaluated in RCTs [57].
Results concerning the association between modifiable factors and ICUAW were inconsistent, reflecting the complex interplay of various therapeutic interventions. Critical factors, including drug dosage, timing of administration, duration of drug usage, and specific pathology being treated, underscore the nuanced impact of these variables on patient outcomes [2, 58, 59].
All reviews concerning the use of aminoglycosides showed significant associations between ICUAW and aminoglycoside use, but half of the meta-analysis exhibited high heterogeneity. Aminoglycosides affect neuromuscular transmission and neurotoxicity and may be involved in the development of ICUAW. Despite the lack of evidence, experts recommend careful monitoring of aminoglycoside levels in the blood and appropriate dosing [2, 60].
Although the results for hyperglycemia were contradictory, glucose variability should be considered in the prevention and treatment of myopathies in critically ill patients [2, 61, 62]. Establishing standards for glycemic control (between 90 and 144 mg/dl) [60] and using intensive insulin therapy may reduce ICUAW [62].
Meta-analyses have produced conflicting results regarding the association between NMBAs and neuromuscular complications. Some studies suggest that NMBAs are not significantly associated with muscle weakness when used alone. However, concurrent use of NMBAs and corticosteroids may elevate the risk of muscle weakness [59]. It is noteworthy that the administration of neuromuscular blockers may affect muscle nerve excitability, potentially leading to muscle weakness in critically ill patients. This interaction with neuromuscular function could pose a risk factor for the development of ICUAW, particularly when combined with other factors such as the duration of mechanical ventilation and illness severity. It is critical to acknowledge that factors like the duration of NMBA infusion, specific patient demographics (e.g., septic patients with multiorgan dysfunction), and simultaneous corticosteroid use might modify the risk associated with NMBAs. Furthermore, some NMBA compounds may share structural similarities with steroids, possibly intensifying the risk of developing myopathies. In summary, while NMBAs may not independently constitute a risk factor for ICUAW in most cases, their use in conjunction with factors such as corticosteroids and extended infusion periods might contribute to neuromuscular complications in critically ill patients [58, 59, 63].
Corticosteroids are commonly used in intensive care units and have been linked to ICUAW, despite the lack of consistent results in meta-analyses [22, 23, 29, 39]. However, excessive administration of corticosteroids can cause muscle dysfunction and nerve damage, promote the breakdown of muscle proteins, and increase protein loss. They can also have side effects such as lipodystrophy, and their use may increase the absorption and turnover of fatty acids in adipose tissue, which is closely related to the onset of ICUAW [59, 60, 63,64,65].
In relation to RRT and acute kidney injury (AKI), a recent literature review highlights the pathophysiological mechanisms, such as protein degradation, inflammation, and metabolic pathway alterations, through which AKI and its treatment with RRT–AKI may contribute to muscle loss, suggesting a relationship with ICU-AW. Preclinical and clinical data indicate that both AKI and RRT–AKI could influence the development of ICU-AW [66].
Norepinephrine is used in the ICU as a vasoconstrictor and positive inotropic agent to manage shock and sepsis, thereby improving arterial perfusion and pressure. Only one systematic review addressed its use as a potential factor in the development of ICUAW. Primary studies indicate that norepinephrine is significantly associated with an increased risk of ICUAW, with a dose-dependent effect that increases risk with each cumulative dose. Therefore, it is recommended to limit norepinephrine exposure and shorten its administration in clinical practice to reduce the incidence of ICUAW [45, 67].
A single review has demonstrated an association between nutritional intake and ICUAW [35]. Malnutrition and nutritional imbalance may increase the risk of ICUAW. Interestingly, the timing of total parenteral nutrition (TPN) administration appears to influence risk; early TPN may increase the likelihood, while early caloric restriction and delayed TPN administration may mitigate it [3]. It is important to mention that recent research findings indicate that early mobilization combined with timely nutrition support significantly reduced the incidence of ICUAW compared to early mobilization alone or standard care [68].
The impact of other medical treatments, such as the use of Propofol [69] or prolonged use of extracorporeal membrane oxygenation ECMO [70], also may contribute to ICUAW and should be more investigated.
Strategic interventions and proactive monitoring for modifiable risk factors: effective management of modifiable risk factors such as hyperglycemia, neuromuscular blockade, corticosteroids, aminoglycosides, and nutritional support is crucial for minimizing ICUAW risks. Implementing systematic glycemic control strategies tailored to individual patient conditions and refining guidelines for neuromuscular blocking agents are essential to balance benefits against the risks of prolonged use. Additionally, precise protocols for the timing and dosage of aminoglycosides require frequent monitoring to prevent ICUAW while effectively treating underlying conditions. Early detection and consistent monitoring enable clinicians to tailor interventions that mitigate risks and improve outcomes, necessitating regular evaluation of drug dosages, treatment timing, and ongoing patient conditions to adjust treatment protocols effectively.
The early detection and consistent monitoring of modifiable risk factors are critical for preventing and managing ICUAW. This proactive approach enables clinicians to tailor interventions that mitigate risk and improve patient outcomes. Regular evaluation of variables such as drug dosages, treatment timing, and ongoing patient conditions is essential for adjusting treatment protocols and ensuring effective management of ICUAW.
In an effort to identify patients at risk of developing ICUAW, various predictive models have been developed [52, 60, 64, 65] A recent systematic review by Zhang et al. [63], identified 11 risk models for ICUAW. These models incorporate a variety of predictors based on the type of diseases of the participants, conceptual definitions, and diagnostic tools used. Additionally, some studies have incorporated more specific variables, such as electrodiagnostic tests and ultrasound of the quadriceps rectus femoris muscle (QRF).
The evaluation of these models shows that their values in the area under the receiver operating characteristic curve (ROC) range from 0.7 to 0.923, indicating a moderate to high discriminatory capacity between patients with and without ICUAW. However, it is noted that most of the models analyzed exhibit certain biases, such as lack of blinding, incomplete reporting, insufficient sample sizes, lack of external validation, and inadequate calibration of the models. Therefore, it is concluded that although some models prove effective in predicting ICUAW, it is crucial to address these deficiencies and conduct additional studies to validate and refine the accuracy of these predictive models before their widespread implementation in clinical settings.
Our findings suggest that predictive models for ICUAW should be flexible and incorporate both modifiable and nonmodifiable factors associated with the condition. It is vital to consider factors that have demonstrated a consistent association, such as age, female gender, and organ failure. Additionally, it is essential to account for factors that may not have a conclusive association due to heterogeneity found in systematic reviews or their absence, yet have a significant pathophysiological basis in the development of ICUAW as discussed in this text. Prominent among these factors are comorbidities such as obesity, frailty, high lactate levels, hyperglycemia, the use of NMBAs, corticosteroids, aminoglycosides, renal replacement therapy, norepinephrine, and nutritional intake.
Strengths and limitations
The findings of this review are primarily based on individual reviews. Any biases, methodological errors, or limitations present in the original reviews could impact the conclusions. The associations identified in this study may be affected by the heterogeneity highlighted in the meta-analyses and the lack of meta-analyses for some factors. We only reported the extent of overlap among the meta-analyses and did not devise a strategy to resolve this aspect. Nevertheless, we generated the overlap analysis matrices and a map delineating the primary studies incorporated in each systematic review (Supplementary Material), which can be utilized for subsequent in-depth analyses.
It is important to note that this review highlighted factors described in selected systematic reviews, leading to limited discussion of other potential factors not addressed in those reviews. Many of these unaddressed factors are related to therapies performed in the ICU, whose causal relationships remain unclear. However, the discussion briefly mentions conclusions from primary studies and narrative reviews which emphasize their possible implications with ICUAW.
A comprehensive literature search was conducted using a sensitive approach to identify all relevant reviews related to ICUAW. Nevertheless, the search may be limited by the omission of other databases or publications in nonconventional languages (primarily Asian languages), which could result in the absence of relevant reviews.
Conclusions
This overview identifies nonmodifiable risk factors for ICU-acquired weakness, such as advanced age, female sex, and organ failure, with the need for targeted monitoring in these patient groups. While modifiable factors like glucose control, neuromuscular blockade, corticosteroid use, aminoglycosides, renal replacement therapy, and norepinephrine show variable impacts on ICUAW risk, it is important to note that some risk factors have yielded contradictory results and high heterogeneity. Furthermore, certain factors remain under-researched, highlighting a persistent need for studies with a more personalized focus that encompass all potential factors contributing to the development of ICUAW. The development of preventive approaches tailored to the complexities of ICUAW is also essential. Our findings underline the necessity of individualized treatment strategies to enhance patient outcomes in the ICU.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The detailed data extracted from the systematic reviews supporting the conclusions of this article are included in the supplementary material. An additional table with extended data is available at the following link: https://l1nk.dev/ZxMLC
Abbreviations
- ICUAW:
-
Intensive Care Unit-Acquired Weakness
- MRC:
-
Medical Research Council
- CIPNM:
-
Critical illness polyneuromyopathy
- CIP:
-
Critical illness polyneuropathy
- CIM:
-
Critical illness myopathy
- PROSPERO:
-
International Prospective Register of Systematic Reviews
- JBI:
-
Joanna Briggs Institute
- PRIOR:
-
Preferred reporting items for overviews of reviews
- PRISMA:
-
Preferred reporting items for systematic reviews and meta-analyses
- CCA:
-
Corrected covered area
- ccaR:
-
R package used for overlap analyses
- ROBIS:
-
Risk of bias in systematic reviews
- 6MWT:
-
6-Minute walk test
- APACHE II:
-
Acute physiology and chronic health evaluation II
- SIRS:
-
Systemic inflammatory response syndrome
- SOFA:
-
Sequential organ failure assessment
- MOF:
-
Multiple organ failure
- MV:
-
Mechanical ventilation
- MIP:
-
Maximal inspiratory pressure
- MEP:
-
Maximal expiratory pressure
- RRT:
-
Renal replacement therapy
- AKI:
-
Acute kidney injury
- TPN:
-
Total parenteral nutrition
- ECMO:
-
Extracorporeal membrane oxygenation
- QRF:
-
Quadriceps rectus femoris
- ROC:
-
Receiver operating characteristic
References
Bolton C, Gilbert J, Angelika F, Sibbald W. Polineuropathy in critically ill patients. Neurol Neurosurg Psychiatry. 1984;47:1223–31.
Hermans G, Van den Berghe G. Clinical review: intensive care unit acquired weakness. Crit Care. 2015;19(1):274.
Vanhorebeek I, Latronico N, Van den Berghe G. ICU-acquired weakness. Intensive Care Med. 2020;46(4):637–53.
Wieske L, Dettling-Ihnenfeldt DS, Verhamme C, Nollet F, van Schaik IN, Schultz MJ, et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19(1):196.
Appleton RT, Kinsella J, Quasim T. The incidence of intensive care unit-acquired weakness syndromes: a systematic review. J Intensive Care Soc. 2015;16(2):126–36.
Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364(14):293–304.
Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94.
Needham DM, Davidson J, Cohen H, Hopkins RO, Weinert C, Wunsch H, et al. Improving long-term outcomes after discharge from intensive care unit: report from a stakeholders’ conference. Crit Care Med. 2012;40(2):502–9.
Liu K, Tronstad O, Flaws D, Churchill L, Jones AYM, Nakamura K, et al. From bedside to recovery: exercise therapy for prevention of post-intensive care syndrome. J Intensive Care. 2024;12(1):11.
Fan E, Cheek F, Chlan L, Gosselink R, Hart N, Herridge MS, et al. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190(12):1437–46.
Piva S, Fagoni N, Latronico N. Intensive care unit-acquired weakness: unanswered questions and targets for future research. F1000Res. 2019;8:508. https://doi.org/10.12688/f1000research.17376.1.
Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z, editors. JBI Manual for Evidence Synthesis. JBI; 2024. Available from: https://synthesismanual.jbi.global. https://doi.org/10.46658/JBIMES-24-01.
Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40.
Gates M, Gates A, Pieper D, Fernandes RM, Tricco AC, Moher D, et al. Reporting guideline for overviews of reviews of healthcare interventions: development of the PRIOR statement. BMJ. 2022;378: e070849.
Covidence systematic review software Melbourne, Australia. www.covidence.org.
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev. 2022;18(2): e1230.
Pieper D, Antoine SL, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368–75.
Bougioukas KI, Diakonidis T, Mavromanoli AC, Haidich AB. ccaR: a package for assessing primary study overlap across systematic reviews in overviews. Res Synth Methods. 2023;14(3):443–54.
Whiting P, Davies P, Savović J, Caldwell D, Churchill R, Group R. Evidence to inform the development of ROBIS, a new tool to assess the risk of bias in systematic reviews. J Clin Epidemiol. 2015. https://doi.org/10.1016/j.jclinepi.2015.06.005.
Whiting P. ROBIS tool and resources 2023. https://www.bristol.ac.uk/population-health-sciences/projects/robis/resources/.
Yang T, Li ZQ, Li HL, Zhou JX, Chen GQ. Aminoglycoside use and intensive care unit-acquired weakness: a systematic review and meta-analysis. PLoS ONE. 2020;15(3): e0230181.
Yang T, Li Z, Jiang L, Xi X. Corticosteroid use and intensive care unit-acquired weakness: a systematic review and meta-analysis. Crit Care. 2018;22(1):187.
Yang T, Li Z, Jiang L, Wang Y, Xi X. Risk factors for intensive care unit-acquired weakness: a systematic review and meta-analysis. Acta Neurol Scand. 2018;138(2):104–14.
Wei XB, Wang ZH, Liao XL, Guo WX, Qin TH, Wang SH. Role of neuromuscular blocking agents in acute respiratory distress syndrome: an updated meta-analysis of randomized controlled trials. Front Pharmacol. 2020;10:1637.
Tarazan N, Alshehri M, Sharif S, Al Duhailib Z, Moller MH, Belley-Cote E, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: updated systematic review and meta-analysis of randomized trials. Intensive Care Med Exp. 2020;8(1):61.
Shao S, Kang H, Tong Z. Early neuromuscular blocking agents for adults with acute respiratory distress syndrome: a systematic review, meta-analysis and meta-regression. BMJ Open. 2020;10(11): e037737.
Price DR, Mikkelsen ME, Umscheid CA, Armstrong EJ. Neuromuscular blocking agents and neuromuscular dysfunction acquired in critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(11):2070–8.
Medrinal C, Combret Y, Hilfiker R, Prieur G, Aroichane N, Gravier FE, et al. ICU outcomes can be predicted by noninvasive muscle evaluation: a meta-analysis. Eur Respir J. 2020. https://doi.org/10.1183/13993003.02482-2019.
Yang Z, Wang X, Wang F, Peng Z, Fan Y. A systematic review and meta-analysis of risk factors for intensive care unit acquired weakness. Medicine. 2022;101(43): e31405.
Bellaver P, Schaeffer AF, Leitao CB, Rech TH, Nedel WL. Association between neuromuscular blocking agents and the development of intensive care unit-acquired weakness (ICU-AW): a systematic review with meta-analysis and trial sequential analysis. Anaesth Crit Care Pain Med. 2023;42(3): 101202.
Ydemann M, Eddelien HS, Lauritsen AØ. Treatment of critical illness polyneuropathy and/or myopathy—a systematic review. Dan Med J 2012;59(10).
Sánchez Solana L, Goñi Bilbao I, Ruiz García P, Díaz Agea JL, Leal CC. Acquired neuromuscular dysfunction in the intensive care unit. Enfermería Intensiva (English ed). 2018;29(3):128–37.
Prentice CE, Paratz JD, Bersten AD. Differences in the degree of respiratory and peripheral muscle impairment are evident on clinical, electrophysiological and biopsy testing in critically ill adults: a qualitative systematic review. Crit Care Resusc. 2010;12(2):111–20.
Mc Kittrick A, Kornhaber R, Harats M, Cleary M, Visentin DC, Haik J. Critical care polyneuropathy in burn injuries: an integrative review. Burns. 2017;43(8):1613–23.
Lambell KJ, King SJ, Forsyth AK, Tierney AC. Association of energy and protein delivery on skeletal muscle mass changes in critically ill adults: a systematic review. J Parent Enteral Nutr. 2018;42(7):1112–22.
Hohl M-L. Critical illness polyneuropathy and myopathy: a review. World Crit Care Nurs. 2006. https://doi.org/10.1891/1748-6254.5.2.38.
de Rooij SE, Abu-Hanna A, Levi M, de Jonge E. Factors that predict outcome of intensive care treatment in very elderly patients: a review. Crit Care. 2005;9(4):R307–14.
De Jonghe B, Cook D, Sharshar T, Lefaucheur J-P, Carlet J, Outin H. Acquired neuromuscular disorders in critically ill patients: a systematic review. Intensive Care Med. 1998;24:1242–50.
Annoni R, Jones J, Seraphim Ferreira D, Berney S, Denehy L. Risk factors for intensive care acquired weakness: a systematic review and meta-analysis. Crit Care. 2017;21(S2).
Jung C, Bruno RR, Wernly B, Wolff G, Beil M, Kelm M. Frailty as a prognostic indicator in intensive care. Dtsch Arztebl Int. 2020;117(40):668–73.
Muscedere J, Waters B, Varambally A, Bagshaw SM, Boyd JG, Maslove D, et al. The impact of frailty on intensive care unit outcomes: a systematic review and meta-analysis. Intensive Care Med. 2017;43(8):1105–22.
Engelhardt LJ, Grunow JJ, Wollersheim T, Carbon NM, Balzer F, Spranger J, et al. Sex-specific aspect of skeletal muscle metabolism in the clinical context of intensive care unit-acquired weakness. J Clin Med. 2022. https://doi.org/10.3390/jcm11030846.
de Jonghe B, Sharshar T, Lefaucheur J-P, Authier F-J, Durand-Zaleski I, Boussarsar M, et al. Paresis adquired in the intensive care unit. JAMA. 2002. https://doi.org/10.1001/jama.288.22.2859.
Trudzinski FC, Neetz B, Bornitz F, Muller M, Weis A, Kronsteiner D, et al. Risk factors for prolonged mechanical ventilation and weaning failure: a systematic review. Respiration. 2022;101(10):959–69.
Yang T, Li Z, Jiang L, Xi X. Hyperlactacidemia as a risk factor for intensive care unit-acquired weakness in critically ill adult patients. Muscle Nerve. 2021;64(1):77–82.
Segaran S, Wandrag L, Stotz M, Terblanche M, Hickson M. Does BMI impact on muscle wasting. J Hum Nutr Diet. 2017;30(2):227–35.
Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13(2):781–94.
Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol. 2018. https://doi.org/10.1242/jeb.163840.
Zhao Y, Li Z, Yang T, Wang M, Xi X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PLoS ONE. 2018;13(6): e0198669.
Hogue CW Jr, Stearns JD, Colantuoni E, Robinson KA, Stierer T, Mitter N, et al. The impact of obesity on outcomes after critical illness: a meta-analysis. Intensive Care Med. 2009;35(7):1152–70.
Bagshaw SM, McDermid RC. The role of frailty in outcomes from critical illness. Curr Opin Crit Care. 2013;19(5):496–503.
Zhong F, Zhang H, Peng Y, Lin X, Chen L, Lin Y. A predictive nomogram for intensive care-acquired weakness after cardiopulmonary bypass. Ann Thorac Cardiovasc Surg. 2023. https://doi.org/10.5761/atcs.oa.23-00029.
Lee JA, Yanagawa B, An KR, Arora RC, Verma S, Friedrich JO, et al. Frailty and pre-frailty in cardiac surgery: a systematic review and meta-analysis of 66,448 patients. J Cardiothorac Surg. 2021;16(1):184.
Yamada K, Kitai T, Iwata K, Nishihara H, Ito T, Yokoyama R, et al. Predictive factors and clinical impact of ICU-acquired weakness on functional disability in mechanically ventilated patients with COVID-19. Heart Lung. 2023;60:139–45.
Schmidt DM, Piva TC, Glaeser SS, Martins D, Pinheiro P, Friedman G, et al. Intensive care unit-acquired weakness in patients with COVID-19: occurrence and associated factors. Phys Ther. 2022. https://doi.org/10.1093/ptj/pzac028.
Goossens C, Marques MB, Derde S, Vander Perre S, Dufour T, Thiessen SE, et al. Premorbid obesity, but not nutrition, prevents critical illness-induced muscle wasting and weakness. J Cachexia Sarcopenia Muscle. 2017;8(1):89–101.
Perrella A, Geen O, Scott S, Kaushik R, Ahuja M, Rochwerg B. Exploring the impact of age, frailty, and multimorbidity on ICU interventions: a systematic review. Can J Anesth. 2023;70:S84–6.
Hraiech S, Forel JM, Papazian L. The role of neuromuscular blockers in ARDS: benefits and risks. Curr Opin Crit Care. 2012;18(5):495–502.
Bourenne J, Hraiech S, Roch A, Gainnier M, Papazian L, Forel JM. Sedation and neuromuscular blocking agents in acute respiratory distress syndrome. Ann Transl Med. 2017;5(14):291.
Wieske L, Witteveen E, Verhamme C, Dettling-Ihnenfeldt DS, van der Schaaf M, Schultz MJ, et al. Early prediction of intensive care unit-acquired weakness using easily available parameters: a prospective observational study. PLoS ONE. 2014;9(10): e111259.
Morgeli R, Wollersheim T, Engelhardt LJ, Grunow JJ, Lachmann G, Carbon NM, et al. Critical illness myopathy precedes hyperglycaemia and high glucose variability. J Crit Care. 2021;63:32–9.
Hermans G, Wilmer A, Meersseman W, Milants I, Wouters PJ, Bobbaers H, et al. Impact of intensive insulin therapy on neuromuscular complications and ventilator dependency in the medical intensive care unit. Am J Respir Crit Care Med. 2007;175(5):480–9.
Zhang W, Tang Y, Liu H, Yuan LP, Wang CC, Chen SF, et al. Risk prediction models for intensive care unit-acquired weakness in intensive care unit patients: a systematic review. PLoS ONE. 2021;16(9): e0257768.
Witteveen E, Wieske L, Sommers J, Spijkstra JJ, de Waard MC, Endeman H, et al. Early prediction of intensive care unit-acquired weakness: a multicenter external validation study. J Intensive Care Med. 2020;35(6):595–605.
Penuelas O, Muriel A, Frutos-Vivar F, Fan E, Raymondos K, Rios F, et al. Prediction and outcome of intensive care unit-acquired paresis. J Intensive Care Med. 2018;33(1):16–28.
Teixeira JP, Mayer KP, Griffin BR, George N, Jenkins N, Pal CA, et al. Intensive care unit-acquired weakness in patients with acute kidney injury: a contemporary review. Am J Kidney Dis. 2023;81(3):336–51.
Wolfe KS, Patel BK, MacKenzie EL, Giovanni SP, Pohlman AS, Churpek MM, et al. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest. 2018;154(4):781–7.
Zhou W, Yu L, Fan Y, Shi B, Wang X, Chen T, et al. Effect of early mobilization combined with early nutrition on acquired weakness in critically ill patients (EMAS): a dual-center, randomized controlled trial. PLoS ONE. 2022;17(5): e0268599.
Abdelmalik PA, Rakocevic G. Propofol as a risk factor for ICU-acquired weakness in septic patients with acute respiratory failure. Can J Neurol Sci. 2017;44(3):295–303.
Chen X, Lei X, Xu X, Huang M. Intensive Care Unit-Acquired Weakness in Patients With Extracorporeal Membrane Oxygenation Support: Frequency and Clinical Characteristics. Front. Med. 2022; 9:792201
Acknowledgements
Rocío Fuentes-Aspe, a Ph.D. student in the Doctoral Program in Biomedical Research Methodology and Public Health at Universitat Autònoma de Barcelona, Spain, would like to acknowledge the program for its methodological support in this work. We would also like to acknowledge the methodological support provided by the Dirección de Investigación at Universidad de La Frontera, DIUFRO DI21-0076.
Funding
Universidad de La Frontera provided funding for this research (Proyecto DIUFRO DI21-0076). No additional external funding was received for this study. The funding body had no role in the design of the study; collection, analysis, and interpretation of data; or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed and conceived by RFA and PS, with input from RGA, FGS, and GMN. RGA, FGS and GMN contributed to the data collection. All analyses were performed by RFA in close consultation with PS. The manuscript was drafted by RF and PS and edited with input from all authors. All authors reviewed and contributed to the discussion of findings and the writing and/or editing of the manuscript and gave final approval to the version submitted for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Fuentes-Aspe, R., Gutierrez-Arias, R., González-Seguel, F. et al. Which factors are associated with acquired weakness in the ICU? An overview of systematic reviews and meta-analyses. j intensive care 12, 33 (2024). https://doi.org/10.1186/s40560-024-00744-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40560-024-00744-0